Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units Possessing Highly Oleophobic/Superhydrophobic, Highly Oleophobic/Superhydrophilic, and Superoleophilic/Superhydrophobic Characteristics on the Modified Surfaces
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
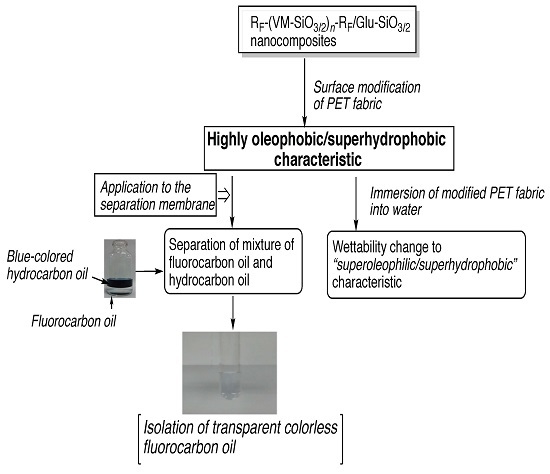
2.3. Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units [RF-(VM-SiO3/2)n-RF/Glu-SiO3/2]
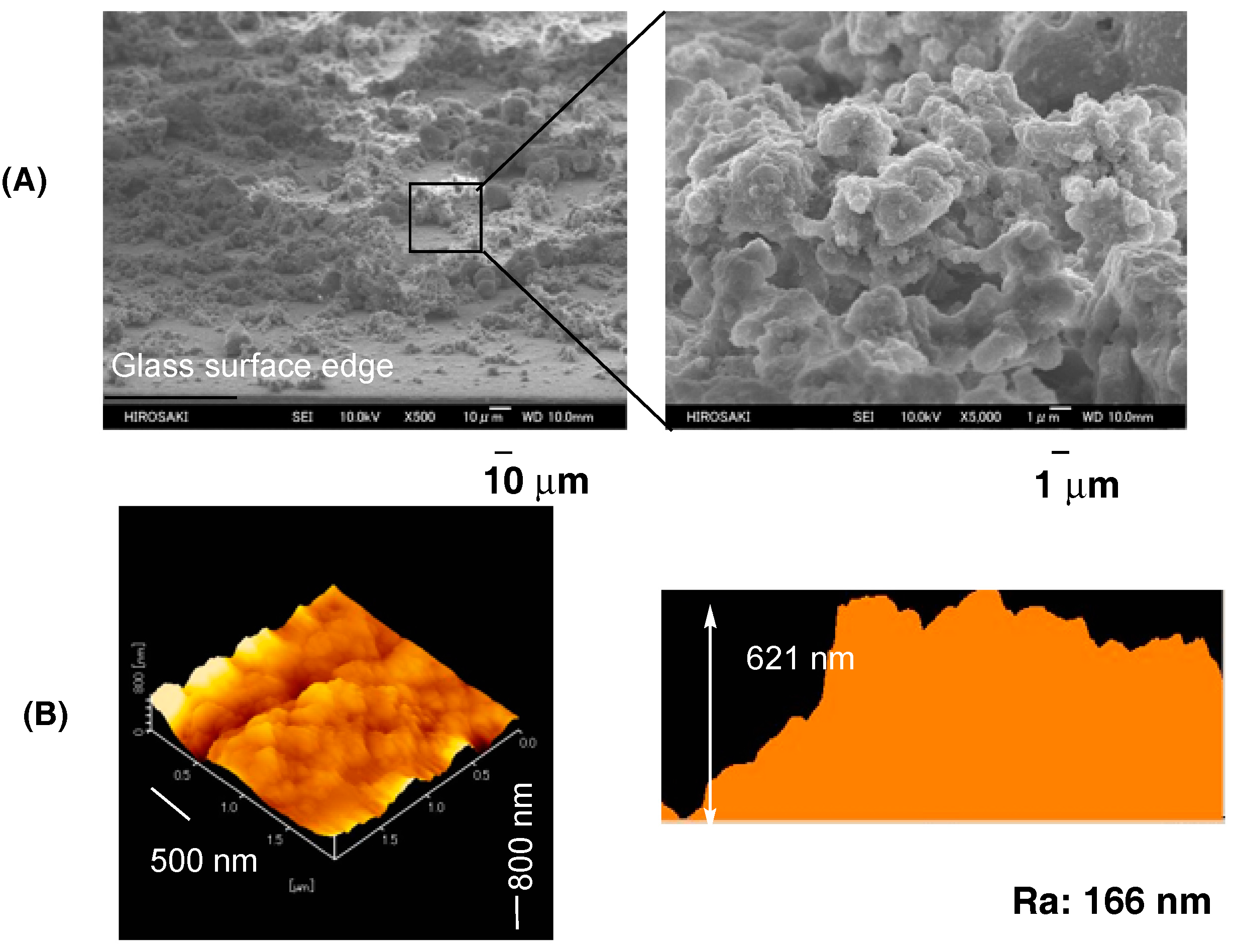
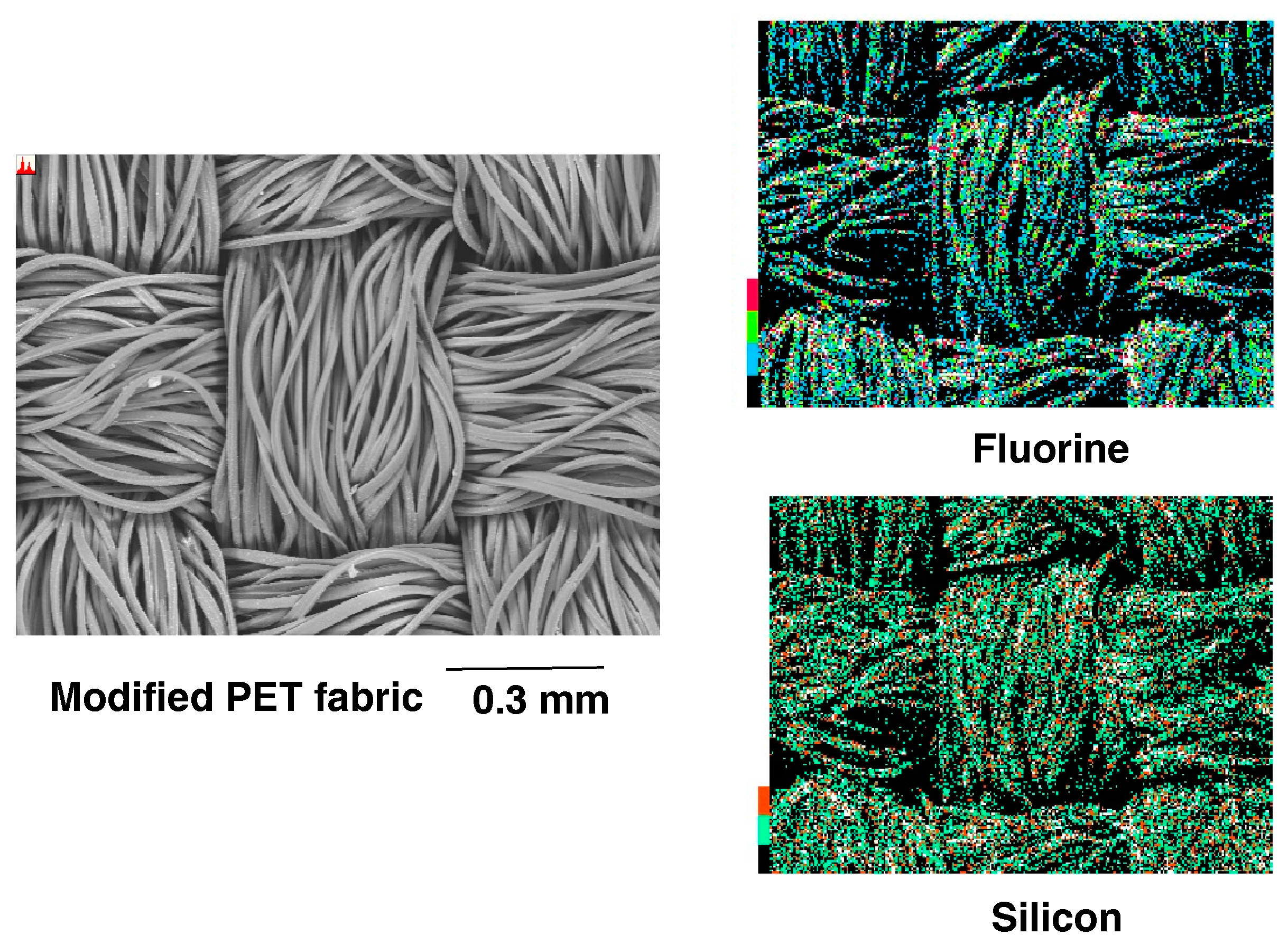
2.4. Preparation of the Modified Glass Treated with the RF-(VM-SiO3/2)n-RF/Glu-SiO3/2 Nanocomposites by Dipping Method
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RF-(VM)n-RF | Fluoroalkyl end-capped vinyltrimethoxysilane oligomer |
| Glu-Si(OEt)3 | N-(3-triethoxysilylpropyl)gluconamide |
| RF-(VM-SiO3/2)n-RF/Glu-SiO3/2 | Fluoroalkyl end-capped vinyltrimethoxysilane oligomeric silica nanocomposites containing gluconamide units |
| PET | Polyethylene terephthalate |
| RF-(VM-SiO3/2)n-RF | Fluoroalkyl end-capped vinyltrimethoxysilane oligomeric silica nanoparticles |
References
- Sawada, H. Fluorinated Peroxides. Chem. Rev. 1996, 96, 1779–1808. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H. Chemistry of fluoroalkanoyl peroxides, 1980–1998. J. Fluor. Chem. 2000, 105, 219–220. [Google Scholar] [CrossRef]
- Sawada, H. Novel self-assembled molecular aggregates formed by fluoroalkyl end-capped oligomers and their application. J. Fluor. Chem. 2003, 121, 111–130. [Google Scholar] [CrossRef]
- Sawada, H. Synthesis of self-assembled fluoroalkyl end-capped oligomeric aggregates—Applications of these aggregates to fluorinated oligomeric nanocomposites. Prog. Polym. Sci. 2007, 32, 509–533. [Google Scholar] [CrossRef]
