1. Introduction
Polymer vesicles prepared by self-association of block copolymers, which are of great interest because of their potential application in fields such as materials science and biochemistry. Usually, polymer vesicles are prepared by self-assembly of amphiphilic block copolymers by the solvent switch method [
1,
2] or the organic-solvent free method [
3]. For the solvent switch method, an amphiphilic diblock copolymer is dissolved in an organic solvent that can be mixed with water, such as dimethylsulfoxide (DMSO),
N,N-diethylformamide (DMF), tetrahydrofuran (THF), or 1,4-dioxane, to prepare a homogeneous polymer solution, followed by the gradual addition water to the organic solvent solution. The hydrophilic block chains become solvated to form the vesicle shell, which stabilizes the polymer vesicle, whereas the hydrophobic blocks associate to form the vesicle membrane. It is difficult to control vesicle size using this method because the self-assembly process depends heavily on the rate of solvent mixing. Moreover, further purification using dialysis is required to remove the organic solvent. This process is time-consuming and economically unfavorable. Furthermore, many factors, such as the initial polymer concentration, organic solvent characteristics, additives used, and temperature, affect the morphology of the polymer aggregates [
4]. Polymer vesicle preparation without the use of an organic solvent usually involves rehydration of the block copolymer in water. The diblock copolymer is dissolved directly in water to form the polymer vesicle, but long and vigorous agitation is usually necessary to fully hydrate the block copolymer. However, this method results in broad size distributions [
5,
6].
Kataoka and Kishimura [
7] reported the elegant formation of polyion complex vesicles (PICsomes) from oppositely charged diblock copolymers of cationic poly(ethylene glycol) (PEG)-
block-poly((5-aminopentyl)-α,β-aspartamide) (PEG-P(Asp-AP)) and anionic PEG-
