Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
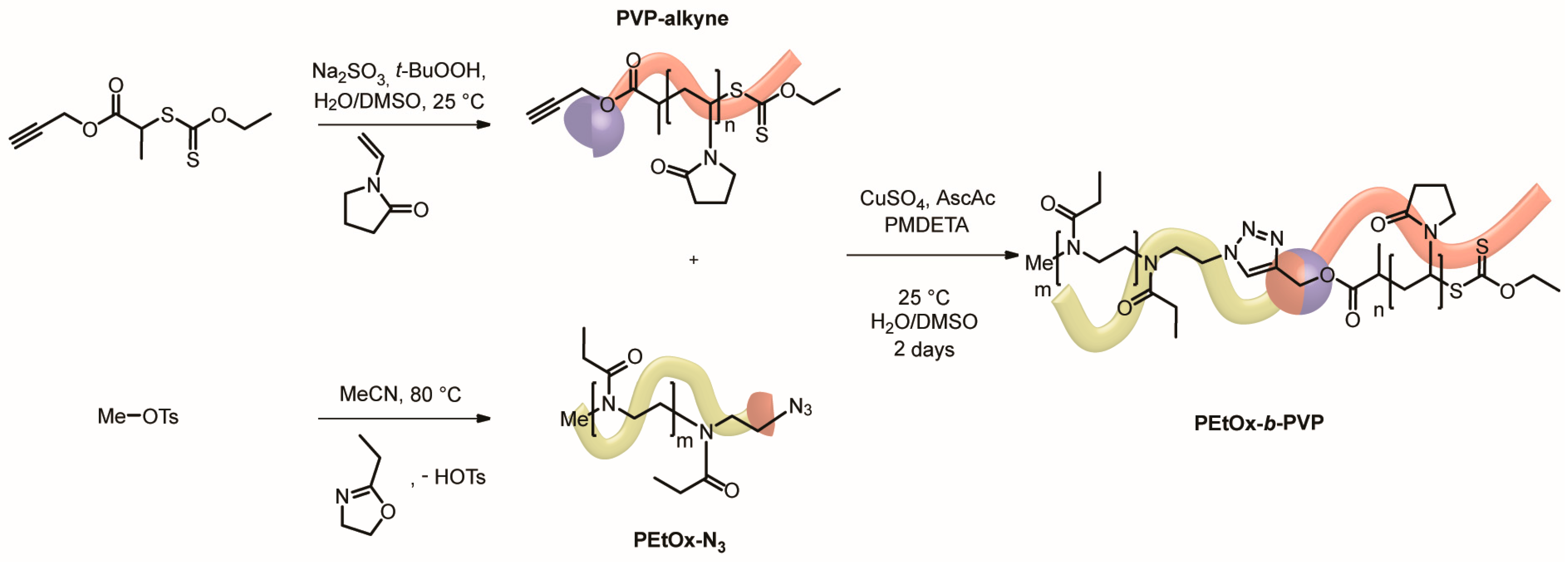
2.2. Synthesis of PVP14k-alkyne
2.3. Synthesis of PVP14k-b-PEtOx22k
2.4. Investigations of Self-Assembly of PVPxxk-b-PEtOx22k in Water
2.5. Characterization Methods
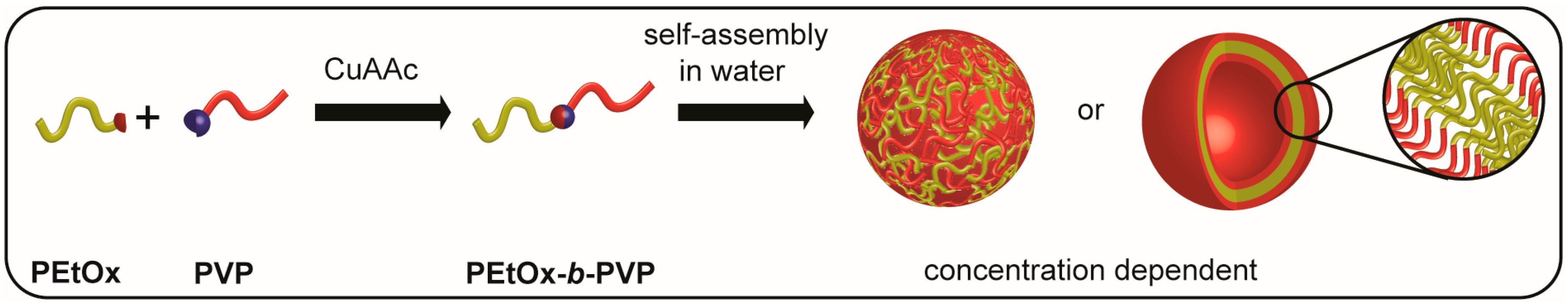
3. Results
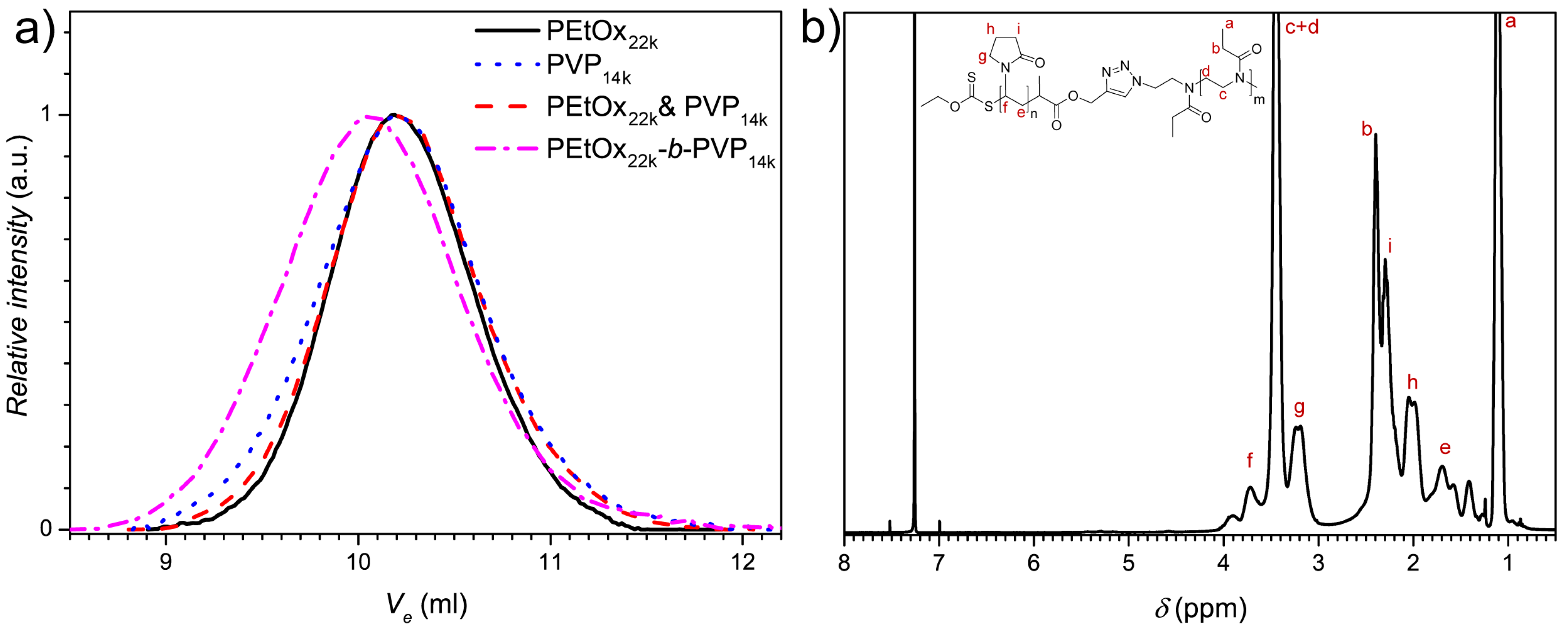
3.1. Synthesis of PEtOx22k-b-PVPxxk
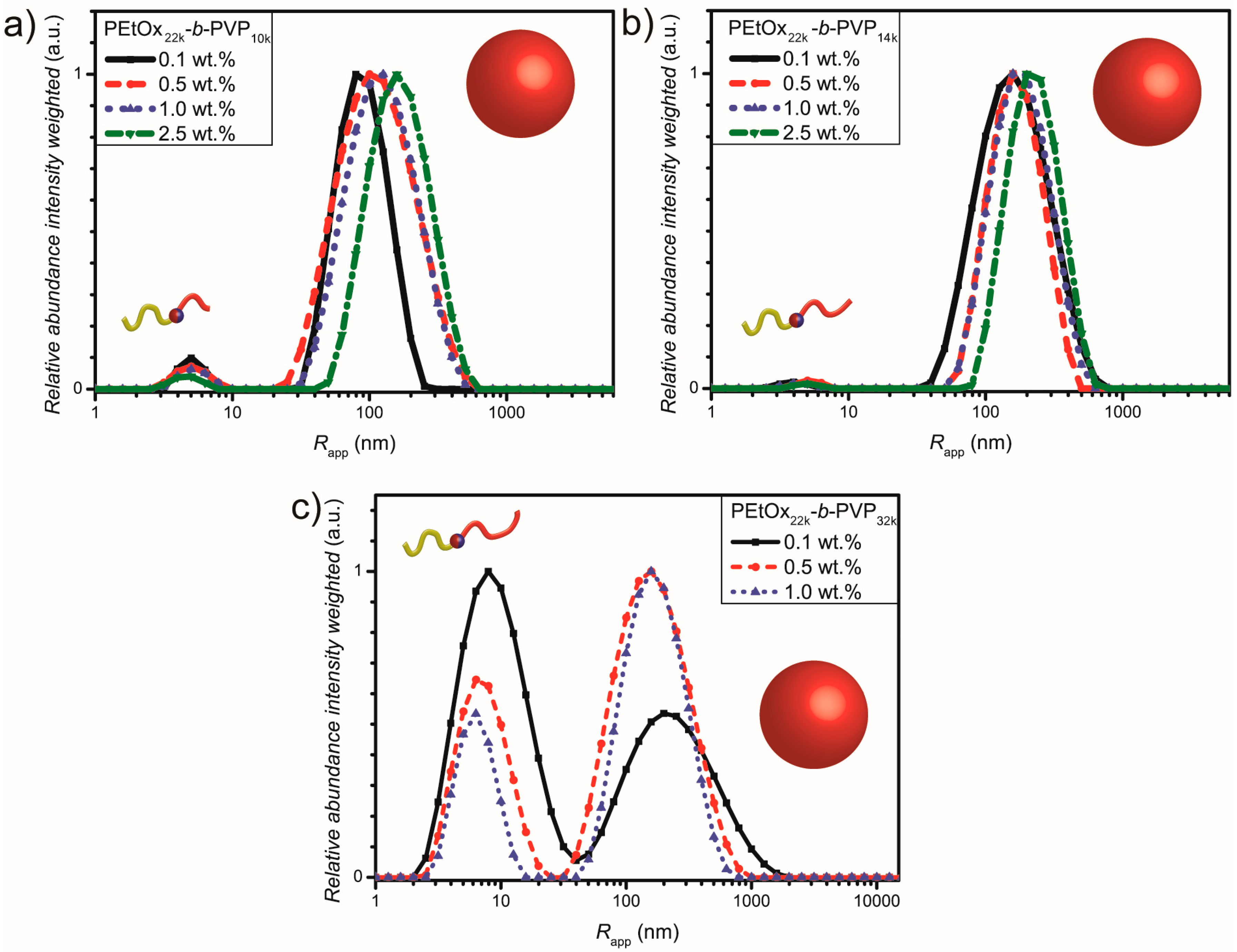
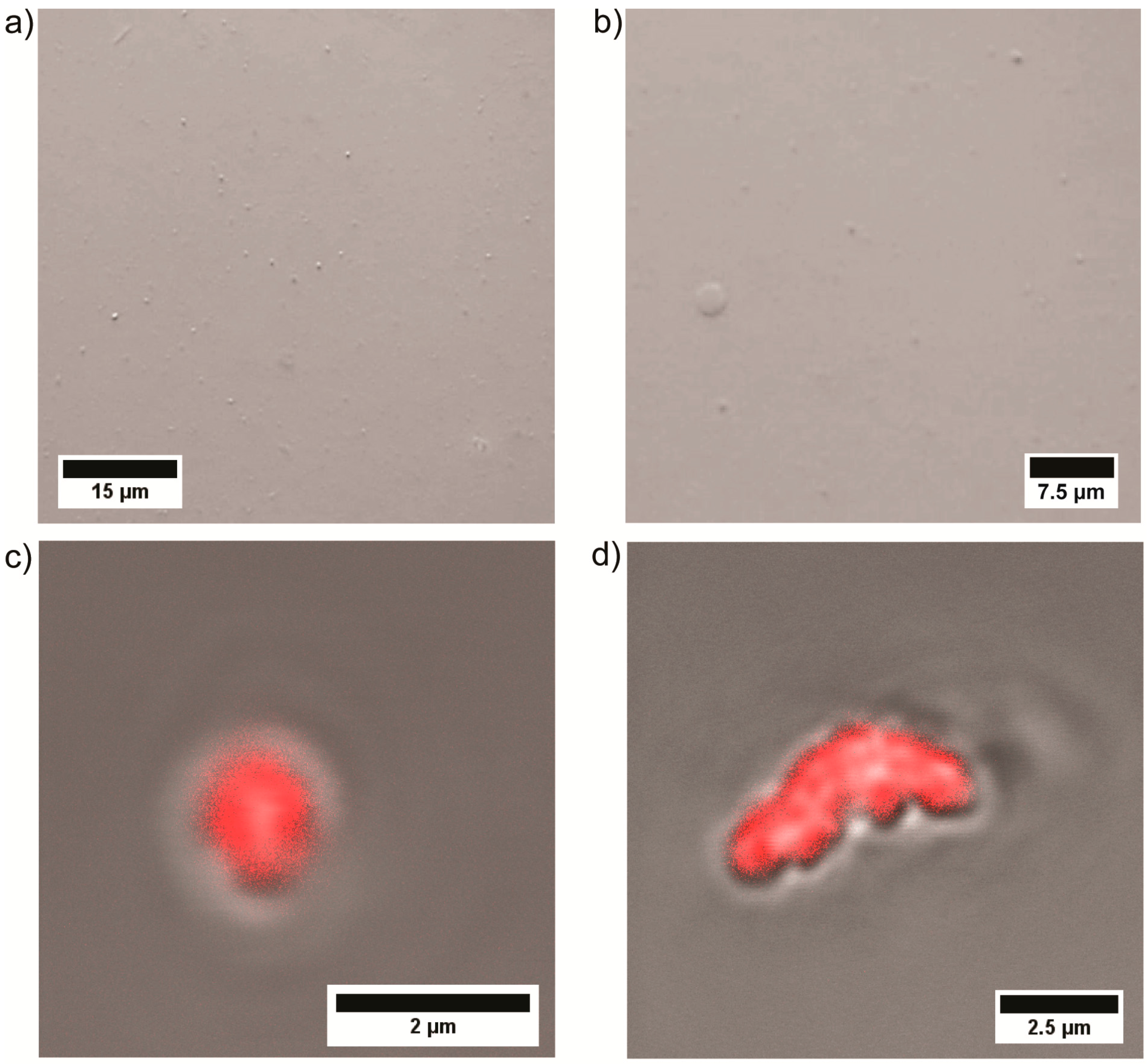
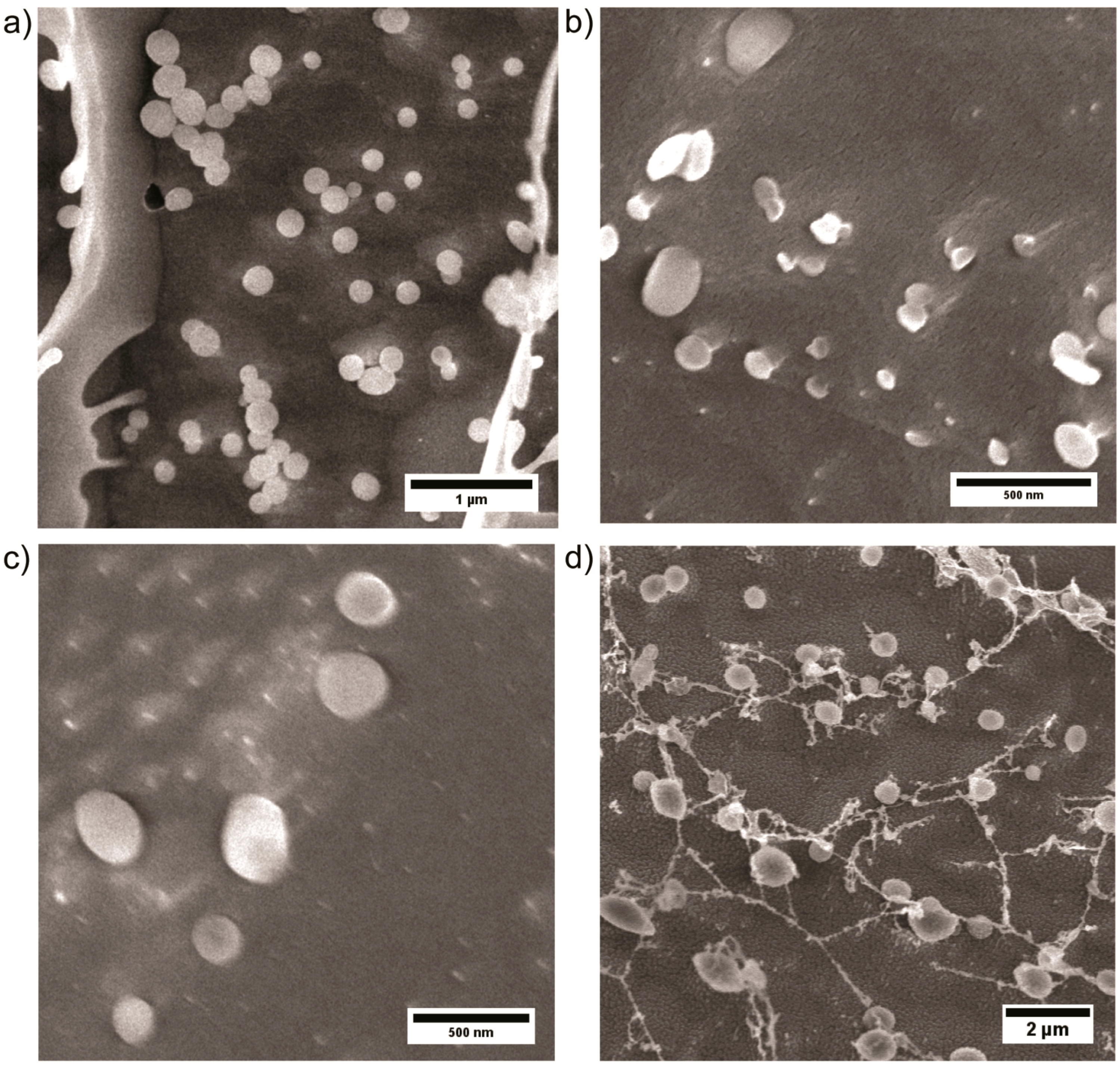
3.2. Self Assembly of PEtOx22k-b-PVPxxk in Water
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suh, H.S.; Kim, D.H.; Moni, P.; Xiong, S.; Ocola, L.E.; Zaluzec, N.J.; Gleason, K.K.; Nealey, P.F. Sub-10-nm patterning via directed self-assembly of block copolymer films with a vapour-phase deposited topcoat. Nat. Nanotechnol. 2017, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Lennon, E.M.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. Evolution of Block Copolymer Lithography to Highly Ordered Square Arrays. Science 2008, 322, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; van Hest, J.C.M. A Polymersome Nanoreactor with Controllable Permeability Induced by Stimuli-Responsive Block Copolymers. Adv. Mater. 2009, 21, 2787–2791. [Google Scholar] [CrossRef]
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE-i-PP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Matsen, M.W. Phase Behavior of Block Copolymer/Homopolymer Blends. Macromolecules 1995, 28, 5765–5773. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Elbert, J.; Scheid, D.; Hawker, C.J.; Klinger, D.; Gallei, M. Metallopolymer-Based Shape Anisotropic Nanoparticles. ACS Macro Lett. 2015, 4, 731–735. [Google Scholar] [CrossRef]
