A Conjugated Polyelectrolyte with Pendant High Dense Short-Alkyl-Chain-Bridged Cationic Ions: Analyte-Induced Light-Up and Label-Free Fluorescent Sensing of Tumor Markers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Fluorescence Measurements
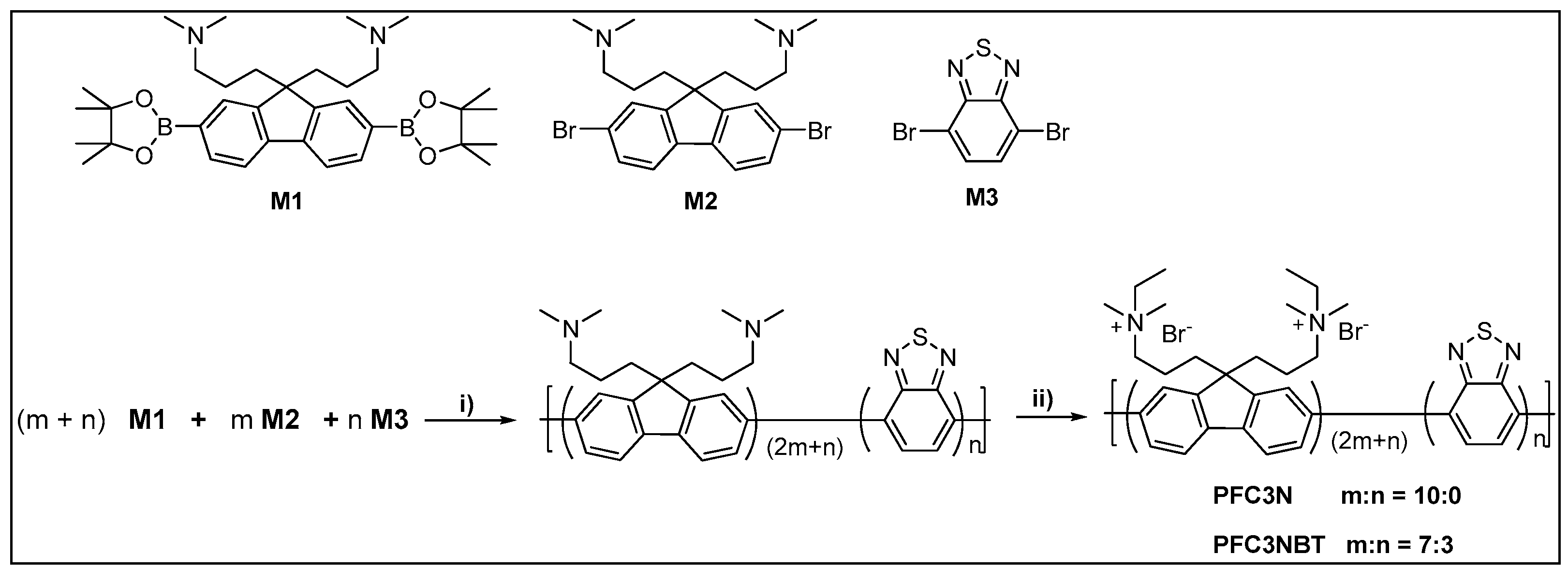
2.3. Preparation of Poly({9,9-bis[3’-(N,N-dimethylamino)propyl]-2,7-fluorene-4,7-benzothiadiazole})
2.4. Synthesis of Poly(9,9-bis{3’-[(N,N-dimethyl)-N-ethylammonium}-propyl]-2,7-fluorene Dibromide)
3. Results and Discussion
3.1. Design Principle of PFC3NBT
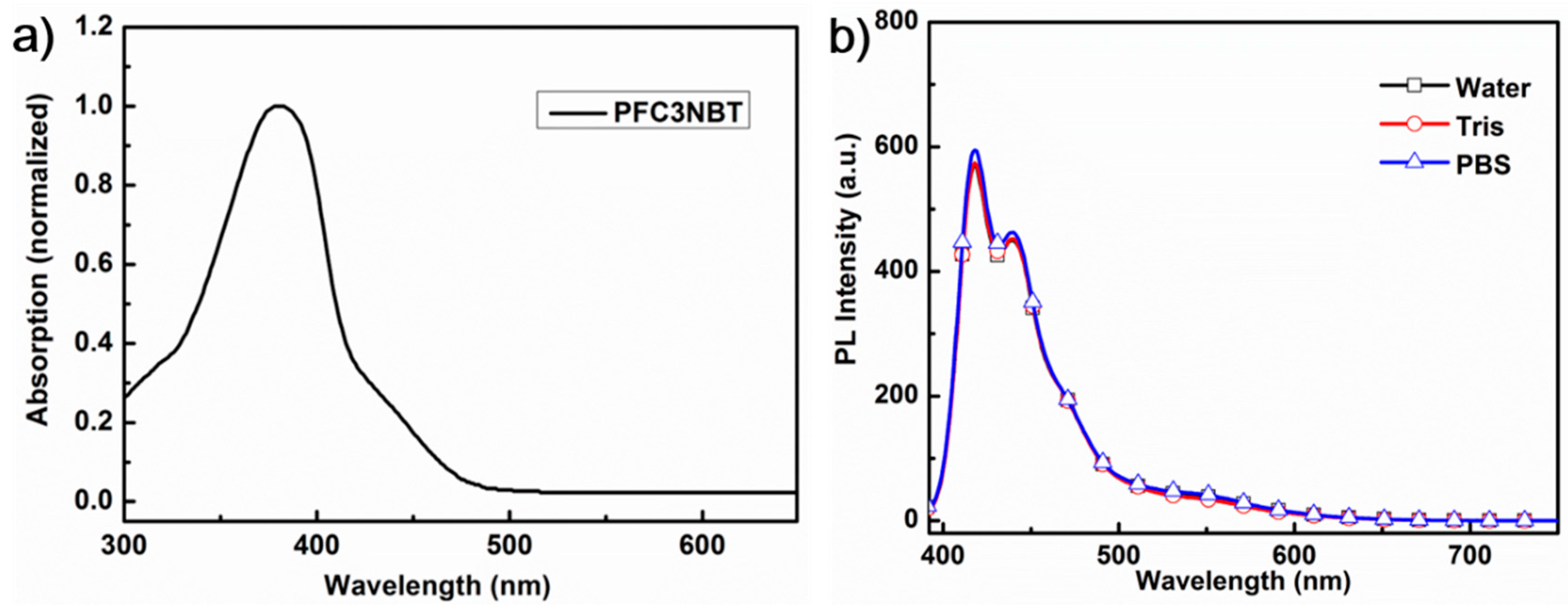
3.2. UV-Vis Absorption and Photoluminescence (PL) Spectra
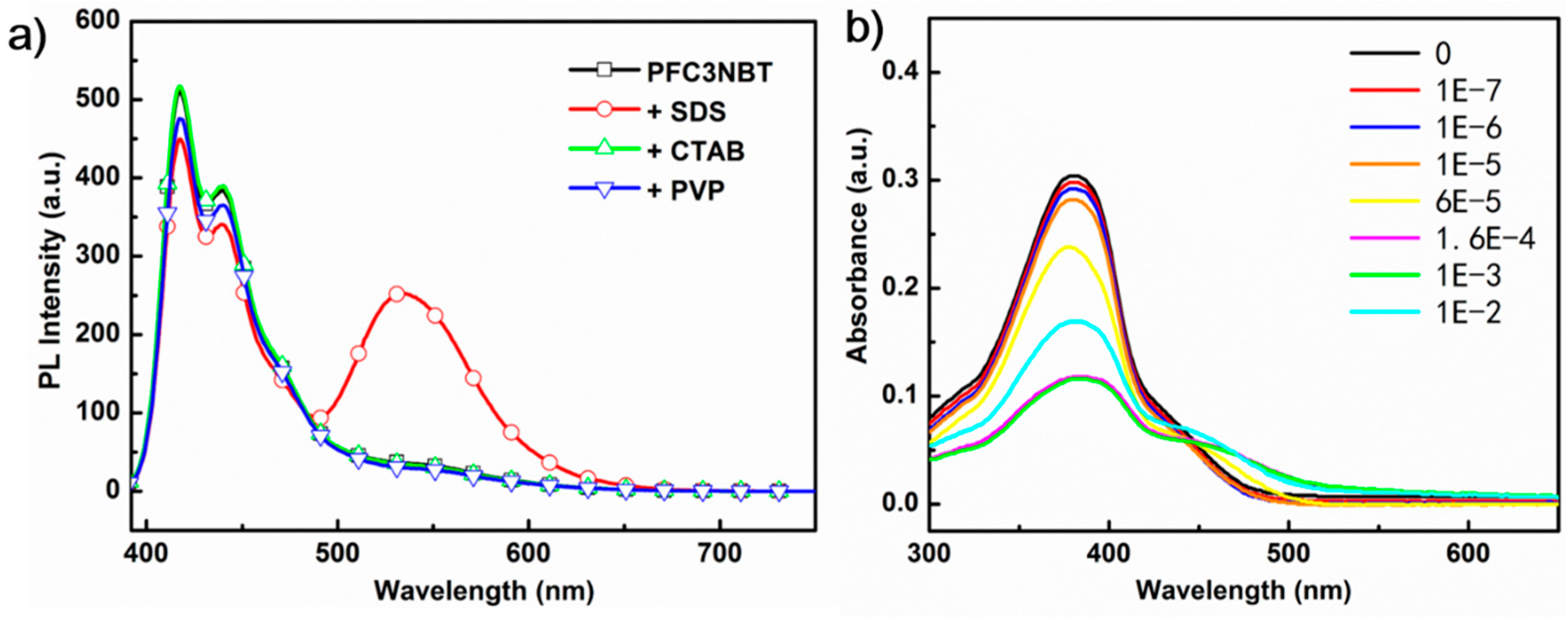
3.3. PL Response of PFC3NBT to Sodium Dodecyl Sulfate (SDS)
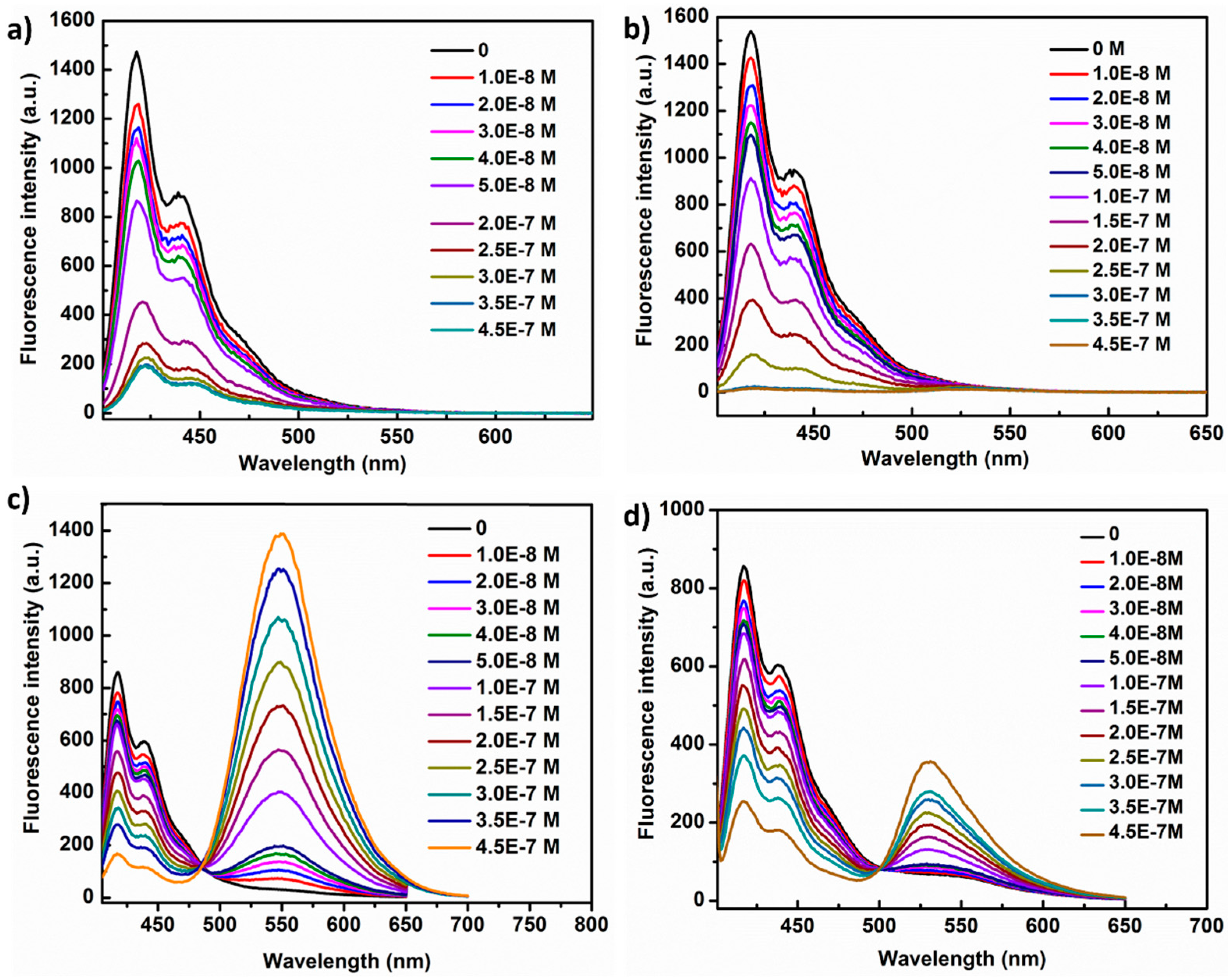
3.4. PL Response of PFC3NBT and PFC3N to DNAs
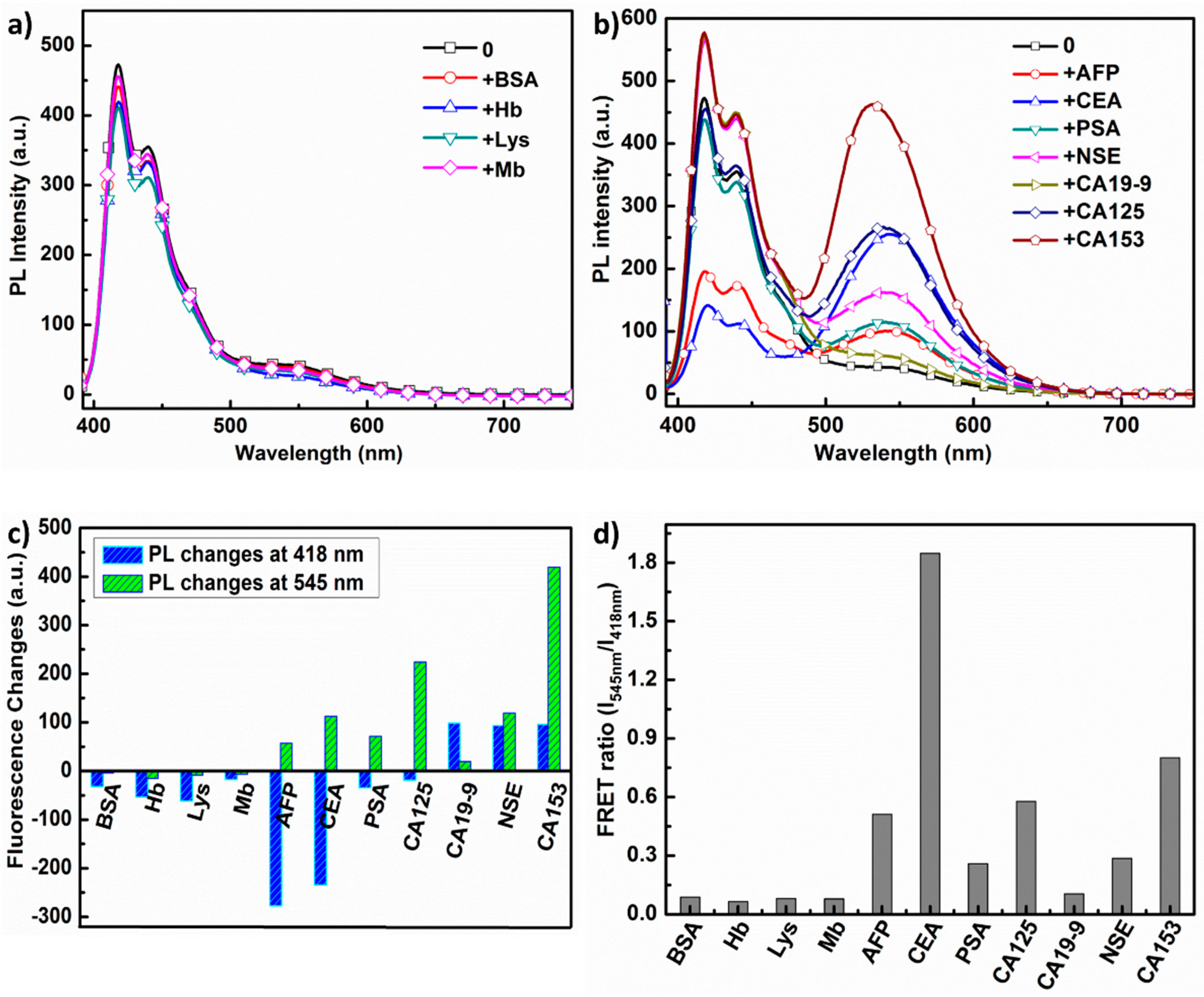
3.5. PL Responses of PFC3NBT to Different Proteins
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA detection using water-soluble conjugated polymers and peptide nucleic acid probes. Proc. Natl. Acad. Sci. USA 2002, 99, 10954–10957. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying gluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Interpolyelectrolyte complexes of conjugated copolymers and DNA: Platforms for multicolor biosensors. J. Am. Chem. Soc. 2004, 126, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Liu, L.B.; Yang, Q.; Lv, F.T.