The Significant Influence of Bacterial Reaction on Physico-Chemical Property Changes of Biodegradable Natural and Synthetic Polymers Using Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
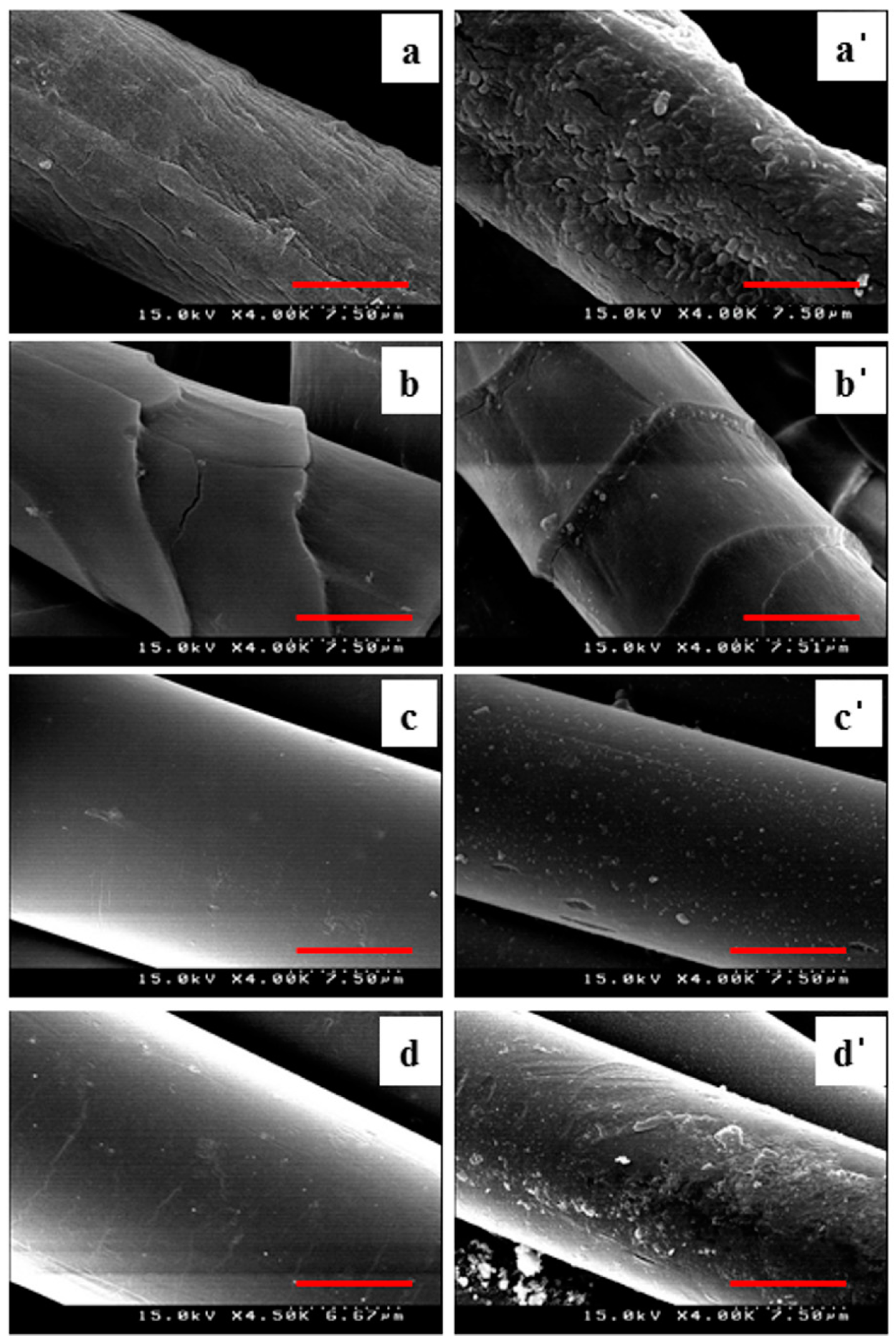
2.3. SEM Analysis
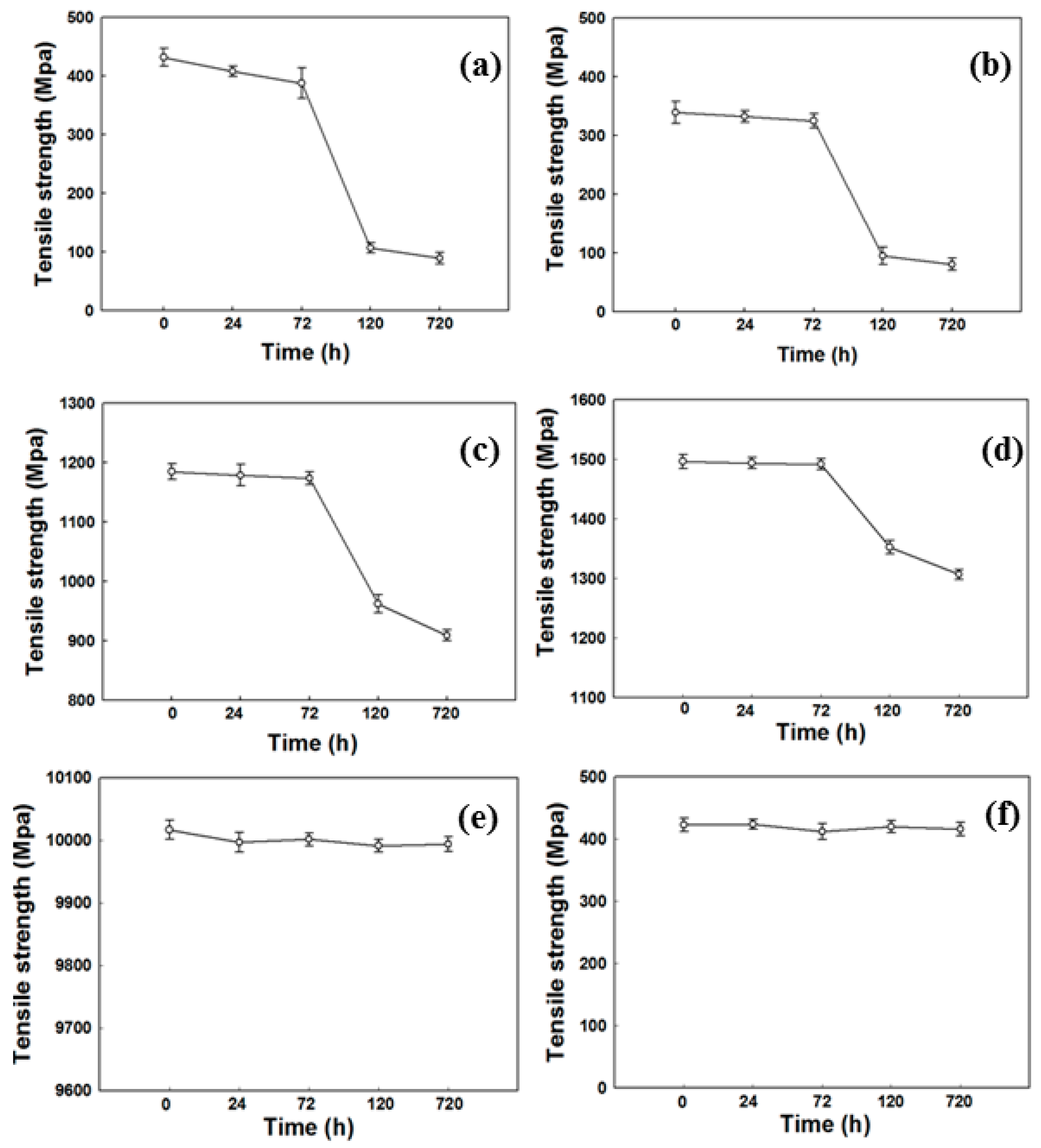
2.4. Mechanical Strength
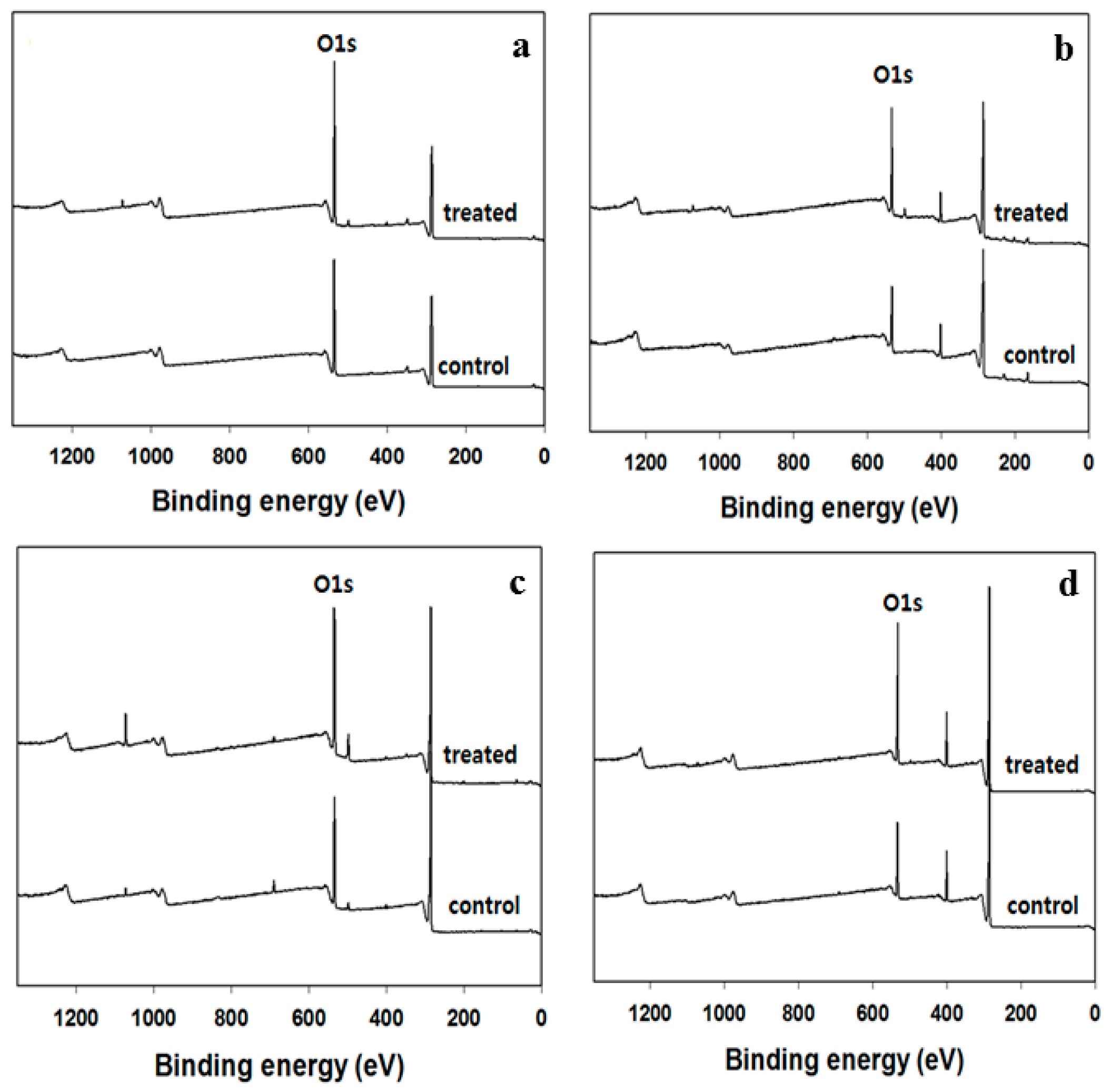
2.5. XPS Analysis
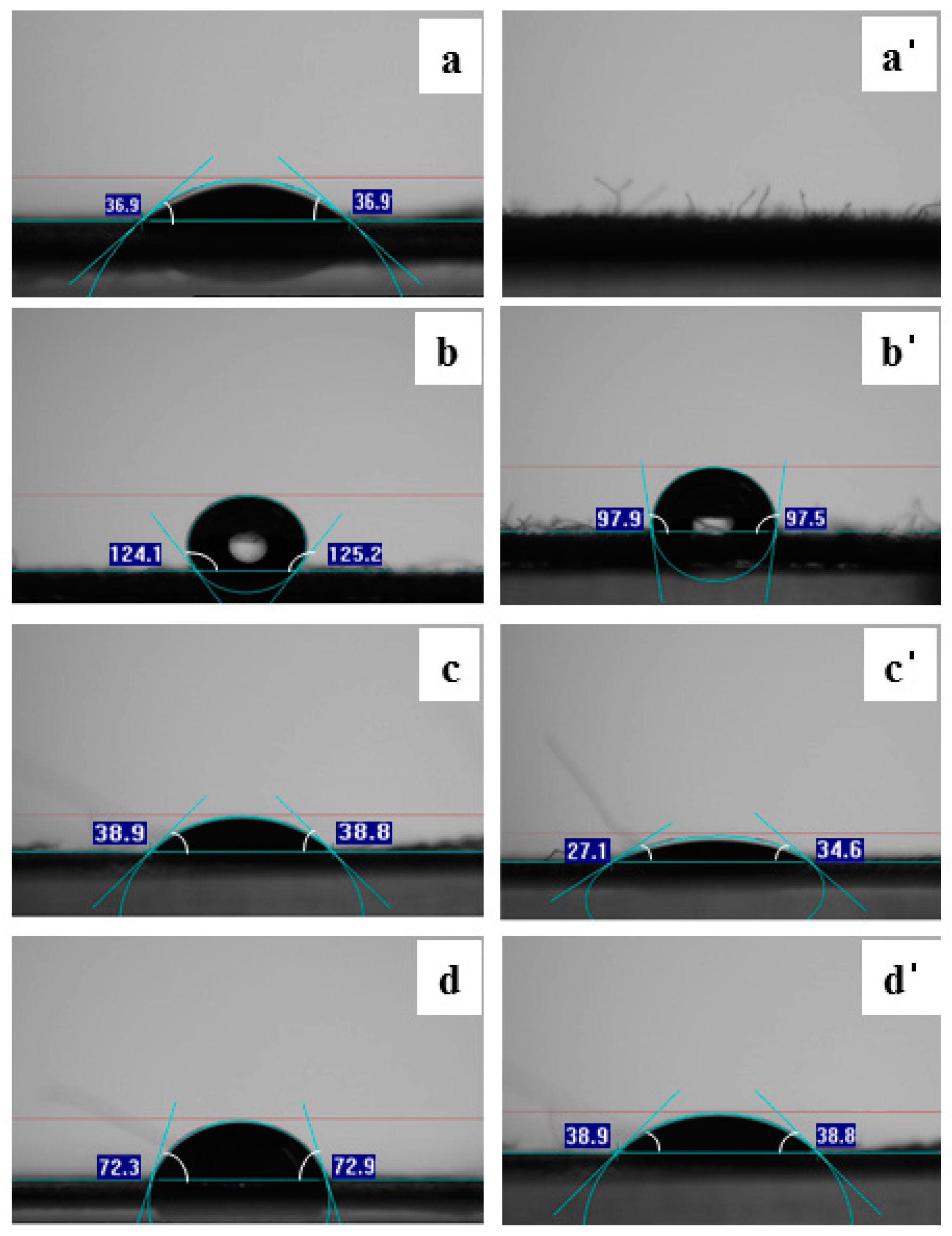
2.6. Surface Property
3. Results and Discussion
3.1. Analysis of Physical Property
3.2. Surface Morphology
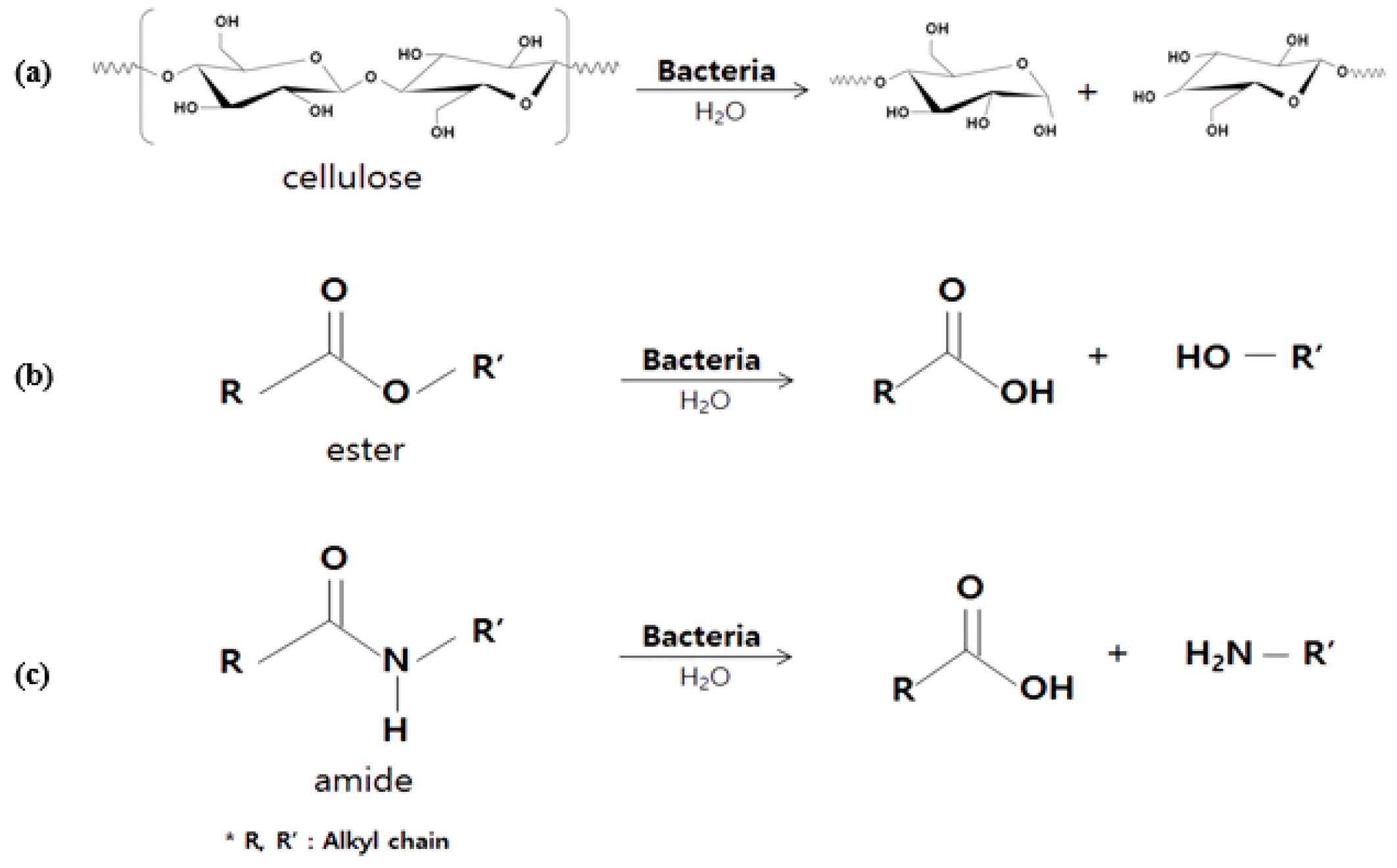
3.3. Reaction of E. coli with Fiber Materials
3.4. Topographical Properties of the Surface
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Höfer, D. Antimicrobial textiles, skin-borne flora and odour. Curr. Probl. Dermatol. 2006, 33, 67–77. [Google Scholar] [PubMed]
- Teufe, L.; Pipal, A.; Schuster, K.C.; Staudinger, T.; Redl, B. Material dependent growth of human skin bacteria ontextiles investigated using challenge tests and DNA genotyping. J. Appl. Microbiol. 2009, 108, 450–461. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Laing, R.M.; Brooks, H.J.L.; Niven, B.E. Odor intensity in apparel fabrics and the link with bacterial populations. Text. Res. J. 2007, 77, 449–456. [Google Scholar] [CrossRef]
