Electrochemical Polymerization of Hydroquinone on Graphite Felt as a Pseudocapacitive Material for Application in a Microbial Fuel Cell
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of PHQ–GF and PHQ–AGF Electrodes and Electrochemical Tests
2.2. MFC Operation and Performance Tests
2.3. Physical and Electrochemical Characterizations
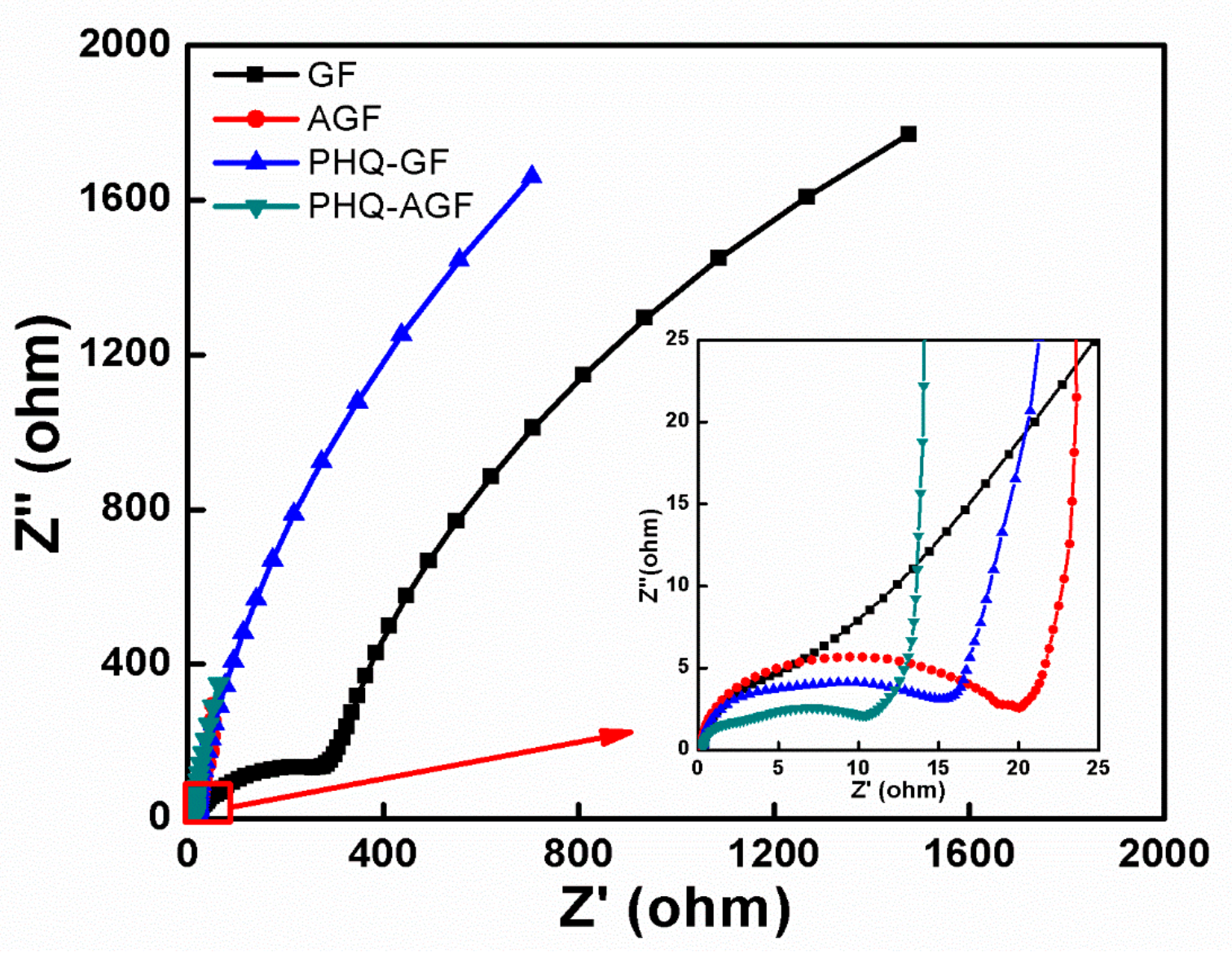
3. Results and Discussion
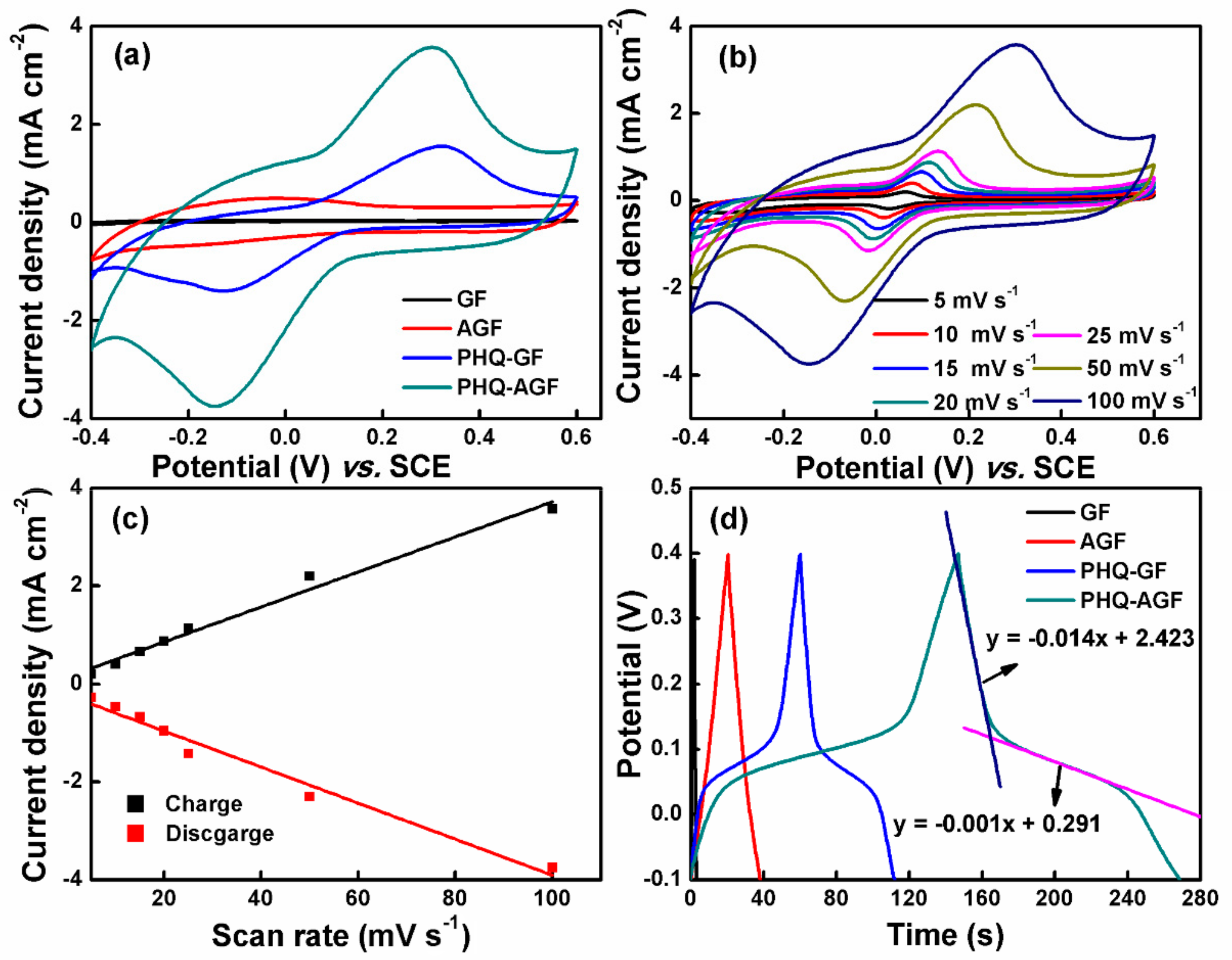
3.1. Electrochemical Capacitance Performance of the Composite Films
3.2. Analysis of Oxygen-Containing Functional Groups
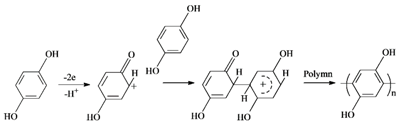
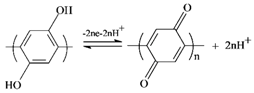
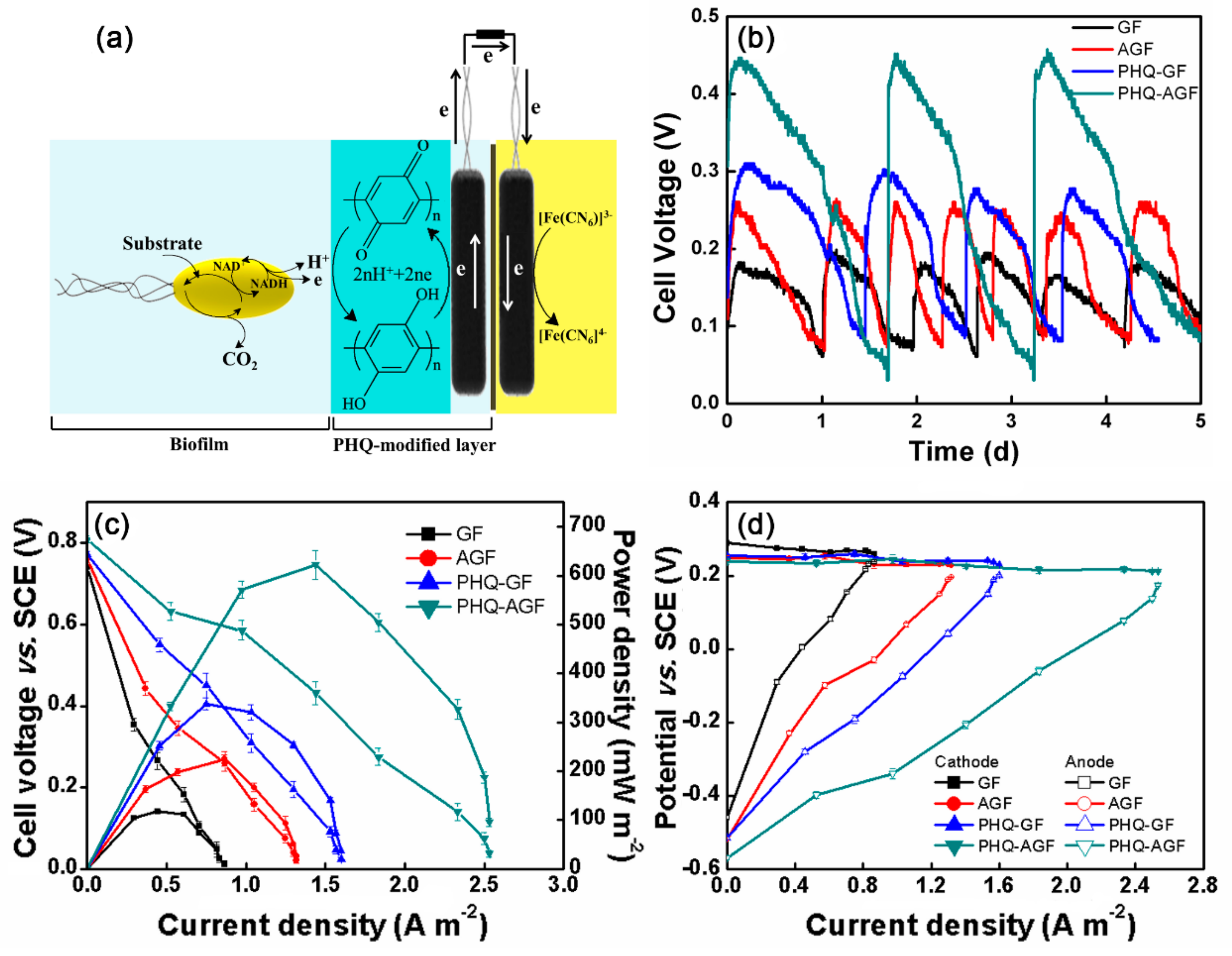
3.3. Proposed Mechanism of Hydroquinone Electropolymerization on the Graphite Felt Surface
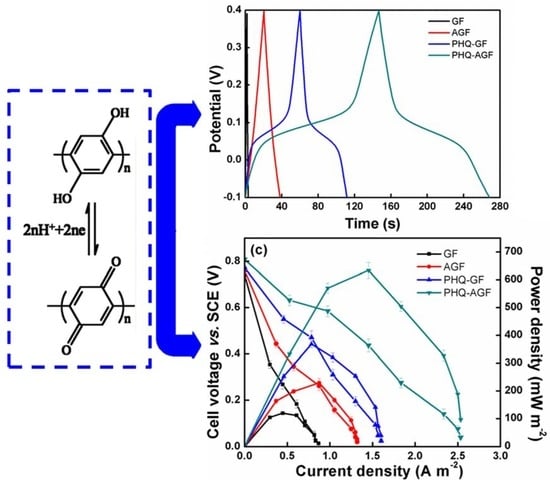
3.4. MFC Power Performance
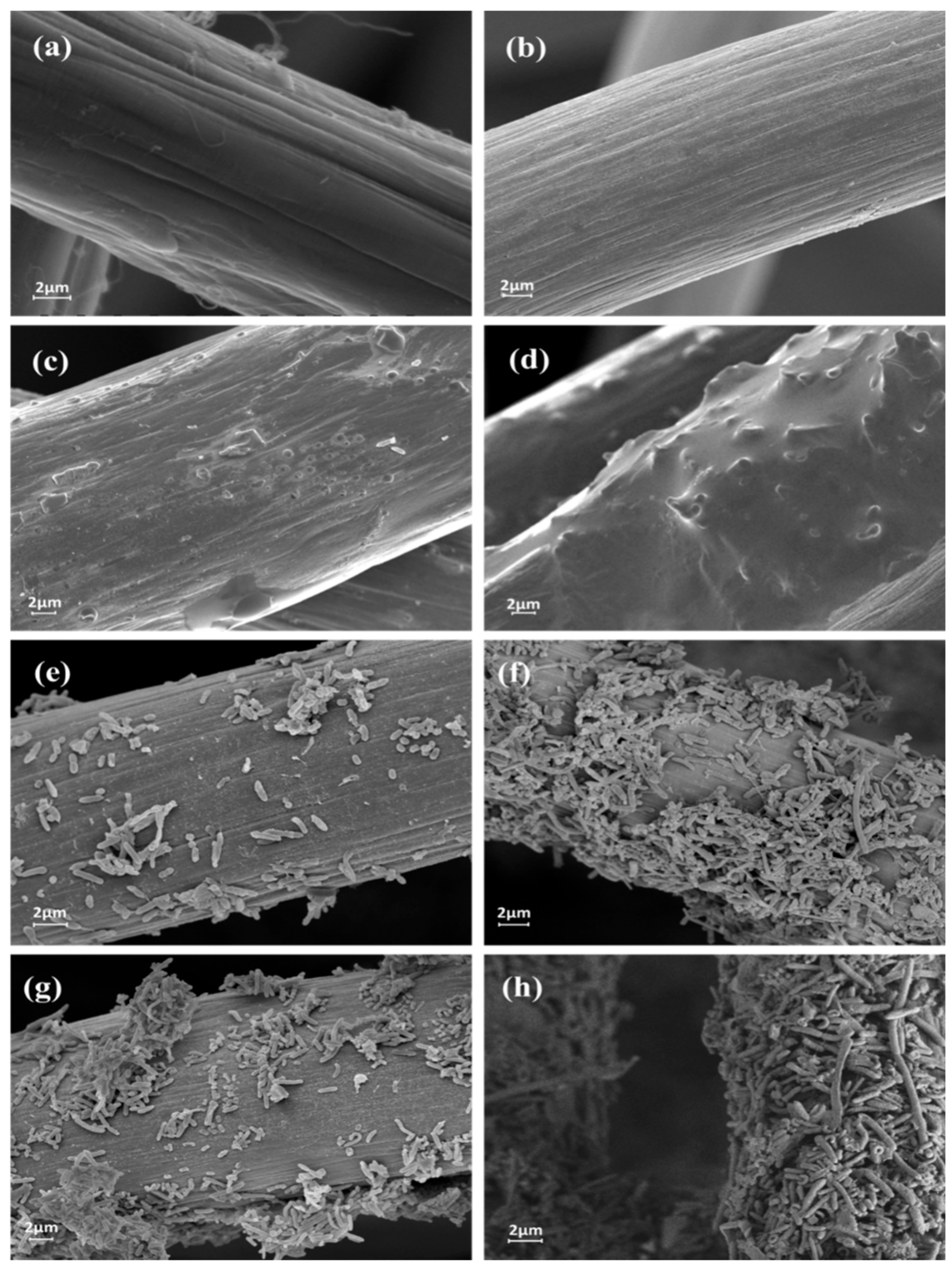
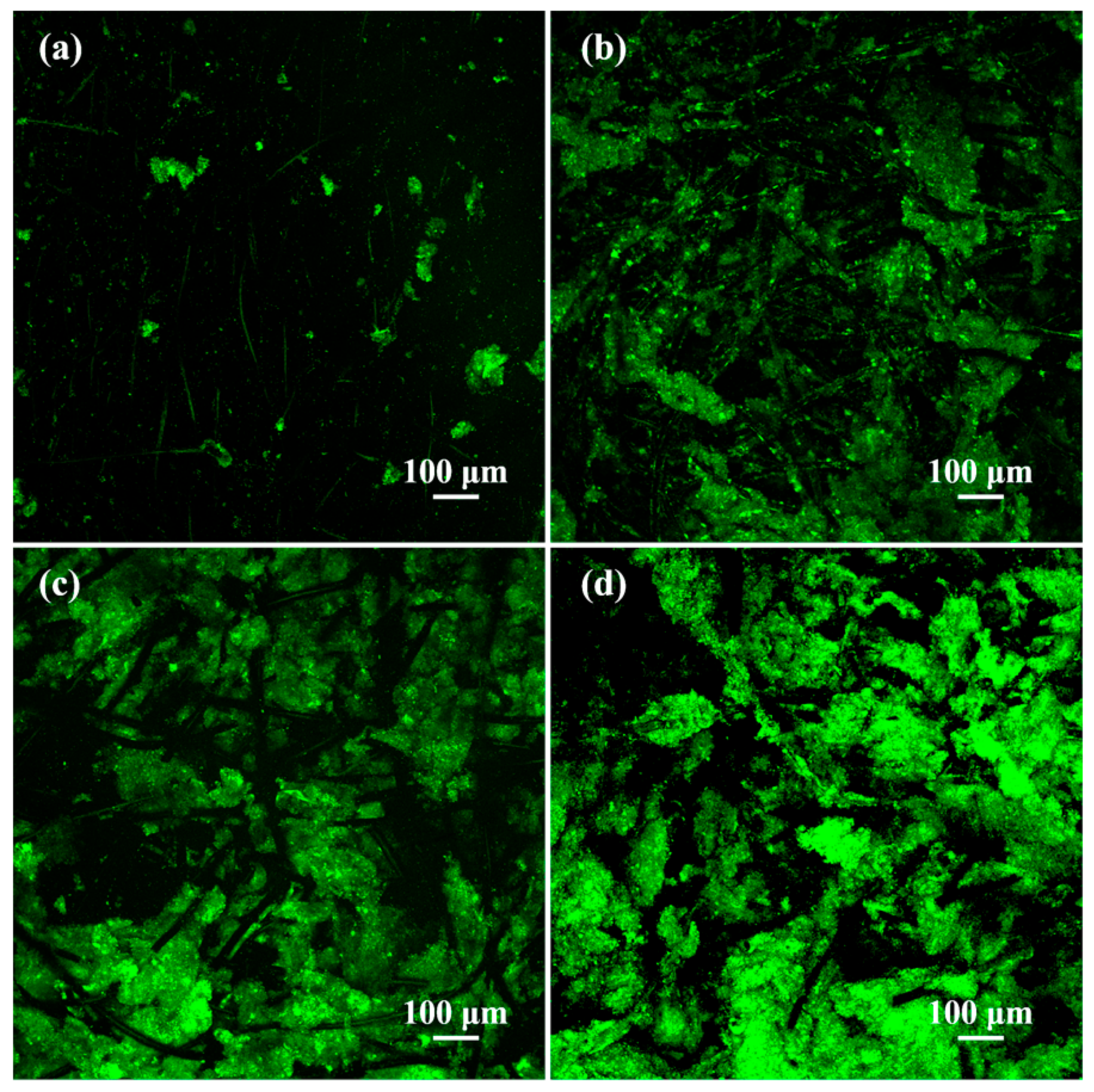
3.5. Morphological and Electrochemical Characterizations of the Inoculated Anodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vonlanthen, D.; Lazarev, P.; See, K.A.; Wudl, F.; Heeger, A.J. A stable polyaniline-benzoquinone-hydroquinone supercapacitor. Adv. Mater. 2014, 26, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Arcila-Velez, M.R.; Roberts, M.E. Redox solute doped polypyrrole for high-charge capacity polymer electrodes. Chem. Mater. 2014, 26, 1601–1607. [Google Scholar] [CrossRef]