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, B.S.; Heeger, A.J.; Bazan, G.C. DNA hybridization detection with water-soluble conjugated polymers and chromophore-labeled single-stranded DNA. J. Am. Chem. Soc. 2003, 125, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.R.; Schanze, K.S. Amplified fluorescence sensing of protease activity with conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA 2004, 101, 7505–7510. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Liu, B. Fluorescent conjugated polyelectrolytes for bioimaging. Adv. Funct. Mater. 2011, 21, 3408–3423. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J. A universal method to produce low–work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhang, K.; Huang, F.; Bazan, G.C.; Cao, Y. Amino N-oxide functionalized conjugated polymers and their amino-functionalized precursors: new cathode interlayers for high-performance optoelectronic devices. Adv. Funct. Mater. 2012, 22, 2846–2854. [Google Scholar] [CrossRef]
- Meng, B.; Fu, Y.; Xie, Z.; Liu, J.; Wang, L. Phosphonate-functionalized donor polymer as an underlying interlayer to improve active layer morphology in polymer solar cells. Macromolecules 2014, 47, 6246–6251. [Google Scholar] [CrossRef]
- Duarte, A.; Pu, K.; Liu, B.; Bazan, G.C. Recent advances in conjugated polyelectrolytes for emerging optoelectronic applications. Chem. Mater. 2011, 23, 501–515. [Google Scholar] [CrossRef]