- Boott, C.E.; Gwyther, J.; Harniman, R.L.; Hayward, D.W.; Manners, I. Scalable and uniform 1D nanoparticles by synchronous polymerization, crystallization and self-assembly. Nat. Chem. 2017. [Google Scholar] [CrossRef]
- Gröschel, A.H.; Schacher, F.H.; Schmalz, H.; Borisov, O.V.; Zhulina, E.B.; Walther, A.; Müller, A.H.E. Precise hierarchical self-assembly of multicompartment micelles. Nat. Commun. 2012, 3, 710. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Schmidt, B.V.K.J.; Barner-Kowollik, C.; Piñol, M.; Oriol, L. A Novel Photoresponsive Azobenzene-Containing Miktoarm Star Polymer: Self-Assembly and Photoresponse Properties. Macromolecules 2014, 47, 3693–3700. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and Liposomes: A Self-Assembly Principle Beyond Lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Blasco, E.; Schmidt, B.V.K.J.; Barner-Kowollik, C.; Pinol, M.; Oriol, L. Dual thermo- and photo-responsive micelles based on miktoarm star polymers. Polym. Chem. 2013, 4, 4506–4514. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Lunn, D.J.; Winnik, M.A.; Manners, I. Colour-tunable fluorescent multiblock micelles. Nat. Commun. 2014, 5, 3372. [Google Scholar] [CrossRef] [PubMed]
- Barner-Kowollik, C.; Goldmann, A.S.; Schacher, F.H. Polymer Interfaces: Synthetic Strategies Enabling Functionality, Adaptivity, and Spatial Control. Macromolecules 2016, 49, 5001–5016. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Supramolecular X- and H-shaped star block copolymers via cyclodextrin-driven supramolecular self-assembly. Polym. Chem. 2014, 5, 2461–2472. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Skrabania, K.; Kristen, J.; Laschewsky, A.; Akdemir, Ö.; Hoth, A.; Lutz, J.-F. Design, Synthesis, and Aqueous Aggregation Behavior of Nonionic Single and Multiple Thermoresponsive Polymers. Langmuir 2007, 23, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Hsueh, H.-Y.; Jeng, U.S.; Ho, R.-M. Functionalized Nanoporous Gyroid SiO2 with Double-Stimuli-Responsive Properties as Environment-Selective Delivery Systems. Macromolecules 2014, 47, 3041–3051. [Google Scholar] [CrossRef]