- Kuwahara, T.; Yamazaki, H.; Kondo, M.; Shimomura, M. Modification of an enzyme electrode by electrodeposition of hydroquinone for use as the anode of a glucose fuel cell. Appl. Surf. Sci. 2012, 258, 6321–6325. [Google Scholar] [CrossRef]
- Babanova, S.; Matanovic, I.; Chavez, M.S.; Atanassov, P. Role of Quinones in electron transfer of PQQ–glucose dehydrogenase anodes-mediation or orientation effect. J. Am. Chem. Soc. 2015, 137, 7754–7762. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Annamalai, S.K. Selective immobilization of hydroquinone on carbon nanotube modified electrode via phenol electro-oxidation method and its hydrazine electro-catalysis and Escherichia coli antibacterial activity. Electrochim. Acta 2012, 62, 207–217. [Google Scholar] [CrossRef]
- Feng, C.; Ma, L.; Li, F.; Mai, H.; Lang, X.; Fan, S. A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, H.; Du, Z.; Ng, H.Y. Spontaneous modification of graphite anode by anthraquinone-2-sulfonic acid for microbial fuel cells. Bioresour. Technol. 2014, 164, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, X.; Sheng, C.; Chen, X.; Kong, Y.; Tao, Y.; Chen, Z. Selective determination of hydroquinone in the presence of catechol based on over-oxidized poly(hydroquinone). J. Solid State Electrochem. 2012, 16, 3159–3164. [Google Scholar] [CrossRef]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Xie, D.; Yu, H.; Li, C.; Ren, Y.; Wei, C.; Feng, C. Competitive microbial reduction of perchlorate and nitrate with a cathode directly serving as the electron donor. Electrochim. Acta 2014, 133, 217–223. [Google Scholar] [CrossRef]
- Feng, C.; Lv, Z.; Yang, X.; Wei, C. Anode modification with capacitive materials for a microbial fuel cell: An increase in transient power or stationary power. Phys. Chem. Chem. Phys. 2014, 16, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- Śliwak, A.; Grzyb, B.; Ćwikła, J.; Gryglewicz, G. Influence of wet oxidation of herringbone carbon nanofibers on the pseudocapacitance effect. Carbon 2013, 64, 324–333. [Google Scholar] [CrossRef]
- Wang, G.; Liang, R.; Liu, L.; Zhong, B. Improving the specific capacitance of carbon nanotubes-based supercapacitors by combining introducing functional groups on carbon nanotubes with using redox-active electrolyte. Electrochim. Acta 2014, 115, 183–188. [Google Scholar] [CrossRef]