- Sawada, H. Preparation and Application of Novel Fluoroalkyl End-capped Oligomeric Nanocomposites. Polym. Chem. 2012, 3, 46–65. [Google Scholar] [CrossRef]
- Sawada, H.; Tanimura, T.; Katayama, S.; Kawase, T. Aggregation of fluoroalkyl units: Synthesis of gelling fluoroalkylated end-capped oligomers containing hydroxy segments possessing metal ion binding and releasing abilities. J. Chem. Soc. Chem. Commun. 1997, 1391–1392. [Google Scholar] [CrossRef]
- Sawada, H.; Tanimura, T.; Katayama, S.; Kawase, T.; Tomita, T.; Baba, M. Synthesis and Properties of Gelling Fluoroalkylated End-Capped Oligomers Containing Hydroxy Segments. Polym. J. 1998, 30, 797–804. [Google Scholar] [CrossRef]
- Sawada, H.; Nakamura, Y.; Katayama, S.; Kawase, T. Gelation of Fluoroalkylated End-Capped Oligomers Containing Triol Segments under Non-Crosslinked Conditions, and Binding or Releasing of Metal Ions by These Oligomers. Bull. Chem. Soc. Jpn. 1997, 70, 2839–2845. [Google Scholar] [CrossRef]
- Sawada, H.; Murai, Y.; Kurachi, M.; Kawase, T.; Minami, T.; Kyokane, J.; Tomita, T. Synthesis and antibacterial activity of novel fluoroalkyl end-capped oligomers containing ammonium segments: Application to new fluorinated gelling materials with antibacterial activity. J. Mater. Chem. 2002, 12, 188–194. [Google Scholar] [CrossRef]
- Narain, R.; Jhurry, D. Synthesis and characterization of novel polymers derived from gluconolactone. Polym. Int. 2001, 51, 85–91. [Google Scholar] [CrossRef]
- Carter, A.; Morton, D.W.; Kiely, D.E. Synthesis of some poly(4-alkyl-4-azaheptamethylene-d-glucaramides). J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3892–3899. [Google Scholar] [CrossRef]