- Schacher, F.; Rudolph, T.; Wieberger, F.; Ulbricht, M.; Müller, A.H.E. Double Stimuli-Responsive Ultrafiltration Membranes from Polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) Diblock Copolymers. ACS Appl. Mater. Interfaces 2009, 1, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Kocak, G.; Tuncer, C.; Butun, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Elbert, J.; Barner-Kowollik, C.; Gallei, M. Individually Addressable Thermo- and Redox-Responsive Block Copolymers by Combining Anionic Polymerization and RAFT Protocols. Macromol. Rapid Commun. 2014, 35, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Hirschbiel, A.F.; Schmidt, B.V.K.J.; Krolla-Sidenstein, P.; Blinco, J.P.; Barner-Kowollik, C. Photochemical Design of Stimuli-Responsive Nanoparticles Prepared by Supramolecular Host–Guest Chemistry. Macromolecules 2015, 48, 4410–4420. [Google Scholar] [CrossRef]
- Ge, Z.; Xie, D.; Chen, D.; Jiang, X.; Zhang, Y.; Liu, H.; Liu, S. Stimuli-Responsive Double Hydrophilic Block Copolymer Micelles with Switchable Catalytic Activity. Macromolecules 2007, 40, 3538–3546. [Google Scholar] [CrossRef]
- Brosnan, S.M.; Schlaad, H.; Antonietti, M. Aqueous Self-Assembly of Purely Hydrophilic Block Copolymers into Giant Vesicles. Angew. Chem. Int. Ed. 2015, 54, 9715–9718. [Google Scholar] [CrossRef] [PubMed]
- Mace, C.R.; Akbulut, O.; Kumar, A.A.; Shapiro, N.D.; Derda, R.; Patton, M.R.; Whitesides, G.M. Aqueous Multiphase Systems of Polymers and Surfactants Provide Self-Assembling Step-Gradients in Density. J. Am. Chem. Soc. 2012, 134, 9094–9097. [Google Scholar] [CrossRef] [PubMed]