- Feng, F.D.; He, F.; An, L.L.; Wang, S.; Li, Y.L.; Zhu, D.B. Fluorescent conjugated polyelectrolytes for biomacromolecule detection. Adv. Mater. 2008, 20, 2959–2964. [Google Scholar] [CrossRef]
- Liu, X.F.; Tang, Y.L.; Wang, L.H.; Zhang, J.; Song, S.P.; Fan, C.H.; Wang, S. Optical detection of mercury(II) in aqueous solutions by using conjugated polymers and label-free oligonucleotides. Adv. Mater. 2007, 19, 1471–1474. [Google Scholar] [CrossRef]
- Li, C.; Numata, M.; Takeuchi, M.; Shinkai, S. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP. Angew. Chem. Int. Ed. 2005, 44, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.Y.; Mikhailovsky, A.; Bazan, G.C. Design of cationic conjugated polyelectrolytes for DNA concentration determination. J. Am. Chem. Soc. 2007, 129, 11134–11145. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers. Chem. Mater. 2004, 16, 4467–4476. [Google Scholar] [CrossRef]
- Zhan, R.Y.; Liu, B. Benzothiadiazole-containing conjugated polyelectrolytes for biological sensing and imaging. Macromol. Chem. Phys. 2015, 216, 131–144. [Google Scholar] [CrossRef]
- Wang, S.; Gaylord, B.S.; Bazan, G.C. Fluorescein provides a resonance gate for FRET from conjugated polymers to DNA intercalated dyes. J. Am. Chem. Soc. 2004, 126, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.D.; Liu, L.B.; Wang, S. Fluorescent conjugated polymer-based FRET technique for detection of DNA methylation of cancer cells. Nat. Protoc. 2010, 5, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, F.; Yu, M.H.; Wang, S. Analyte-induced aggregation of a water-soluble conjugated polymer for fluorescent assay of oxalic acid. Macromol. Rapid Commun. 2007, 28, 1905–1911. [Google Scholar] [CrossRef]