- Rennie, P.J.; Gower, D.B.; Holland, K.T.; Mallet, A.I.; Watkins, W. The skin microflora and the formation of human axillary odour. Int. J. Cosmet. Sci. 1990, 12, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.; Dey, A.; Ghosh, S.; Bajpai, S.; Jha, M.K. Quantification of bacterial adherence on different textile fabrics. Int. Biodeterior. Biodegrad. 2011, 65, 1169–1174. [Google Scholar] [CrossRef]
- Obendorf, K.; Kjm, J.; Koniz, R.F. Measurement of odor development due to bacterial action on antimicrobial polyester fabrics. AATCC Rev. 2007, 7, 35–40. [Google Scholar]
- Szostak-Kotowa, J. Biodeterioration of Textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Merry, J. The adherence of Staphylococcus aureus, Staphylococcus epidermidis, and E. coli on cotton, polyester and their blends. J. Appl. Bacteriol. 1986, 60, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.E.; Cavaco-Paulo, A. Enzyme Applications for Fiber Processing, 1st ed.; ACS Symposium Series Book; American Chemistry Society: Washington, DC, USA, 1998; pp. 13–22. [Google Scholar]
- Parvinzadeh, M. Effect of proteolytic enzyme on dyeing of wool with madder. Enzym. Microb. Technol. 2007, 40, 1719–1722. [Google Scholar] [CrossRef]
- Gubitz, G.M.; Cavaco-Paulo, A. Enzymes go big: Surface hydrolysis and functionalisation of synthetic polymers. Trends Biotechnol. 2008, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Rouette, H.K. Encyclopedia of Textile Finishing, 1st ed.; Springer: Berlin, Germany, 2001; pp. 134–158. [Google Scholar]
- Cavaco-Paulo, A.; Gubitz, G.M. Textile Processing with Enzyme, 1st ed.; Woodhead Publishing: Washington, DC, USA, 2003; pp. 34–54. [Google Scholar]
- Silva, C.; Matama, T.; Guebitz, G.M.; Cavaco-Paulo, A. Influence of organic solvents on cutinase stability and accessibility to polyamide fibers. J. Polym. Sci. Polym. Chem. 2015, 43, 2749–2753. [Google Scholar] [CrossRef]
- Kumar, A.; Purtell, C.; Lepola, M. Enzymatic Treatment of Man-Made Cellulosic. Fabr. Text. Chem. Color. 1994, 26, 25–28. [Google Scholar]
- Sarkar, A.K.; Etters, J.N. Enzymatic hydrolysis of cotton fibers: Modeling using an empirical equation. J. Cotton Sci. 2004, 8, 254–260. [Google Scholar]