- Yamamoto, K.; Asada, T.; Nishide, H.; Tsuchida, E. The Preparation of poly (dihydroxyphenylene) through the electro-oxidative polymerization of hydroquinone. Bull. Chem. Soc. Jpn. 1990, 63, 1211–1216. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G.; Inganäs, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.K.; Lee, H.; Lee, S.B.; Park, H.S. Superior pseudocapacitive behavior of confined lignin nanocrystals for renewable energy-storage materials. ChemSusChem 2014, 7, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Barathi, P.; Kumar, A.S. Electrochemical Oxidation of Hazardous Tetracene to Highly redox active anthraquinone and hydroquinone derivatives on a carbon nanotube-modified electrode and its selective hydrogen peroxide sensing. Electroanalysis 2014, 26, 2342–2349. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Liu, C.; Han, N.; Xu, X.; Zhao, F.; Fan, J.; Li, Y. Polyanthraquinone-based nanostructured electrode material capable of high-performance pseudocapacitive energy storage in aprotic electrolyte. Nano Energy 2015, 15, 654–661. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Kang, N.; Zuo, Y.J.; Hilliou, L.; Ashokkumar, M.; Hemar, Y. Viscosity and hydrodynamic radius relationship of high-power ultrasound depolymerised starch pastes with different amylose content. Food Hydrocoll. 2016, 52, 183–191. [Google Scholar] [CrossRef]