- Ohno, K.; Fukuda, T.; Kitano, H. Free radical polymerization of a sugar residue-carrying styryl monomer with a lipophilic alkoxyamine initiator: Synthesis of a well-defined novel glycolipid. Macromol. Chem. Phys. 1998, 199, 2193–2197. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Leon, O.; Cerrada, M.L.; Rodriguez-Hernandez, J.; Sanchez-Chaves, M.; Fernandez-Garcia, M. Chemical modification of block copolymers based on 2-hydroxyethyl acrylate to obtain amphiphilic glycopolymers. Eur. Polym. J. 2015, 62, 167–178. [Google Scholar] [CrossRef]
- Goto, M.; Kobayashi, K.; Hachikawa, A.; Saito, K.; Cho, C.; Akaike, T. Micellar Behavior of Sugar-Carrying Polystyrene in Aqueous Solution. Macromol. Chem. Phys. 2001, 202, 1161–1165. [Google Scholar] [CrossRef]
- Wagner, R.; Richter, L.; Wersig, R.; Schmaucks, G.; Weiland, B.; Weissmuller, J.; Reiners, J. Silicon-Modified Carbohydrate Surfactants I: Synthesis of Siloxanyl Moieties Containing Straight-chained Glycosides and Amides. Appl. Organomet. Chem. 1996, 10, 421–435. [Google Scholar] [CrossRef]
- Babiuch, K.; Pretzel, D.; Tolstik, T.; Vollrath, A.; Stanca, S.; Foertsch, F.; Becer, C.R.; Gottschaldt, M.; Biskup, C.; Schubert, U.S. Uptake of Well-Defined, Highly Glycosylated, Pentafluorostyrene-Based Polymers and Nanoparticles by Human Hepatocellular Carcinoma Cells. Macromol. Biosci. 2012, 12, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Richter, L.; Weiland, B.; Reiners, J.; Weissmuller, J. Silicon-Modified Carbohydrate Surfactants II: Siloxanyl Moieties Containing Branched Structures. Appl. Organomet. Chem. 1996, 10, 437–450. [Google Scholar] [CrossRef]