block-poly(α,β-aspartic acid) (PEG-PAsp). The degree of polymerization (DP) for PEG, P(Asp-AP), and PAsp is 45, 75, and 75, respectively. Aqueous solutions of PEG-P(Asp-AP) and PEG-PAsp are prepared separately, and are mixed to prepare the PICsomes. In general, PEG is used as a hydrophilic segment for biocompatible materials because PEG suppresses non-specific protein adsorption due to its solvophilicity, large exclusion volume effect, and high mobility. The PICsome composed of PEG-P(Asp-AP) and PEG-PAsp is a potential candidate as a carrier for drug delivery systems (DDS) because the PICsome surface is surrounded by biocompatible PEG shells. However, the preparation of PICsomes involves the time-consuming processes of protection and deprotection during the syntheses of P(Asp-AP) and PAsp. Stuart and co-workers [
8,
9] reported water-soluble polyion complex micelles formed from oppositely charged polymers. Schrage and co-workers [
10] reported PICsomes with a corona of segregated polymer chains formed from oppositely charged block ionomers in THF.
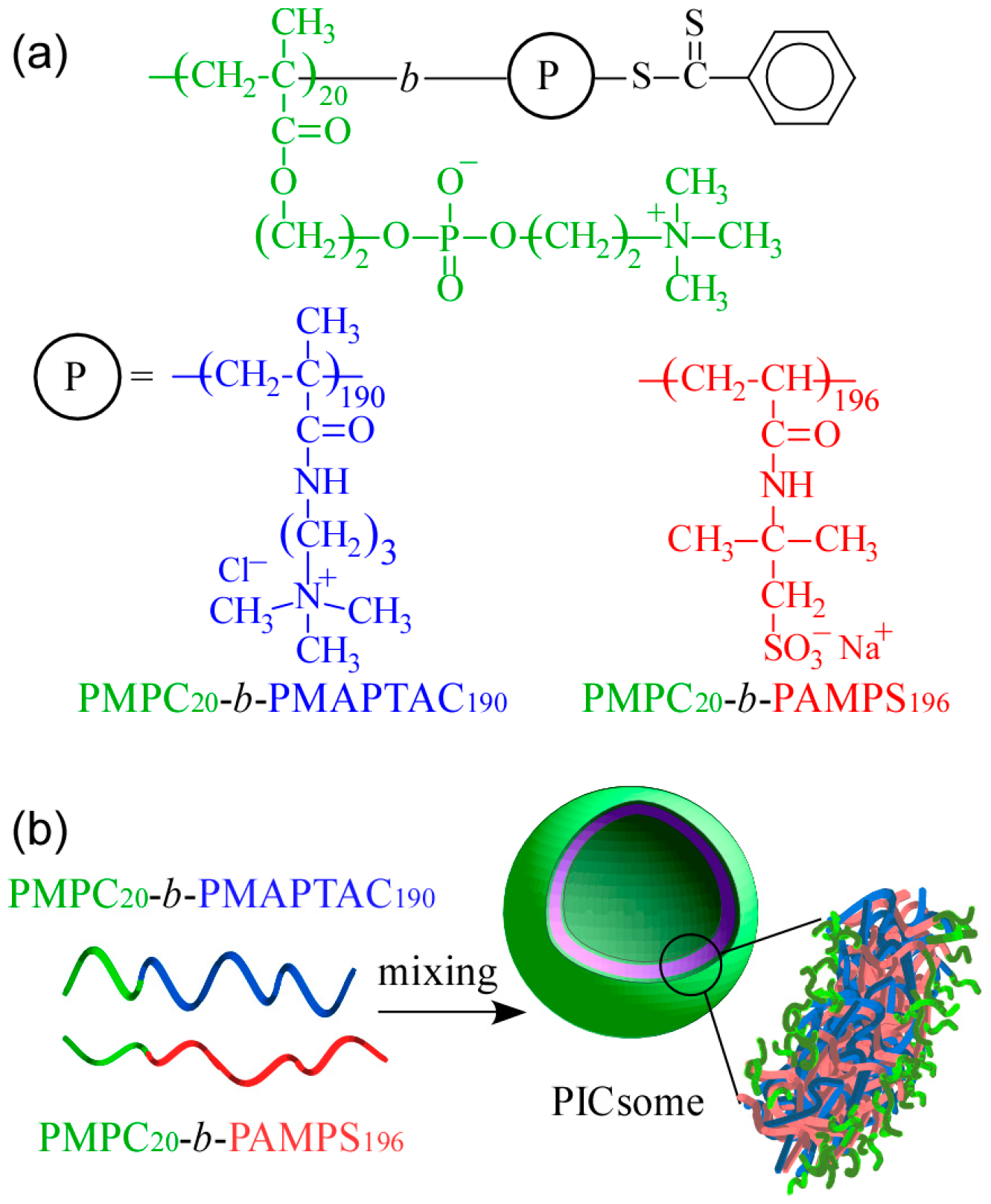
Preparation of a pair of oppositely charged doubly hydrophilic diblock copolymers, PEG-
block-poly((3-(methacrylamido)propyl)trimethylammonium chloride) (PEG-
b-PMAPTAC) and PEG-
block-poly(sodium 2-(acrylamido)-2-methylpropanesulfonate) (PEG-
b-PAMPS), was reported via reversible addition-fragmentation chain transfer (RAFT) radical polymerization using a PEG-based chain transfer agent (CTA) [
11]. A stoichiometrically charged neutral mixture of these oppositely charged diblock copolymers forms water-soluble PIC micelles in water. In addition, preparation of poly(2-(methacryloyloxy)ethyl phosphorylcholine)-
block-PMAPTAC (PMPC-
b-PMAPTAC) and PMPC-
b-PAMPS has been reported via RAFT using PMPC-based CTA with DP = 100 [
12]. Diblock copolymers with different, well-controlled PMAPTAC (DP = 27, 48, and 96) and PAMPS (DP = 27, 45, and 99) block lengths were obtained. The PMPC possesses excellent blood compatibility, that is, PMPC does not induce hemolysis and activation of platelets when it is in contact with blood, due to a polyampholyte containing both positive and negative charges in its phosphorylcholine group [
13]. Mixing aqueous solutions of PMPC-
b-PMAPTAC and PMPC-
b-PAMPS leads to the spontaneous formation of simple core-shell PIC micelles composed of a PIC core and PMPC shells.