- Keating, C.D. Aqueous Phase Separation as a Possible Route to Compartmentalization of Biological Molecules. Acc. Chem. Res. 2012, 45, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Long, M.S.; Keating, C.D. Nanoparticle Conjugation Increases Protein Partitioning in Aqueous Two-Phase Systems. Anal. Chem. 2006, 78, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, O.; Mace, C.R.; Martinez, R.V.; Kumar, A.A.; Nie, Z.; Patton, M.R.; Whitesides, G.M. Separation of Nanoparticles in Aqueous Multiphase Systems through Centrifugation. Nano Lett. 2012, 12, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, R.A.; Nicolai, T.; Chassenieux, C.; Benyahia, L. Stabilization of Water-in-Water Emulsions by Polysaccharide-Coated Protein Particles. Langmuir 2016, 32, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.; Opdam, J.; van ’t Oor, I.S.J.; Soligno, G.; van Roij, R.; Tromp, R.H.; Erné, B.H. Water-in-Water Emulsions Stabilized by Nanoplates. ACS Macro Lett. 2015, 4, 965–968. [Google Scholar] [CrossRef]
- Dewey, D.C.; Strulson, C.A.; Cacace, D.N.; Bevilacqua, P.C.; Keating, C.D. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat. Commun. 2014, 5, 4670. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Lipowsky, R. Giant Vesicles Exposed to Aqueous Two-Phase Systems: Membrane Wetting, Budding Processes, and Spontaneous Tubulation. Adv. Mater. Interfaces 2017, 4, 1600451. [Google Scholar] [CrossRef]
- Esquena, J. Water-in-water (W/W) emulsions. Curr. Opin. Colloid Interface Sci. 2016, 25, 109–119. [Google Scholar] [CrossRef]
- Casse, O.; Shkilnyy, A.; Linders, J.; Mayer, C.; Häussinger, D.; Völkel, A.; Thünemann, A.F.; Dimova, R.; Cölfen, H.; Meier, W.; et al. Solution Behavior of Double-Hydrophilic Block Copolymers in Dilute Aqueous Solution. Macromolecules 2012, 45, 4772–4777. [Google Scholar] [CrossRef]