- Hashimoto, K.; Saito, H.; Ohsawa, R. Glycopolymeric inhibitors of β-glucuronidase I. Synthesis and polymerization of styrene derivatives having pendant D-glucaric moieties. J. Polym. Sci. A 2006, 44, 4895–4903. [Google Scholar] [CrossRef]
- Becer, C.R. The Glycopolymer Code: Synthesis of Glycopolymers and Multivalent Carbohydrate—Lectin Interactions. Macromol. Rapid Commun. 2012, 33, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Audebeau, E.; Norvez, S.; Iliopoulos, I. Modification of Poly(allylamine) for Crosslinking by Borax. Macrmol. Symp. 2013, 331, 152–157. [Google Scholar] [CrossRef]
- Bordege, V.; Munoz-Bonillia, A.; Leon, O.; Cuervo-Rodriguez, R.; Sanchez-Chaves, M.; Fernandez-Garcia, M. Gluconolactone-derivated polymers: Copolymerization, thermal properties, and their potential use as polymeric surfactants. J. Polym. Sci. A 2011, 49, 526–536. [Google Scholar] [CrossRef]
- Sawada, H.; Suzuki, T.; Takashima, H.; Takishita, K. Preparation and properties of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric nanoparticles—A new approach to facile creation of a completely super-hydrophobic coating surface with these nanoparticles. Colloid Polym. Sci. 2008, 286, 1569–1574. [Google Scholar] [CrossRef]
- Sawada, H.; Nakayama, M. Synthesis of fluorine-containing organosilicon oligomers. J. Chem. Soc. Chem. Commun. 1991, 10, 677–678. [Google Scholar] [CrossRef]
- Sawada, H.; Ikematsu, Y.; Kawase, T.; Hayakawa, Y. Synthesis and surface properties of novel fluoroalkylated flip-flop-type silane coupling agents. Langmuir 1996, 12, 3529–3530. [Google Scholar] [CrossRef]