The shapes of self-assemblies formed from amphiphilic block copolymers in water are influenced by the hydrophilic/hydrophobic balance. They change from spherical micelles, thread-like micelles, and vesicles upon an increase in molecular weight of the hydrophobic block [
14,
15]. We would like to confirm PIC aggregates with a small volume fraction of water-soluble part form vesicle structures similar to conventional amphiphilic block copolymers. In the present study, cationic MAPTAC and anionic AMPS were polymerized using short-chain-length PMPC-based CTA with DP = 20 via RAFT radical polymerization to obtain a pair of oppositely charged diblock copolymers. These diblock copolymers were composed of short-chain-length PMPC blocks and long-chain-length charged blocks (PAMPS or PMAPTAC). The DP value of the charged blocks was about 10 times larger than that of the PMPC block. Polyion complex vesicles (PICsomes) were formed by mixing these two oppositely charged diblock copolymers, which were characterized. The expected PICsome structure is shown in
Figure 1. The hydrated PMPC shells were covered on the inside and outside by a PICsome membrane composed of PMAPTAC and PAMPS blocks. This PICsome was thought to have the ability to incorporate nonionic water-soluble guest molecules into its hollow core.
3. Results and Discussion
To obtain oppositely charged diblock copolymers (P
20M
190 and P
20A
196), block copolymerization was conducted using PMPC macro-CTA with DP = 20 via RAFT radical polymerization. The conversions of MAPTAC and AMPS were estimated from
1H NMR measurements after polymerization reached 93.0% and 95.0%, respectively. The molecular characteristics of PMPC, P
20M
190, and P
20A
196 are summarized in
Table 1. The theoretical number-average molecular weight (
Mn(theo)) was calculated using:
where [M]
0 is initial monomer concentration, [CTA]
0 is initial CTA concentration,
xm is the conversion of monomer,
Mm is the molecular weight of the monomer, and
MCTA is the molecular weight of the CTA. The
Mn(NMR) and
Mn(GPC) for PMPC were close to the theoretical
Mn(theo) value, and the molecular weight distribution (