- Blanazs, A.; Warren, N.J.; Lewis, A.L.; Armes, S.P.; Ryan, A.J. Self-assembly of double hydrophilic block copolymers in concentrated aqueous solution. Soft Matter 2011, 7, 6399–6403. [Google Scholar] [CrossRef]
- Willersinn, J.; Drechsler, M.; Antonietti, M.; Schmidt, B.V.K.J. Organized Polymeric Submicron Particles via Self-Assembly and Cross-Linking of Double Hydrophilic Poly(ethylene oxide)-b-poly(N-vinylpyrrolidone) in Aqueous Solution. Macromolecules 2016, 49, 5331–5341. [Google Scholar] [CrossRef]
- Willersinn, J.; Bogomolova, A.; Brunet Cabré, M.; Schmidt, B.V.K.J. Vesicles of double hydrophilic pullulan and poly(acrylamide) block copolymers: A combination of synthetic- and bio-derived blocks. Polym. Chem. 2017, 8, 1244–1254. [Google Scholar] [CrossRef]
- Taubert, A.; Furrer, E.; Meier, W. Water-in-water mesophases for templating inorganics. Chem. Commun. 2004, 2170–2171. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Walta, S.; Rosencrantz, R.R.; Korner, A.; Schulte, C.; Elling, L.; Richtering, W.; Boker, A. Micelles from self-assembled double-hydrophilic PHEMA-glycopolymer-diblock copolymers as multivalent scaffolds for lectin binding. Polym. Chem. 2016, 7, 878–886. [Google Scholar] [CrossRef]
- Rudolph, T.; Crotty, S.; von der Lühe, M.; Pretzel, D.; Schubert, U.; Schacher, F. Synthesis and Solution Properties of Double Hydrophilic Poly(ethylene oxide)-block-poly(2-ethyl-2-oxazoline) (PEO-b-PEtOx) Star Block Copolymers. Polymers 2013, 5, 1081. [Google Scholar] [CrossRef]
- Rueda, J.C.; Komber, H.; Cedrón, J.C.; Voit, B.; Shevtsova, G. Synthesis of New Hydrogels by Copolymerization of Poly(2-methyl-2-oxazoline) Bis(macromonomers) and N-Vinylpyrrolidone. Macromol. Chem. Phys. 2003, 204, 947–953. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Huang, X.; Voit, B. Engineering Functional Polymer Capsules toward Smart Nanoreactors. Chem. Rev. 2016, 116, 1053–1093. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, M.; Kempe, K.; Schubert, U.S. Covalently cross-linked poly(2-oxazoline) materials for biomedical applications—From hydrogels to self-assembled and templated structures. J. Mater. Chem. B 2015, 3, 526–538. [Google Scholar] [CrossRef]
- Wendler, F.; Rudolph, T.; Gorls, H.; Jasinski, N.; Trouillet, V.; Barner-Kowollik, C.; Schacher, F.H. Maleimide-functionalized poly(2-ethyl-2-oxazoline): Synthesis and reactivity. Polym. Chem. 2016, 7, 2419–2426. [Google Scholar] [CrossRef]
- Mero, A.; Pasut, G.; Via, L.D.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, J.F.; McDaniel, J.R.; Chilkoti, A.; Jordan, R.; Luxenhofer, R. Maleimide-Functionalized Poly(2-Oxazoline)s and Their Conjugation to Elastin-Like Polypeptides. Macromol. Biosci. 2016, 16, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Washio, I.; Chen, J.; Cai, H.; Li, Z.-Y.; Xia, Y. Poly(vinyl pyrrolidone): A Dual Functional Reductant and Stabilizer for the Facile Synthesis of Noble Metal Nanoplates in Aqueous Solutions. Langmuir 2006, 22, 8563–8570. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xie, C.; Chen, W.; Yang, C.; Wu, W.; Jiang, X. Platinum-Incorporating Poly(N-vinylpyrrolidone)-poly(aspartic acid) Pseudoblock Copolymer Nanoparticles for Drug Delivery. Biomacromolecules 2015, 16, 2059–2071. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly(N-vinylpyrrolidone)-Modified Surfaces for Biomedical Applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kempe, K.; Krieg, A.; Becer, C.R.; Schubert, U.S. “Clicking” on/with polymers: A rapidly expanding field for the straightforward preparation of novel macromolecular architectures. Chem. Soc. Rev. 2012, 41, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Barner-Kowollik, C.; Du