- Zhou, J.H.; Sui, Z.J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.C.; Yuan, W.K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Yamamoto, K.; Asada, T.; Nishide, H.; Tsuchida, E. Electro-oxidative polymerization of p-dialkoxybenzenes and its mechanism. Polym. Bull. 1988, 19, 533–538. [Google Scholar] [CrossRef]
- Boota, M.; Hatzell, K.B.; Kumbur, E.C.; Gogotsi, Y. Towards high-energy-density pseudocapacitive flowable electrodes by the incorporation of hydroquinone. ChemSusChem 2015, 8, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramos, J.; Alligrant, T.M.; Clingenpeel, A.; Alvarez, J.C. Comparing the hydrogen-bonding effect of bronsted bases in solution and when they are covalently bound to the surface of glassy carbon electrodes in the electrochemical behavior of hydroquinone. J. Phys. Chem. C 2012, 116, 20447–20457. [Google Scholar] [CrossRef]
- Ahammad, A.S.; Rahman, M.M.; Xu, G.R.; Kim, S.; Lee, J.J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly (thionine) modified glassy carbon electrode. Electrochim. Acta 2011, 56, 5266–5271. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, P.; Jiang, Y.; Huang, X. Enhanced power generation of microbial fuel cell using manganese dioxide-coated anode in flow-through mode. J. Power Sources 2015, 273, 580–583. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, P.; Yang, X.; Jiang, Y.; Bian, Y.; Chen, C.; Zhang, X.; Huang, X. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens. Bioelectron. 2016, 81, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Deng, L.; Chen, Y.; Yuan, Y. MnO2/Polypyrrole/MnO2 multi-walled-nanotube-modified anode for high-performance microbial fuel cells. Electrochim. Acta 2016, 196, 280–285. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional manganese ferrite/polyaniline hybrid as electrode material for enhanced energy recovery in microbial fuel cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Yuan, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; He, W.; Qu, Y.; Ren, N.; Feng, Y. Enhanced electricity generation for microbial fuel cell by using electrochemical oxidation to modify carbon cloth anode. J. Power Sources 2014, 265, 391–396. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, C.; Zhou, W.; Yu, H. Municipal sludge-derived carbon anode with nitrogen-and oxygen-containing functional groups for high-performance microbial fuel cells. J. Power Sources 2016, 307, 105–111. [Google Scholar] [CrossRef]