- Oikawa, Y.; Saito, T.; Yamada, M.; Sugita, M.; Sawada, H. Preparation and surface property of fluoroalkyl end-capped vinyltrimethoxysilane oligomer/talc composite-encapsulated organic compounds: Application for the separation of oil and water. ACS Appl. Mater. Interfaces 2015, 7, 13782–13793. [Google Scholar] [CrossRef] [PubMed]
- Sumino, E.; Saito, T.; Noguchi, T.; Sawada, H. Facile creation of superoleophobic and superhydrophilic surface by using perfluoropolyether dicarboxylic acid/silica nanocomposites. Polym. Adv. Technol. 2015, 26, 345–352. [Google Scholar] [CrossRef]
- Saito, T.; Tsushima, Y.; Sawada, H. Facile creation of superoleophobic and superhydrophilic Surface by using fluoroalkyl end-capped vinyltrimethoxysilane oligomer/calcium silicide nanocomposites development of these nanocomposites to environmental cyclical type-fluorine recycle through formation of calcium fluoride. Colloid Polym. Sci. 2015, 293, 65–73. [Google Scholar]
- Saito, T.; Tsushima, Y.; Honda, T.; Kamiya, T.; Fujita, M.; Sawada, H. Facile creation of modified surface possessing the controlled wettability between superamphiphobic and superoleophobic–superhydrophilic characteristics by using perfluorocarboxamides/calcium carbonate/calcium fluoride nanocomposites: Application to the separation of oil and water. J. Compos. Mater. 2016, 50, 3831–3842. [Google Scholar]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Y.; Jiang, L. Applications of Bio-Inspired Special Wettable Surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Huang, B.; Zhong, M. Fabrication of superhydrophobic and heat-insulating antimony doped tin oxide/polyurethane films by cast replica micromolding. J. Colloid Interface Sci. 2009, 336, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Taurino, R.; Fabbri, E.; Messori, M.; Pilati, F.; Pospiech, D.; Synytska, A. Facile