Prez, F.E.; Espeel, P.; Hawker, C.J.; Junkers, T.; Schlaad, H.; Van Camp, W. “Clicking” Polymers or Just Efficient Linking: What Is the Difference? Angew. Chem. Int. Ed. 2011, 50, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Schlaad, H. Bioinspired Poly(2-oxazoline)s. Polymers 2011, 3, 467. [Google Scholar] [CrossRef]
- Volet, G.; Lav, T.-X.; Babinot, J.; Amiel, C. Click-Chemistry: An Alternative Way to Functionalize Poly(2-methyl-2-oxazoline). Macromol. Chem. Phys. 2011, 212, 118–124. [Google Scholar] [CrossRef]
- Pound, G.; Aguesse, F.; McLeary, J.B.; Lange, R.F.M.; Klumperman, B. Xanthate-mediated copolymerization of vinyl monomers for Amphiphilic and double-hydrophilic block copolymers with poly(ethylene glycol). Macromolecules 2007, 40, 8861–8871. [Google Scholar] [CrossRef]
- Quemener, D.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. RAFT and click chemistry: A versatile approach to well-defined block copolymers. Chem. Commun. 2006, 5051–5053. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Save, M.; Arathoon, B.; Charleux, B. Preparation of a xanthate-terminated dextran by click chemistry: Application to the synthesis of polysaccharide-coated nanoparticles via surfactant-free ab initio emulsion polymerization of vinyl acetate. J. Polym. Sci. A 2008, 46, 2845–2857. [Google Scholar] [CrossRef]
- Chen, F.P.; Ames, A.E.; Taylor, L.D. Aqueous solutions of poly(ethyloxazoline) and its lower consolute phase transition. Macromolecules 1990, 23, 4688–4695. [Google Scholar] [CrossRef]
- Senak, L.; Wu, C.S.; Malawer, E.G. Size Exclusion Chromatography of Poly(vinylpyrrolidone). J. Liq. Chromatogr. 1987, 10, 1127–1150. [Google Scholar] [CrossRef]
- Hussain, H.; Tan, B.H.; Gudipati, C.S.; Liu, Y.; He, C.B.; Davis, T.P. Synthesis and self-assembly of poly(styrene)-b-poly(N-vinylpyrrolidone) amphiphilic diblock copolymers made via a combined ATRP and MADIX approach. J. Polym. Sci. A 2008, 46, 5604–5615. [Google Scholar] [CrossRef]