Mw/
Mn) was narrow (=1.03), indicating the controlled mechanism of the polymerization. Values obtained from
1H NMR to determine the true molecular weight of P
20M
190 and P
20A
196 yielded
Mn(NMR) = 4.95 × 10
4 and 4.85 × 10
4 g/mol, which was in fair agreement with
Mn(theo) = 4.82 × 10
4 and 4.68 × 10
4 g/mol, respectively. The
Mn(GPC) value for P
20M
190 and P
20A
196 deviated markedly from
Mn(theo). Note that the
Mn(GPC) values estimated by GPC were only apparent values because of the inherent error involved in the use of molecular weight standards [poly(2-vinylpyridine) and sodium poly(styrenesulfonate)] for calibrating the GPC data.
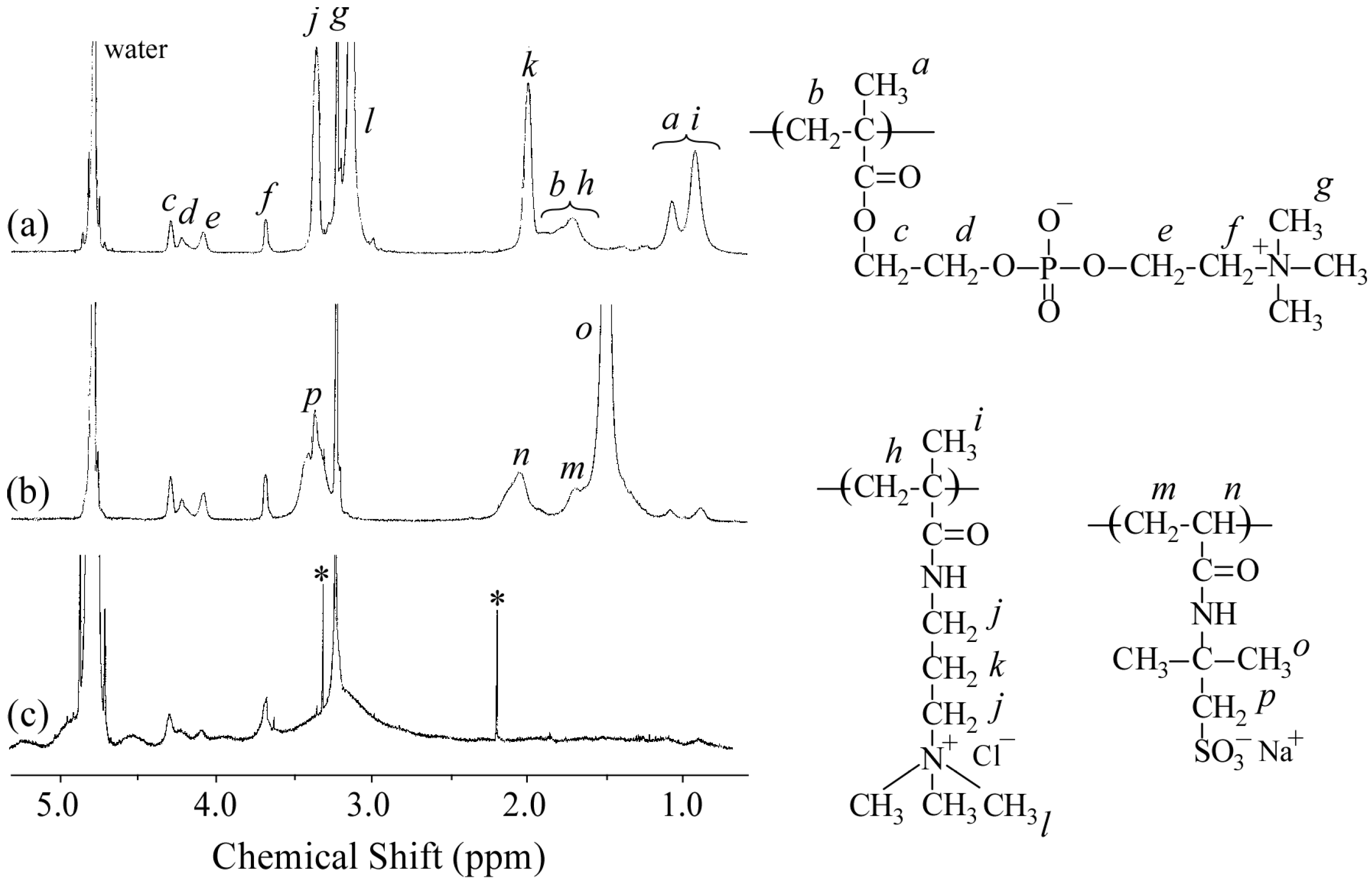
Figure 2a,b show the
1H NMR spectra for P
20M
190 and P
20A
196, respectively. The resonance bands observed in the 0.8–1.2 ppm region and at 1.8 ppm were attributed to the α-methyl protons and main chain methylene protons, respectively (
Figure 2a). The DP and
Mn(NMR) values of the PMAPTAC block in P
20M
190 were determined from the integral intensity ratio of the resonance bands due to pendant methyl protons in the PMAPTAC block at 3.1 ppm and PMPC pendant methylene protons at 3.7 ppm. The resonance bands observed at 1.2–2.2 ppm for P
20A
196 were attributed to the sum of the main chain and pendent methyl groups in the PAMPS block (
Figure 2b). Values for DP and
Mn(NMR) of the PAMPS block in P
20A
196 were calculated from the integral intensity ratio of the pendent methylene protons in the PAMPS block at 3.3 ppm and PMPC pendant methylene protons at 3.7 ppm.
Figure 2c shows the
1H NMR spectrum for the polyion complex vesicle (PICsome) composed of P
20M
190 and P
20A
196 with
f+ = 0.5 in D
2O containing 0.1 M NaCl. The intensity of resonance peaks associated with the PMAPTAC and PAMPS blocks was weak compared with those associated with the PMPC block. These observations suggest that the motion of the PMAPTAC and PAMPS blocks was highly restricted due to formation of the PIC core. The mobility of PMPC chains may be higher than that of the PMAPTAC and PAMPS chains because the PMPC chains form shells surrounding the PIC.