preparation of superhydrophobic coatings by sol-gel processes. J. Colloid Interface Sci. 2008, 325, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Li, Y.; Mabry, J.M.; Tuteja, A. Hierarchically Structured Superoleophobic Surfaces with Ultralow Contact Angle Hysteresis. Adv. Mater. 2012, 24, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, M.; Zhang, D.; Gao, Z.; Wang, C. Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation. Carbohydr. Polym. 2014, 103, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Li, B.; Wang, A. Mechanical- and oil-durable superhydrophobic polyester materials for selective oil absorption and oil/water separation. J. Colloid Interface Sci. 2014, 413, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
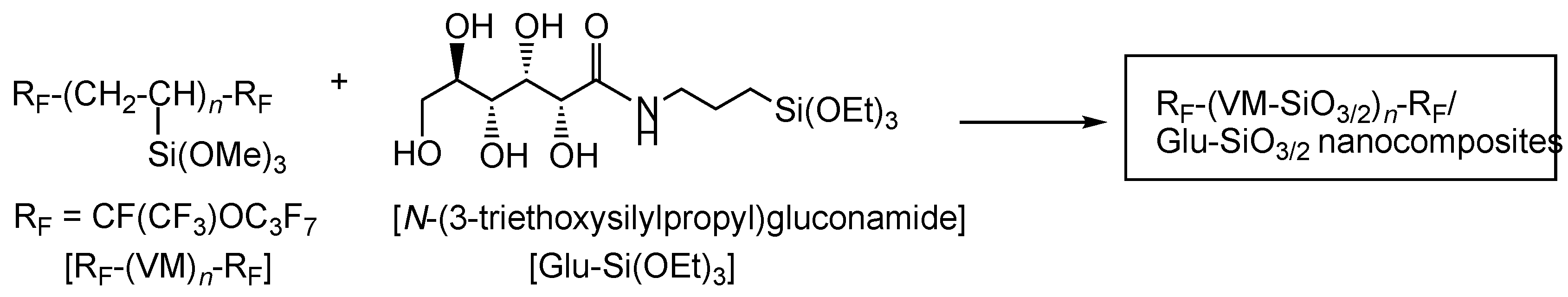
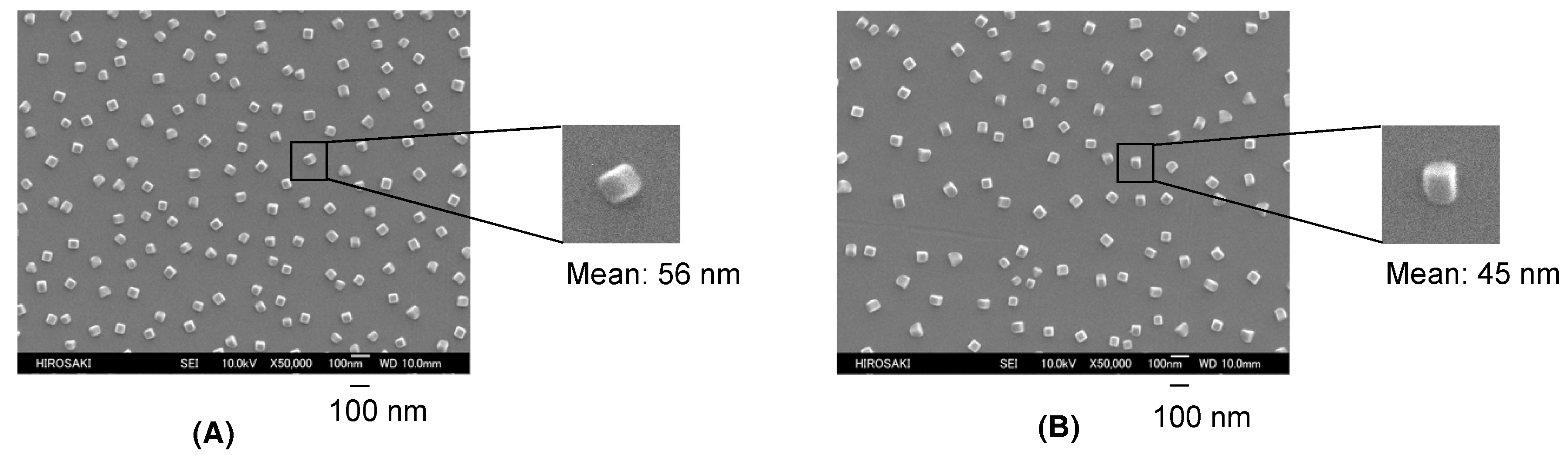
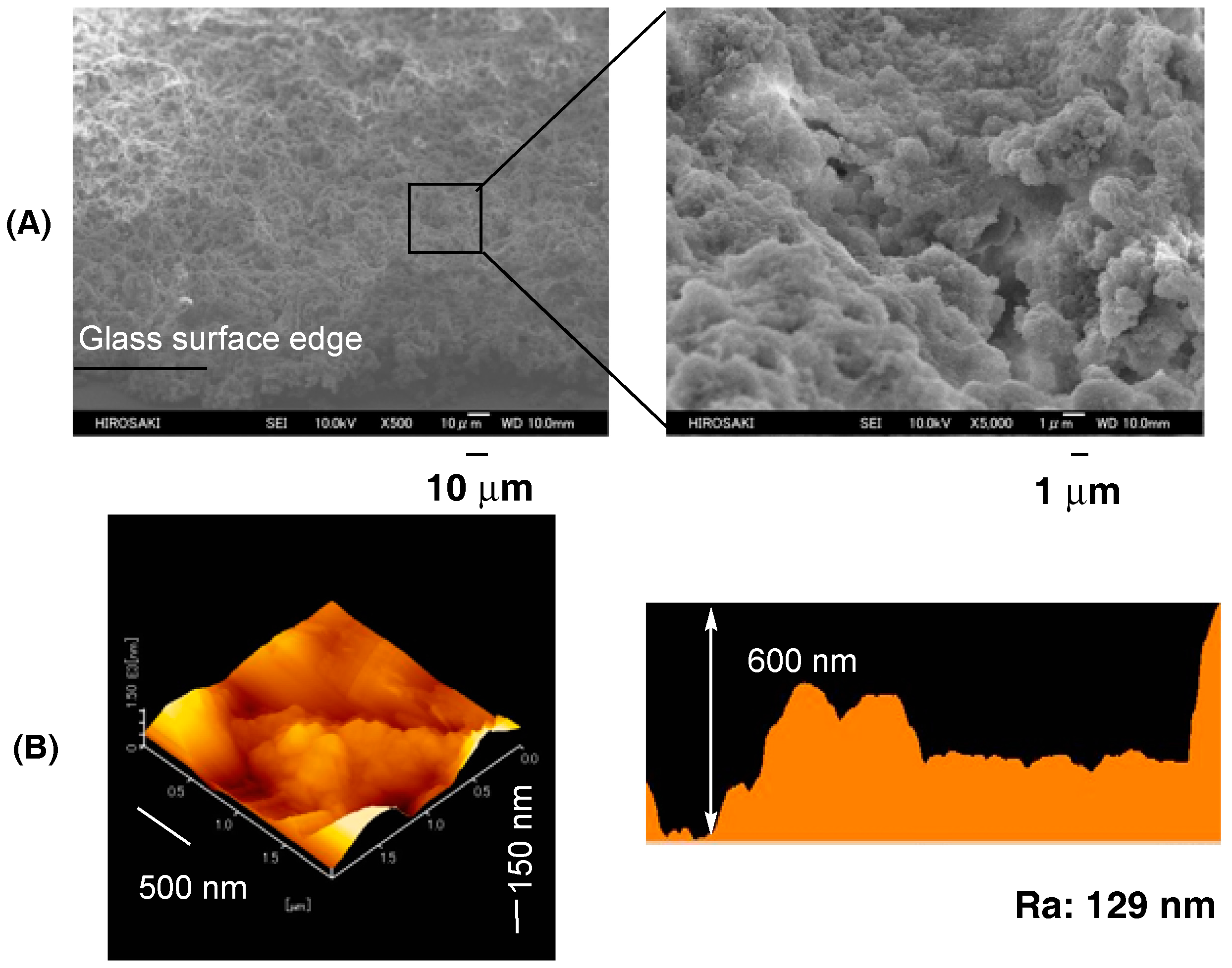
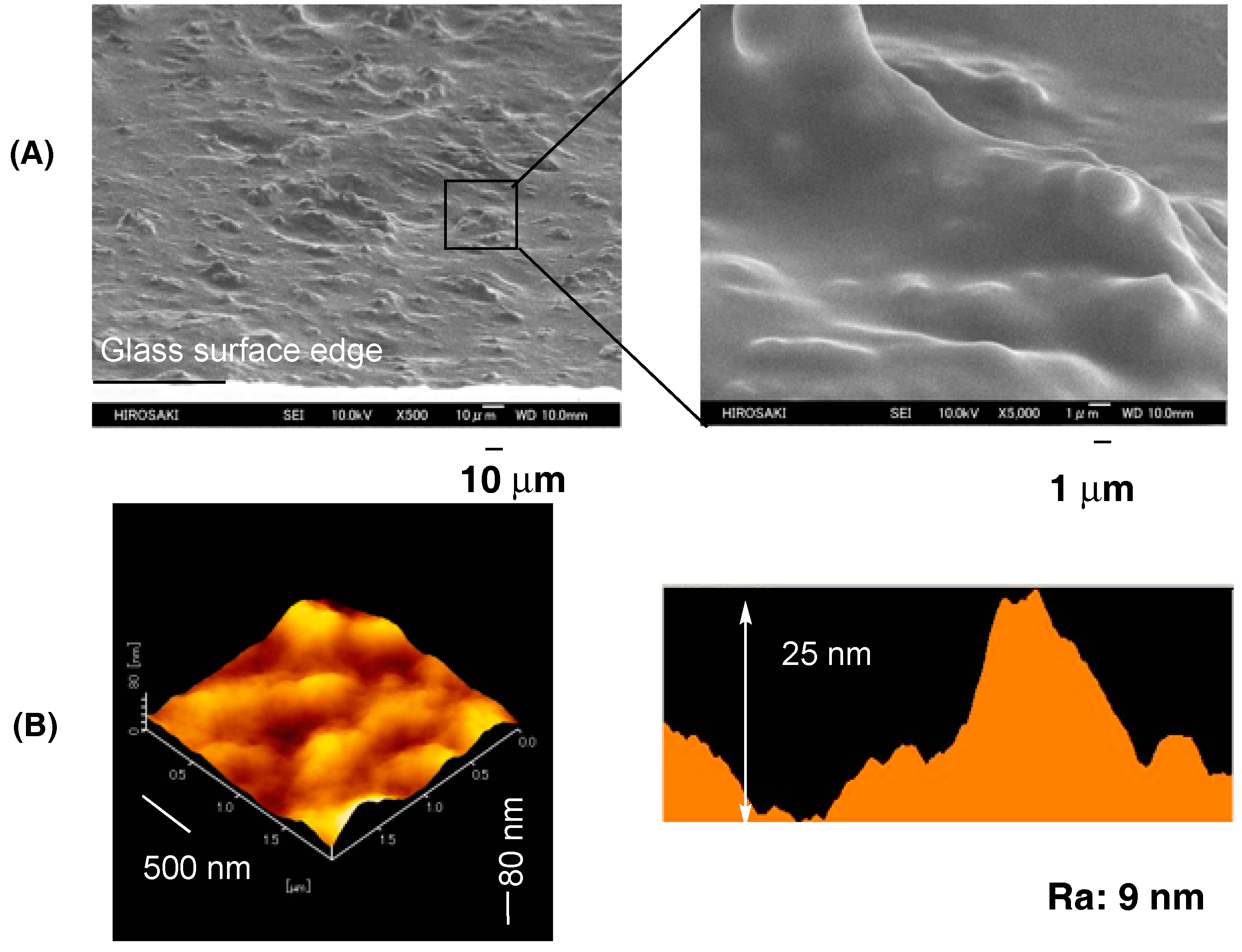
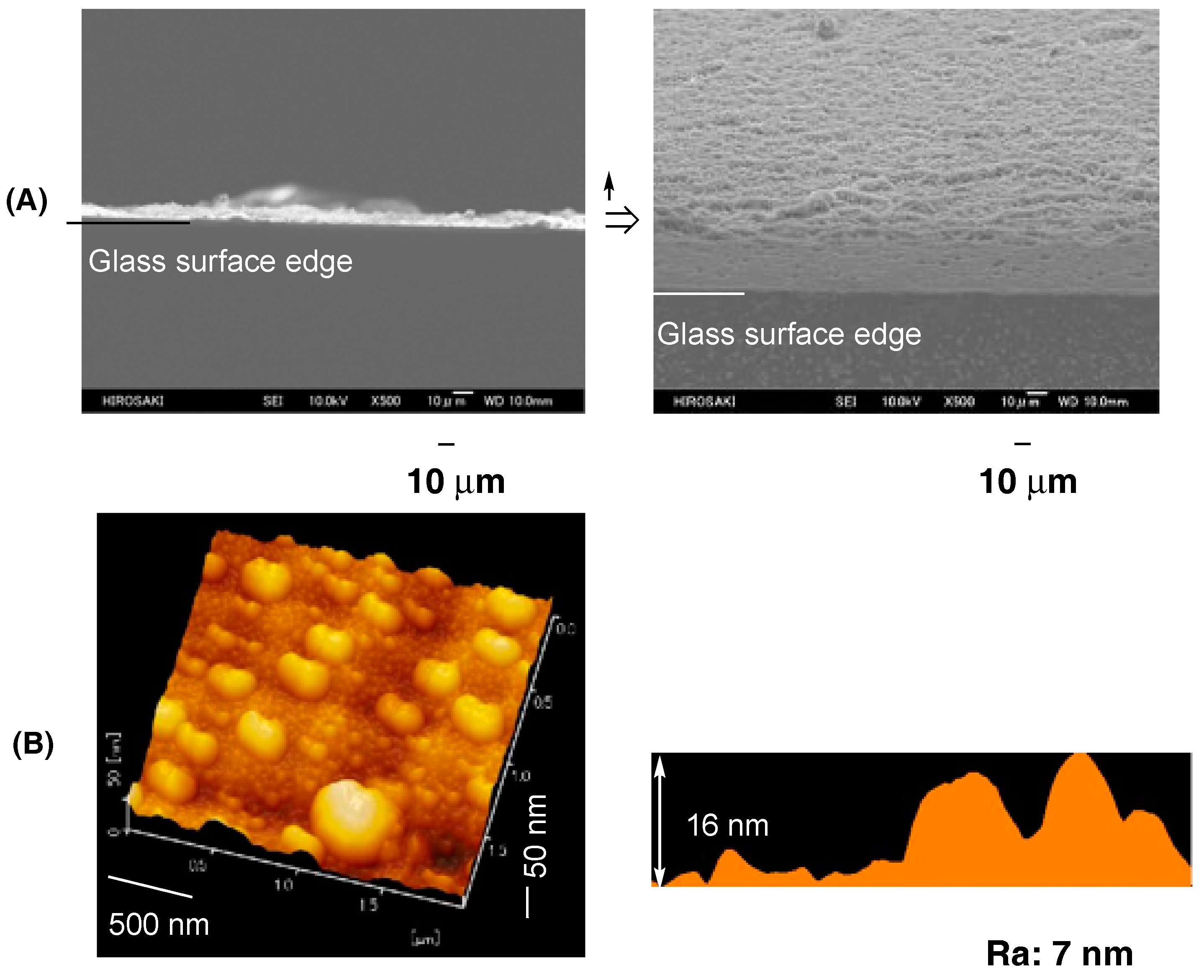
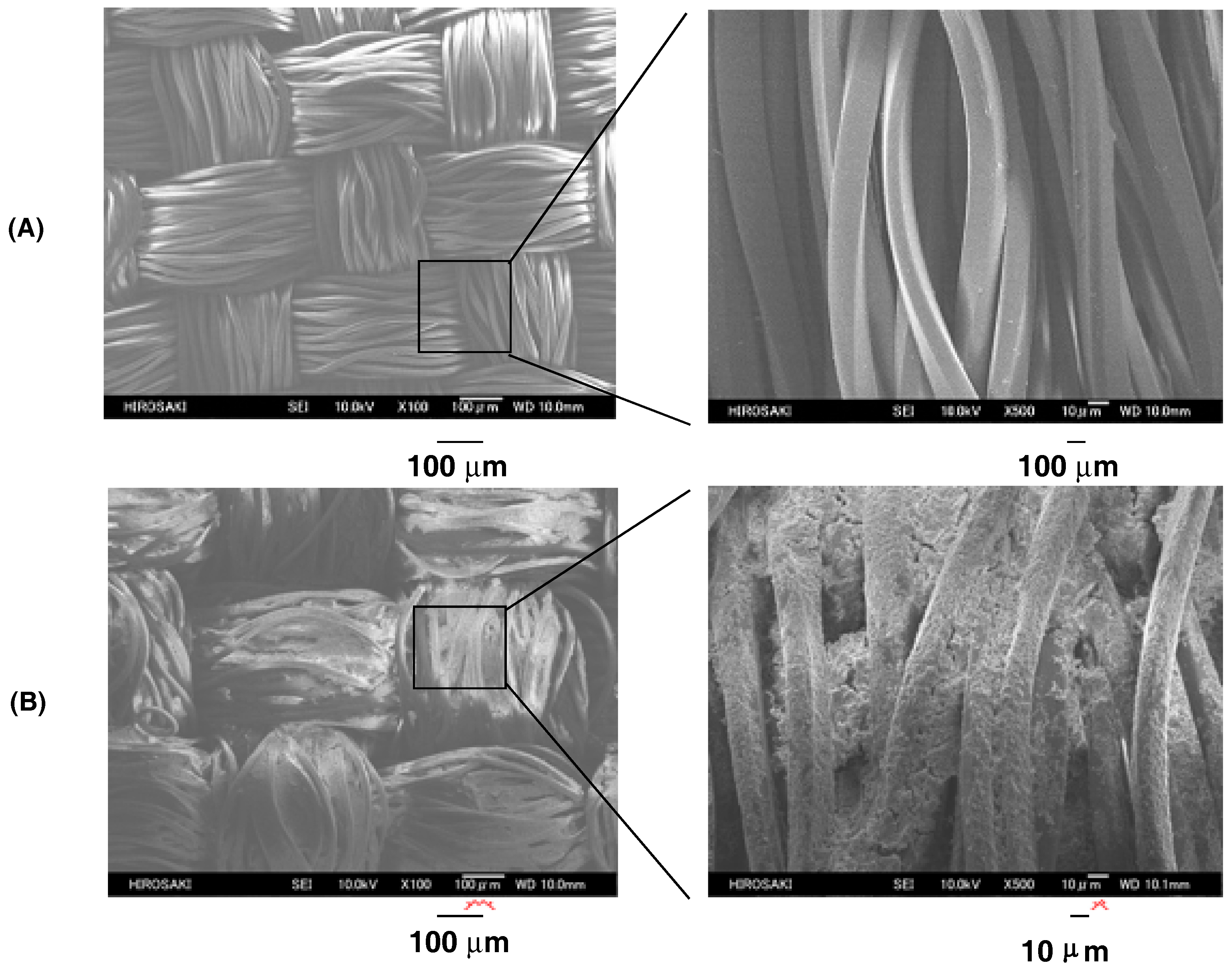
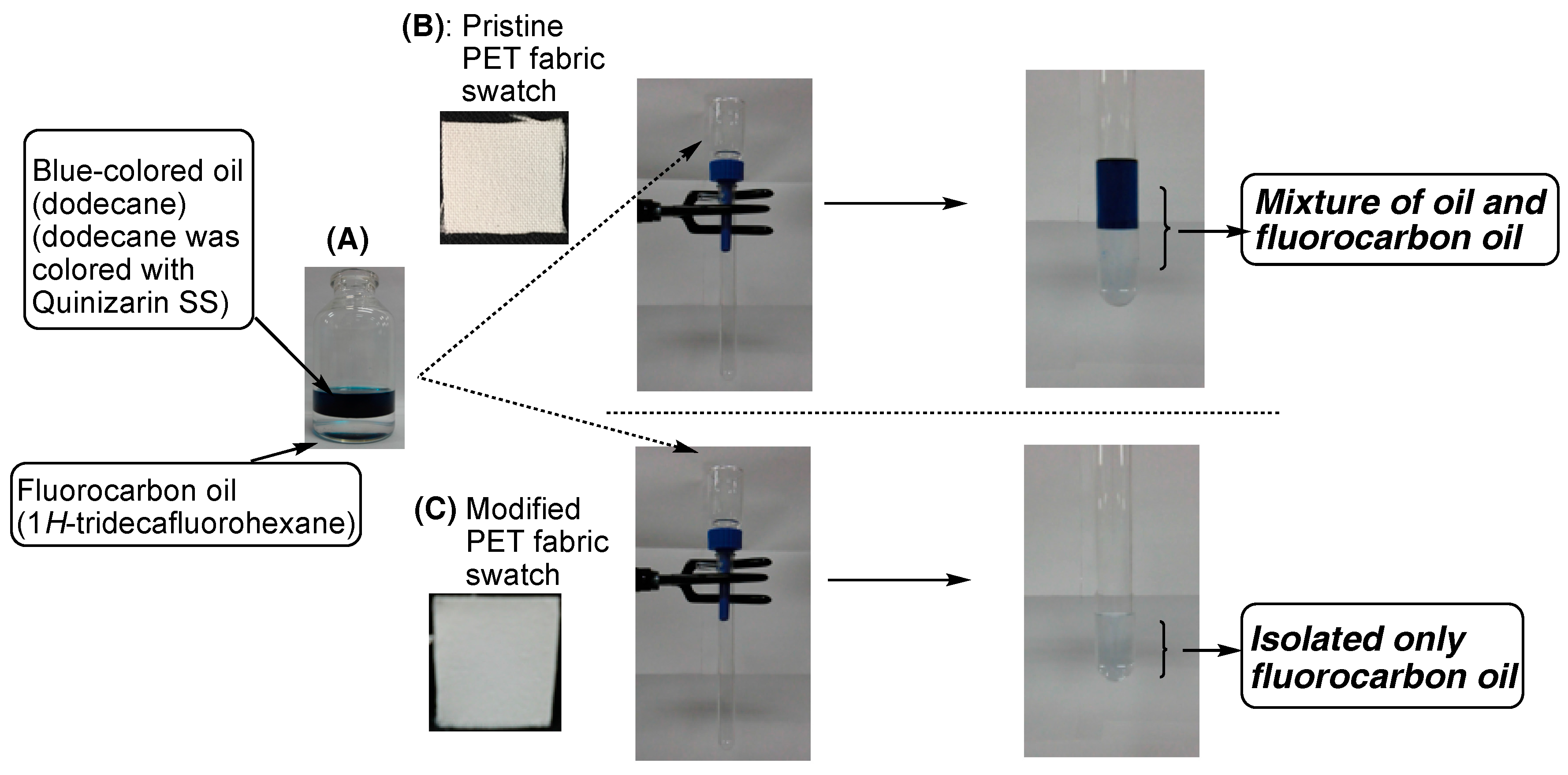

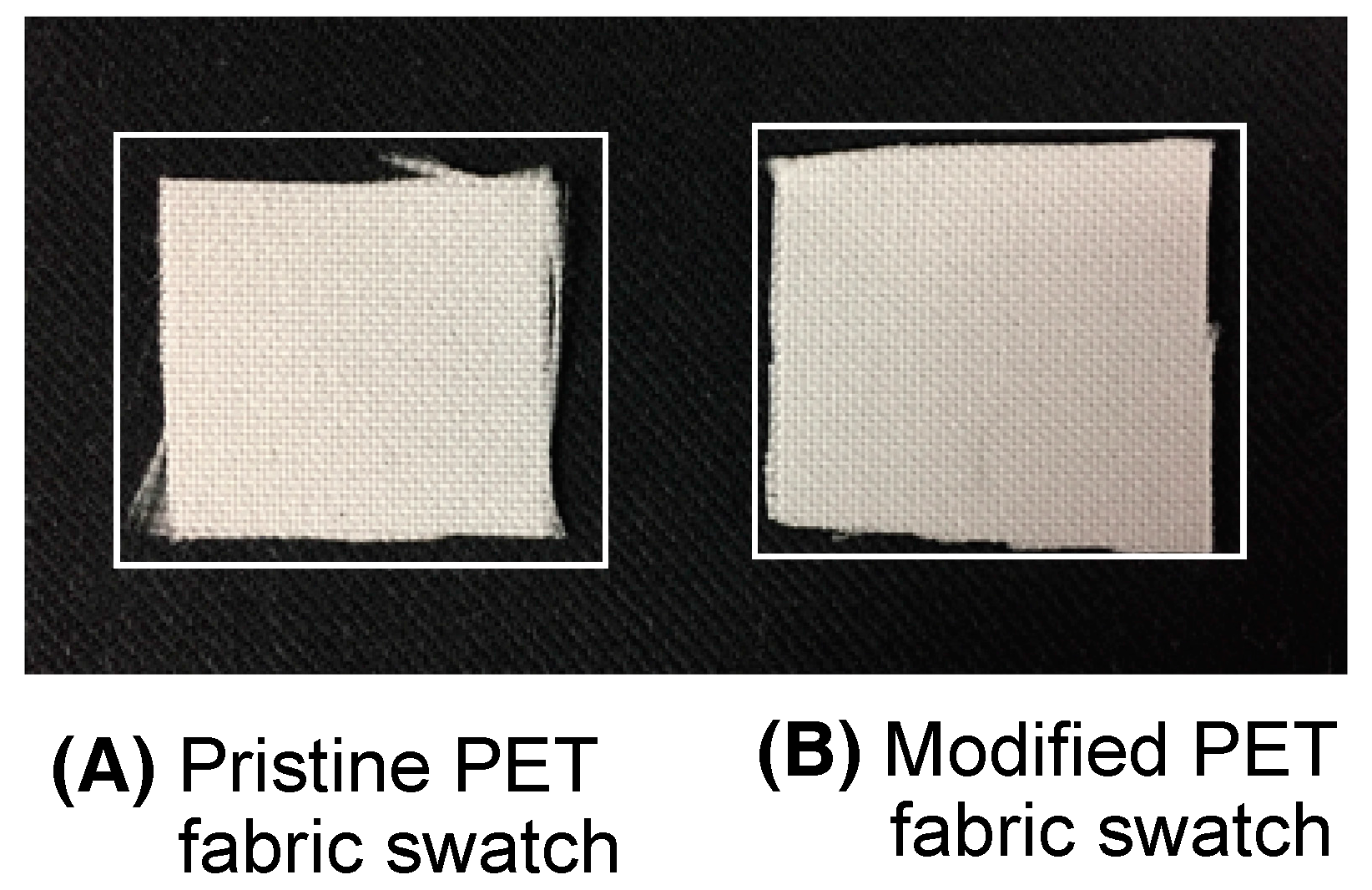


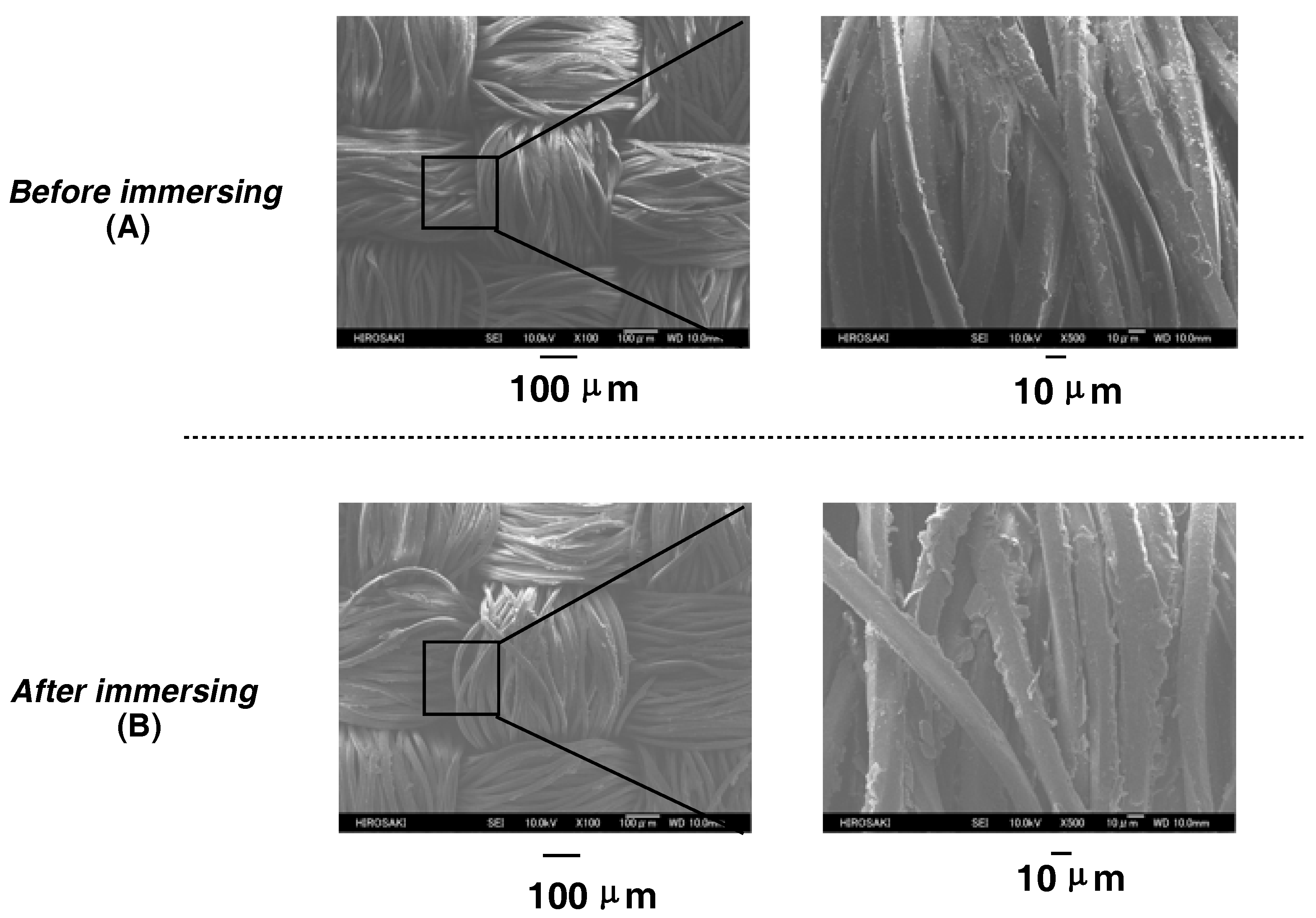
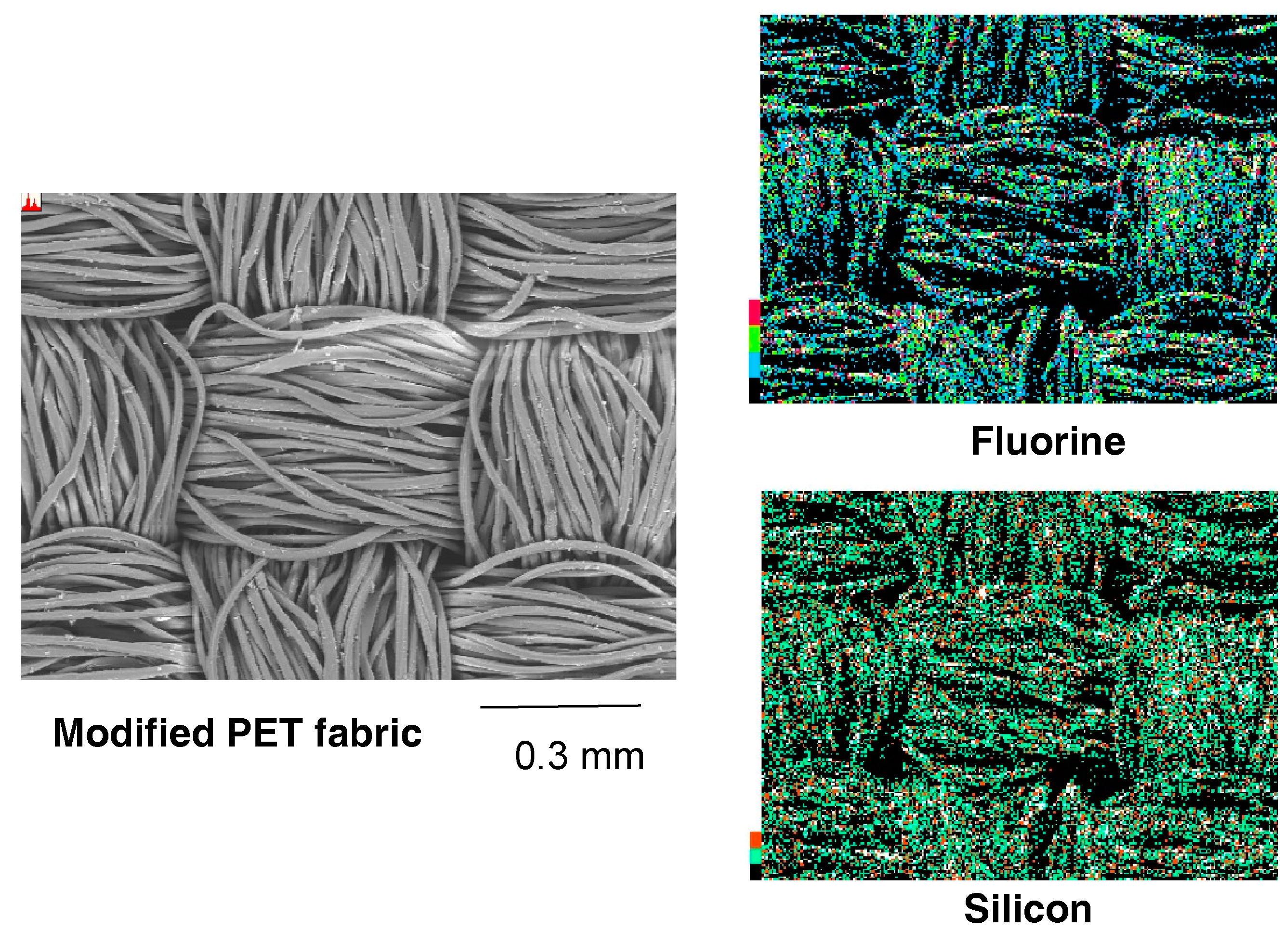
| Run | RF-(VM)n-RF (mg) (mmol) | Glu-Si(OEt)3 (mg) c (mmol) | MeOH (mL) | 25 wt % aq. NH3 (mL) | Yield a (%) | Size of Composites b (nm ± STD) |
|---|---|---|---|---|---|---|
| 1 | 200 (0.27) | 10 (0.01) | 5.0 | 1.0 | 54 | 32.7 ± 3.1 |
| 2 | 200 (0.27) | 25 (0.03) | 5.0 | 1.0 | 39 | 38.1 ± 7.1 |
| 3 | 200 (0.27) | 50 (0.06) | 5.0 | 1.0 | 43 | 53.8 ± 17.3 |
| 4 | 200 (0.27) | 90 (0.11) | 5.0 | 1.0 | 39 | 37.4 ± 9.0 |
| 5 | 200 (0.27) | 170 (0.21) | 5.0 | 1.0 | 31 | 71.8 ± 14.0 |
| 6 | 200 (0.27) | 350 (0.44) | 5.0 | 1.0 | 43 | 35.5 ± 2.3 |