- Wang, L.S.; Zhang, Y.Z.; Gao, P.J.; Shi, D.X.; Liu, H.W.; Gao, H.L. Changes in the structural properties and rate of hydrolysis of cotton fibers during extended enzymatic hydrolysis. Biotechnol. Bioeng. 2006, 93, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Dadashian, F.; Nejad, N.H. Optimization of enzymatic hydrolysis of wool fibers for nanoparticles production using response surface methodology. Adv. Power Technol. 2013, 24, 416–426. [Google Scholar] [CrossRef]
- Marten, E.; Muller, R.; Deckwer, W. Studies on the enzymatic hydrolysis of polyesters: II. Aliphatic-aromatic copolyesters. Polym. Degrad. Stab. 2003, 80, 485–501. [Google Scholar] [CrossRef]
- Herzog, K.; Müller, R.; Deckwer, W. Mechanism and Kinetics of the enzymatic hydrolysis of polyester nanoparticles by lipases. Polym. Degrad. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Kiumarsi, A.; Parvinzadeh, M. Enzymatic hydrolysis of nylon 6 fibre using lipolityc enzyme. J. Appl. Polym. Sci. 2010, 116, 3140–3147. [Google Scholar]
- Wang, L.; Liu, J.; Zhang, Y.; Zhao, Y.; Gao, P. Comparison of domains function between cellobiohydrolase I and endoglucanase I from Trichoderma pseudokoningii S-38 by limited proteolysis. J. Mol. Catal. B 2003, 24–25, 27–38. [Google Scholar] [CrossRef]