- Tang, X.; Guo, K.; Li, H.; Du, Z.; Tian, J. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes. Bioresour. Technol. 2011, 102, 3558–3560. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Liou, J.S.; Liu, J.L.; Chiu, Y.F.; Xu, C.H.; Chen, B.Y.; Chen, J.Z. Feasibility study of surface-modified carbon cloth electrodes using atmospheric pressure plasma jets for microbial fuel cells. J. Power Sources 2016, 336, 99–106. [Google Scholar] [CrossRef]
- Geng, Q.; Guo, Q.; Cao, C.; Wang, L. Investigation into Nano TiO2/ACSPCR for decomposition of aqueous hydroquinone. Ind. Eng. Chem. Res. 2008, 47, 2561–2568. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Basha, C.A.; Kannadasan, T.; Aravinthan, P. Removal of hydroquinone from water by electrocoagulation using flow cell and optimization by response surface methodology. J. Environ. Sci. Health A 2010, 45, 400–412. [Google Scholar] [CrossRef] [PubMed]

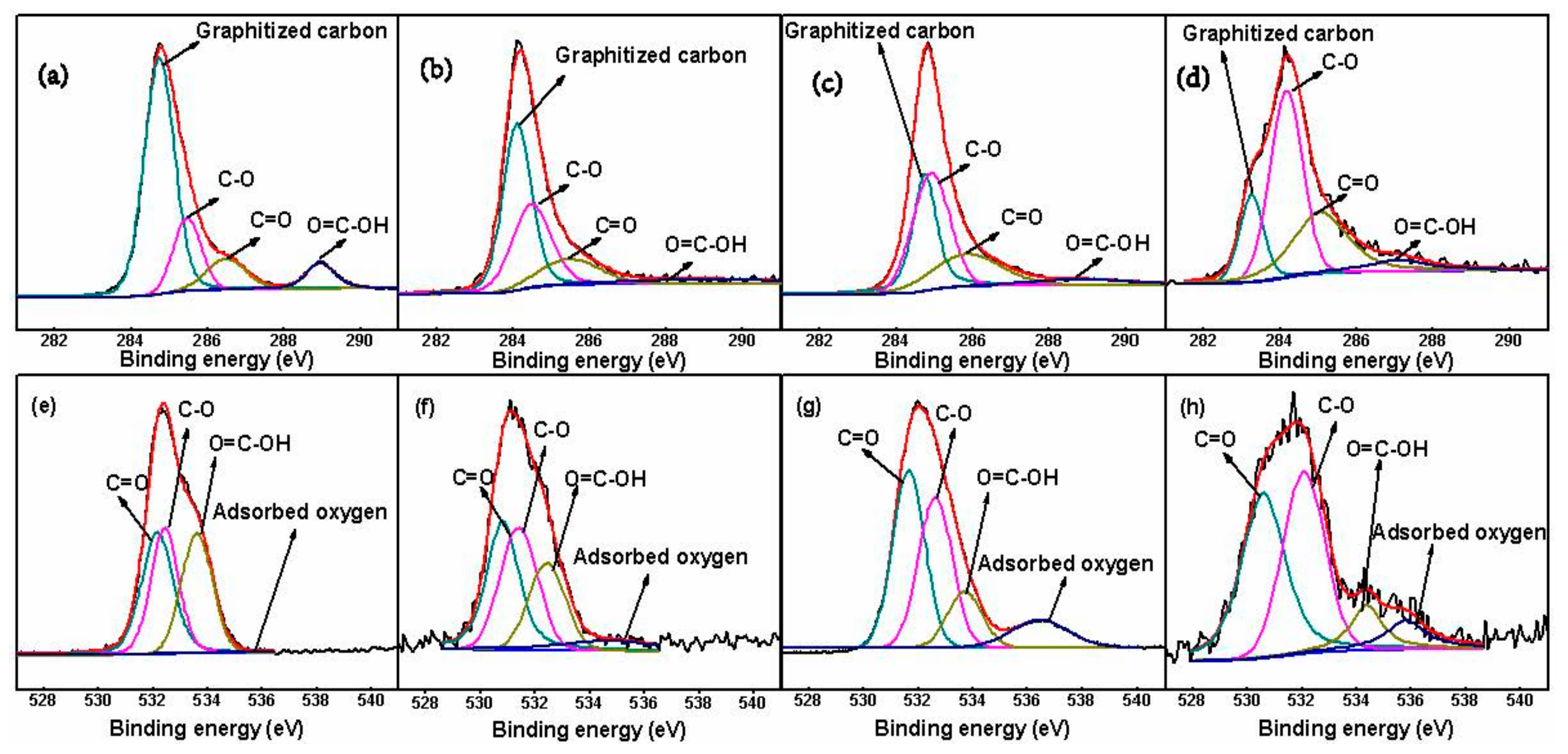
| C1s | O1s | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Graphitized Carbon | C–O | C=O | O=C–OH | C=O | C–O | O=C–OH | Adsorbed Oxygen | ||