- Maynor, M.S.; Nelson, T.L.; O’Sullivan, C.; Lavigne, J.J. A food freshness sensor using the multistate response from analyte-induced aggregation of a cross-reactive poly(thiophene). Org. Lett. 2007, 9, 3217–3220. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.L.; O’Sullivan, C.; Greene, N.T.; Maynor, M.S.; Lavigne, J.J. Cross-reactive conjugated polymers: analyte-specific aggregative response for structurally similar diamines. J. Am. Chem. Soc. 2006, 128, 5640–5641. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G. Optimization of the molecular orbital energies of conjugated polymers for optical amplification of fluorescent sensors. J. Am. Chem. Soc. 2006, 128, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- An, L.L.; Tang, Y.L.; Feng, F.D.; He, F.; Wang, S. Water-soluble conjugated polymers for continuous and sensitive fluorescence assays for phosphatase and peptidase. J. Mater. Chem. 2007, 17, 4147–4152. [Google Scholar] [CrossRef]
- Bao, B.Q.; Yuwen, L.H.; Zheng, X.; Weng, L.X.; Zhu, X.R.; Zhan, X.W.; Wang, L.H. A fluorescent conjugated polymer for trace detection of diamines and biogenic polyamines. J. Mater. Chem. 2010, 20, 9628–9634. [Google Scholar] [CrossRef]