- Brueckner, T.; Eberi, A.; Heumann, S.; Rabe, M.; Guebitz, G.M. Enzymatic and chemical hydrolysis of poly(ethylene terephthalate) fabrics. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6435–6443. [Google Scholar] [CrossRef]
- Floyd, K.A.; Moore, J.L.; Eberly, A.R.; Good, J.A.D.; Shaffer, C.L.; Zaver, H.; Almqvist, F.; Skaar, E.P.; Caprioli, R.M.; Hadjifrangiskou, M. Adhesive fiber stratification in uropathogenic Escherichia coli biofilms unveils oxygen-mediated control of type 1 pili. PLoS Pathog. 2015, 11, e1004697. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Oh, M.; Yeo, W.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhao, C.; Yan, K.; Sun, G. Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohydr. Polym. 2016, 144, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hardin, R.Z. Enzymatic scouring of cotton-surfactants, agitation and selection of enzymes. Text. Chem. Color. 1998, 30, 23–29. [Google Scholar]
- Schmidt, J.; Wei, R.; Oeser, T.; Silva, L.A.D.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, H.C. Analysis of fiber blends. Part II. Determination of blend composition by moisture regain. Text. Res. J. 1980, 50, 359–362. [Google Scholar] [CrossRef]
- Wu, K.; Wu, C.; Chang, J. Biodegradability and mechanical properties of polycaprolactone composites encapsulating phosphate-solubilizing bacterium Bacillus sp. PG01. Process Biochem. 2007, 42, 669–675. [Google Scholar] [CrossRef]
- Bahi, A.; Jones, J.T.; Carr, C.M.; Ulijn, R.V.; Shao, J. Surface characterization of chemically modified wool. Text. Res. J. 2007, 77, 937–945. [Google Scholar] [CrossRef]
- Kawahara, Y.; Yoshioka, T.; Takarada, W.; Kikutani, T.; Tsuji, M. Alkaline hydrolysis kinetics of poly(ethylene terephthalate) fibers. J. Fiber Sci. Technol. 2016, 72, 9–16. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, J. Antibacterial activity of polyacrylonitrile-chitosan electrospun nanofibers. Carbohydr. Polym. 2014, 102, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bharathidasan, T.; Narayanan, T.N.; Sathyanaryanan, S.; Sreejakumari, S.S. Above 170 water contact angle and oleophobicity of fluorinated grapheme oxide based transparent polymeric films. Carbon 2015, 84, 207–213. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.; Kim, S.; Kim, S.; Lee, J.W. The Significant Influence of Bacterial Reaction on Physico-Chemical Property Changes of Biodegradable Natural and Synthetic Polymers Using Escherichia coli. Polymers 2017, 9, 121. https://doi.org/10.3390/polym9040121
Kang C, Kim S, Kim S, Lee JW. The Significant Influence of Bacterial Reaction on Physico-Chemical Property Changes of Biodegradable Natural and Synthetic Polymers Using Escherichia coli. Polymers. 2017; 9(4):121. https://doi.org/10.3390/polym9040121
Chicago/Turabian StyleKang, Chankyu, SamSoo Kim, SooJung Kim, and Jae Woong Lee. 2017. "The Significant Influence of Bacterial Reaction on Physico-Chemical Property Changes of Biodegradable Natural and Synthetic Polymers Using Escherichia coli" Polymers 9, no. 4: 121. https://doi.org/10.3390/polym9040121
APA StyleKang, C., Kim, S., Kim, S., & Lee, J. W. (2017). The Significant Influence of Bacterial Reaction on Physico-Chemical Property Changes of Biodegradable Natural and Synthetic Polymers Using Escherichia coli. Polymers, 9(4), 121. https://doi.org/10.3390/polym9040121