| GF | 64.37% | 17.40% | 10.46% | 7.77% | 35.40% | 31.00% | 30.63% | 2.98% | |
| AGF | 47.40% | 31.95% | 14.29% | 6.36% | 35.86% | 32.34% | 22.56% | 9.25% | |
| PHQ–GF | 32.33% | 40.94% | 21.06% | 5.67% | 39.72% | 35.35% | 13.57% | 11.36% | |
| PHQ–AGF | 16.51% | 45.20% | 30.49% | 7.79% | 43.16% | 38.21% | 10.06% | 8.57% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Feng, C. Electrochemical Polymerization of Hydroquinone on Graphite Felt as a Pseudocapacitive Material for Application in a Microbial Fuel Cell. Polymers 2017, 9, 220. https://doi.org/10.3390/polym9060220
Wang G, Feng C. Electrochemical Polymerization of Hydroquinone on Graphite Felt as a Pseudocapacitive Material for Application in a Microbial Fuel Cell. Polymers. 2017; 9(6):220. https://doi.org/10.3390/polym9060220
Chicago/Turabian StyleWang, Guanwen, and Chunhua Feng. 2017. "Electrochemical Polymerization of Hydroquinone on Graphite Felt as a Pseudocapacitive Material for Application in a Microbial Fuel Cell" Polymers 9, no. 6: 220. https://doi.org/10.3390/polym9060220
APA StyleWang, G., & Feng, C. (2017). Electrochemical Polymerization of Hydroquinone on Graphite Felt as a Pseudocapacitive Material for Application in a Microbial Fuel Cell. Polymers, 9(6), 220. https://doi.org/10.3390/polym9060220