Aggregates formed by electrostatic interactions sometimes depend on the mixing pathway [
24,
25,
26]. We studied PICsome size dependence on the mixing pathway. A standard method is that a P
20M
190 solution was added dropwise to a P
20A
196 solution over a period of 5 min at room temperature with stirring. The P
20A
196 solution was added to the P
20M
190 solution, and the P
20M
190 solution was added to the P
20A
196 solution immediately. These two additional methods had no effect on the size of PICsome with
f+ = 0.5.
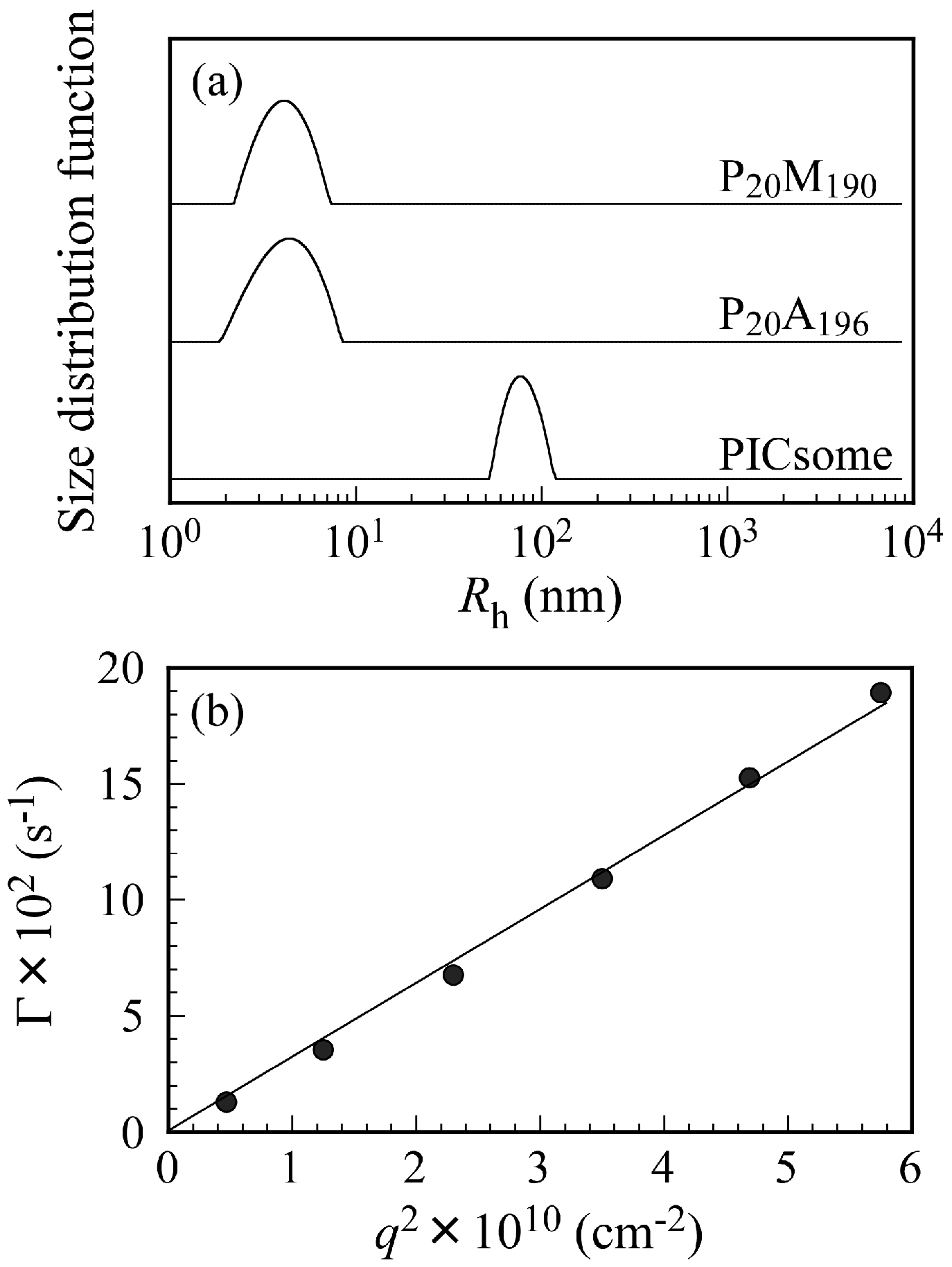
Figure 3a shows
Rh distributions for P
20M
190, P
20A
196, and the PICsome with
f+ = 0.5 in 0.1 M NaCl at
Cp = 0.5 g/L and a scattering angle (θ) = 90°. Unimodal
Rh distributions were observed. The
Rh values for P
20M
190, P
20A
196, and PICsome were 4.3, 4.4, and 78.0 nm, respectively. The
Rh values from 4.3 to 4.4 nm are reasonable for unimers of these block copolymers. If the polymer main chain forms completely planar zigzag structure, the distance between one carbon to the next carbon is about 0.25 nm [
27]. Hence, we can calculate the end-to-end distance of fully expanded polymer chains. The end-to-end distance of fully extended P
20M
190 and P
20A
196 chains were calculated as 52.5 and 54.0 nm, respectively. The
Rh of 78.0 nm found for the PICsome was larger than those expected from the fully extended length of the P
20M
190 and P
20A
196 chains. These observations indicate that the shape of the PICsome is not a simple core-shell spherical micelle. Large compound aggregates or vesicles should be formed by mixing P
20M
190 and P
20A
196. Relaxation rates (Γ) measured at different θ plotted against the square of the scattering vector (
q2) are shown in
Figure 3b. A line passing through the origin suggests that all of the relaxation modes were virtually diffusive [
28].
To confirm the stability of the PICsome size, Rh values were measured at various standing times. The Rh values were nearly constant and independent of time until 150 h, suggesting that the structure of the PICsome does not change with time (data not shown). Scattering intensities of the PICsomes were also independent of time.
To further characterize the PICsomes, SLS measurements were performed for θ from 30 to 130° with a 20° increment. The refractive index increment (d