| 7 | 200 (0.27) | 700 (0.88) | 5.0 | 1.0 | 69 | 51.1 ± 6.5 |
| 8 | 200 (0.27) | 1400 (1.75) | 5.0 | 1.0 | 86 | 42.8 ± 8.7 |
| Run * | Feed Ratio (mmol/mmol) (RF-(VM)-RF/Glu-Si(OEt)3) | Dodecane | Contact Angle (Degree) | ||||||
| Water | |||||||||
| Time | |||||||||
| 0 m | 5 m | 10 m | 15 m | 20 m | 25 m | 30 m | |||
| 1 | (0.27/0.01) | 74 | 180 | - ** | - | - | - | - | - |
| 2 | (0.27/0.03) | 112 | 180 | - ** | - | - | - | - | - |
| 3 | (0.27/0.06) | 107 | 142 | 130 | 132 | 131 | 128 | 127 | 104 |
| 4 | (0.27/0.11) | 97 | 137 | 134 | 134 | 129 | 127 | 127 | 122 |
| 5 | (0.27/0.21) | 94 | 135 | 133 | 132 | 132 | 128 | 113 | 101 |
| 6 | (0.27/0.44) | 97 | 137 | 143 | 122 | 91 | 62 | 0 | - ** |
| 7 | (0.27/0.88) | 58 | 133 | 132 | 104 | 0 | - ** | - | - |
| 8 | (0.27/1.75) | 56 | 108 | 106 | 102 | 99 | 93 | 86 | 80 |
| Parent RF-(VM-SIO2)n-RF | 46 | 180 | - ** | - | - | - | - | - | |
| Non-treated glass | 0 | 50 | |||||||
| Run No. | Feed Amounts (mg/mg) of | ||
|---|---|---|---|
| RF-(VM)n-RF/Glu-Si(OEt)3 | 25% aq. Ammonia (mL) | Methanol (mL) | |
| 9 | 200/90 [0.27/0.11(mmol/mmol)] | 0.6 | 15 |
| 10 | 200/170 [0.27/0.21 (mmol/mmol)] | 0.6 | 15 |
| Run | Feed Ratio (mmol/mmol) (RF-(VM)-RF/Glu-Si(OEt)3) | Dodecane | Contact Angle (Degree) | ||||||
| Water | |||||||||
| Time (min) | |||||||||
| 0 m | 5 m | 10 m | 15 m | 20 m | 25 m | 30 m | |||
| Before immersing into water | |||||||||
| 9 | (0.27/0.11) | 102 | 180 | - a | - | - | - | - | - |
| 10 | (0.27/0.21) | 89 | 180 | - a | - | - | - | - | - |
| After immersing into water | |||||||||
| 9 | (0.27/0.11) | 0 | 180 | - a | - | - | - | - | - |
| 10 | (0.27/0.21) | 0 | 180 | - a | - | - | - | - | - |
| Atomic Contents (atm, %) | |||||