- Ďorďovič, V.; Uchman, M.; Procházka, K.; Zhigunov, A.; Pleštil, J.; Nykänen, A.; Ruokolainen, J.; Matějíček, P. Hybrid Nanospheres Formed by Intermixed Double-Hydrophilic Block Copolymer Poly(ethylene oxide)-block-poly(2-ethyloxazoline) with High Content of Metallacarboranes. Macromolecules 2013, 46, 6881–6890. [Google Scholar] [CrossRef]
| Polymer | Mn [g·mol−1] a,b | Ð a,b | Ratio PVP/PEtOx [mol·mol−1] c |
|---|---|---|---|
| PEtOx22k-N3 | 22,200 | 1.24 a | - |
| PVP10k | 9400 a | 1.4 a | - |
| PVP14k | 13,700 a | 1.37 a | - |
| PVP32k | 31,500 a | 1.12 a | - |
| PEtOx22k-b-PVP10k | 18,900 b | 2.15 b | 0.48 |
| PEtOx22k-b-PVP14k | 17,000 b | 1.8 b | 0.60 |
| PEtOx22k-b-PVP32k | 38,800 b | 2.0 b | 1.42 |
| Block copolymer | Rapp [nm] a peak 1 | Abundance peak 1 [a.u.] | Rapp [nm] a peak 2 | Abundance peak 2 [a.u.] |
|---|---|---|---|---|
| PEtOx22k-b-PVP10k | 5.0 | 0.06 | 140 | 1.0 |
| PEtOx22k-b-PVP14k | 4.0 | 0.01 | 197 | 1.0 |
| PEtOx22k-b-PVP32k | 7.0 | 0.53 | 190 | 1.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willersinn, J.; Schmidt, B.V.K.J. Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution. Polymers 2017, 9, 293. https://doi.org/10.3390/polym9070293
Willersinn J, Schmidt BVKJ. Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution. Polymers. 2017; 9(7):293. https://doi.org/10.3390/polym9070293
Chicago/Turabian StyleWillersinn, Jochen, and Bernhard V.K.J. Schmidt. 2017. "Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution" Polymers 9, no. 7: 293. https://doi.org/10.3390/polym9070293
APA StyleWillersinn, J., & Schmidt, B. V. K. J. (2017). Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution. Polymers, 9(7), 293. https://doi.org/10.3390/polym9070293