- Sun, P.F.; Lu, X.M.; Fan, Q.L.; Zhang, Z.Y.; Song, W.L.; Li, B.; Huang, L.; Peng, J.W.; Huang, W. Water-soluble iridium(III)-containing conjugated polyelectrolytes with weakened energy transfer properties for multicolor protein sensing applications. Macromolecules 2011, 44, 8763–8770. [Google Scholar] [CrossRef]
- Wang, H.P.; Lu, P.; Wang, B.L.; Qiu, S.; Liu, M.R.; Hanif, M.; Cheng, G.; Liu, S.Y.; Ma, Y.G. A water-soluble π-conjugated polymer with up to 100 mg·mL−1 solubility. Macromol. Rapid Commun. 2007, 28, 1645–1650. [Google Scholar] [CrossRef]
- Huang, F.; Hou, L.T.; Wu, H.B.; Wang, X.; Shen, H.L.; Cao, W.; Yang, W.; Cao, Y. High-efficiency, environment-friendly electroluminescent polymers with stable high work function metal as a cathode: Green- and yellow-emitting conjugated polyfluorene polyelectrolytes and their neutral precursors. J. Am. Chem. Soc. 2004, 126, 9845–9853. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Lobo, V.M.M.; Pina, J.; Ramos, M.L.; Seixas de Melo, J.; Valente, A.J.; Tapia, M.M.J.; Pradhan, S.; Scherf, U. Fluorescence enhancement of the water soluble poly{1,4-phenylene-[9,9-bis(4-phenoxybutylsulfonate)]fluorene-2,7-diyl} copolymer in n-dodecylpentaoxyethylene glycol ether micelles. Macromolecules 2004, 37, 7425–7427. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.J.; Zhu, J.S.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark antimicrobial mechanisms of cationic phenylene ethynylene polymers and oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar]