|---|---|---|---|---|---|
| C | O | N | F | Si | |
| Before immersing | 39.9 | 33.1 | 20.1 | 6.3 | 0.6 |
| After immersing | 40.0 | 31.6 | 19.7 | 7.9 | 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, S.; Fujii, S.; Saito, T.; Yamazaki, S.; Sawada, H. Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units Possessing Highly Oleophobic/Superhydrophobic, Highly Oleophobic/Superhydrophilic, and Superoleophilic/Superhydrophobic Characteristics on the Modified Surfaces. Polymers 2017, 9, 292. https://doi.org/10.3390/polym9070292
Katayama S, Fujii S, Saito T, Yamazaki S, Sawada H. Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units Possessing Highly Oleophobic/Superhydrophobic, Highly Oleophobic/Superhydrophilic, and Superoleophilic/Superhydrophobic Characteristics on the Modified Surfaces. Polymers. 2017; 9(7):292. https://doi.org/10.3390/polym9070292
Chicago/Turabian StyleKatayama, Shinsuke, Shogo Fujii, Tomoya Saito, Shohei Yamazaki, and Hideo Sawada. 2017. "Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units Possessing Highly Oleophobic/Superhydrophobic, Highly Oleophobic/Superhydrophilic, and Superoleophilic/Superhydrophobic Characteristics on the Modified Surfaces" Polymers 9, no. 7: 292. https://doi.org/10.3390/polym9070292
APA StyleKatayama, S., Fujii, S., Saito, T., Yamazaki, S., & Sawada, H. (2017). Preparation of Fluoroalkyl End-Capped Vinyltrimethoxysilane Oligomeric Silica Nanocomposites Containing Gluconamide Units Possessing Highly Oleophobic/Superhydrophobic, Highly Oleophobic/Superhydrophilic, and Superoleophilic/Superhydrophobic Characteristics on the Modified Surfaces. Polymers, 9(7), 292. https://doi.org/10.3390/polym9070292