- Heeley, M.E.; Gallaher, J.K.; Nguyen, T.L.; Woo, H.Y.; Hodgkiss, J.M. Surfactant controlled aggregation of conjugated polyelectrolytes. Chem. Commun. 2012, 49, 4235–4237. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Mahale, A.; Arulkashmir, A.; Krishnamoorthy, K. Reversible assembly and disassembly of micelles by a polymer that switches between hydrophilic and hydrophobic wettings. Langmuir 2012, 28, 10097–10104. [Google Scholar] [CrossRef] [PubMed]
- Attar, H.A.; Monkman, A.P. Effect of surfactant on water-soluble conjugated polymer used in biosensor. J. Phys. Chem. B 2007, 111, 12418–12426. [Google Scholar] [CrossRef] [PubMed]
- Monteserin, M.; Burrows, H.D.; Mallavia, R.; Di Paolo, R.E.; Macanita, A.L.; Tapia, M.J. How to change the aggregation in the DNA/surfactant/cationic conjugated polyelectrolyte system through the order of component addition: anionic versus neutralsurfactants. Langmuir 2010, 26, 11705–11714. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Hussain, S.; Iyer, P.K. Aggregation-induced FRET via polymer–surfactant complexation: A new strategy for the detection of spermine. Anal. Chem. 2016, 88, 7358–7364. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeman, C.J.; Jiang, J.; Pan, Z.; Schanze, K.S. Intercalation of alkynylplatinum(II) terpyridine complexes into a helical poly(phenylene ethynylene) sulfonate: application to protein sensing. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Du, Y.; Tseng, Y.-T.; Peng, M.H.; Cai, N.; He, Y.; Chang, H.-T.; Yeung, E.S. Fluorescent gold nanodots based sensor array for proteins discrimination. Anal. Chem. 2015, 87, 4253–4259. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, N.; Wang, Y.; Liu, D.; Zhang, C.; Su, S.; Bao, B.; Zhao, B.; Wang, L. A Conjugated Polyelectrolyte with Pendant High Dense Short-Alkyl-Chain-Bridged Cationic Ions: Analyte-Induced Light-Up and Label-Free Fluorescent Sensing of Tumor Markers. Polymers 2017, 9, 227. https://doi.org/10.3390/polym9060227
Fu N, Wang Y, Liu D, Zhang C, Su S, Bao B, Zhao B, Wang L. A Conjugated Polyelectrolyte with Pendant High Dense Short-Alkyl-Chain-Bridged Cationic Ions: Analyte-Induced Light-Up and Label-Free Fluorescent Sensing of Tumor Markers. Polymers. 2017; 9(6):227. https://doi.org/10.3390/polym9060227
Chicago/Turabian StyleFu, Nina, Yijiao Wang, Dan Liu, Caixia Zhang, Shao Su, Biqing Bao, Baomin Zhao, and Lianhui Wang. 2017. "A Conjugated Polyelectrolyte with Pendant High Dense Short-Alkyl-Chain-Bridged Cationic Ions: Analyte-Induced Light-Up and Label-Free Fluorescent Sensing of Tumor Markers" Polymers 9, no. 6: 227. https://doi.org/10.3390/polym9060227
APA StyleFu, N., Wang, Y., Liu, D., Zhang, C., Su, S., Bao, B., Zhao, B., & Wang, L. (2017). A Conjugated Polyelectrolyte with Pendant High Dense Short-Alkyl-Chain-Bridged Cationic Ions: Analyte-Induced Light-Up and Label-Free Fluorescent Sensing of Tumor Markers. Polymers, 9(6), 227. https://doi.org/10.3390/polym9060227