n/d
Cp) for P
20M
190, P
20A
196, and PICsome in 0.1 M NaCl were determined individually. Values for
Mw(SLS),
Rg, and
A2 were estimated from Zimm plots. Aggregation number (
Nagg) for PICsomes (i.e., number of PMPC shell chains per one PICsome) was calculated by dividing
Mw(SLS) with that of unimers. The structure of the PICsome was also characterized by combining DLS and SLS to determine the
Rg/
Rh ratio. The density (
d) of P
20M
190, P
20A
196, and PICsome can be calculated by:
where
V is a polymer or PICsome volume calculated from 4/3π
Rh3. A summary of the properties of P
20M
190, P
20A
196, and the PICsome, including
Mw(SLS),
Nagg,
Rg,
Rh,
Rg/
Rh,
A2, d
n/d
Cp, and
d, is provided in
Table 2. Values for
Mw(SLS) for P
20M
190 and P
20A
196 were close to those for the corresponding
Mn(theo) and
Mn(NMR) values shown in
Table 1. The
Mw(SLS) value for the PICsome was 4.50 × 10
8 g/mol, estimated from SLS. The value of
Nagg for the PICsome was estimated to be 7770. The
Rg/
Rh ratio is a structure-sensitive parameter that provides information about the density distribution of the particles and thereby about particle morphology [
29,
30]. The
Rg/
Rh ratio equals 0.775 for a homogeneous hard sphere, 1.0 for a thin hard spherical shell (e.g., vesicle), and increases significantly for a less dense structure and for a polydisperse solution because large molecules of a broad distribution will contribute more to
Rg than to
Rh, provided that internal modes of motion are absent [
31]. The large
Rg/
Rh ratios (>4) for P
20M
190 and P
20A
196 suggest that the polymer chains were expanded due to electrostatic repulsions in the pendant ions. The
Rg/
Rh ratio for a polymeric vesicle may be less than or greater than 1.0, depending on the thickness and density of the wall [
32]. The
Rg/
Rh ratio of the PICsome was 1.12, which is close to unity, indicates that the PICsome was a vesicle [
33]. The
A2 value for the PICsome was less than those for P
20M
190 and P
20A
196, suggesting that solubility of the PICsome in 0.1 M NaCl was less than those of the unimers. The
d values for P
20M
190, P
20A
196, and PICsome were calculated to be 0.286, 0.273, and 0.376 g/cm
3, respectively. The
d value for the PICsome was slightly larger than those for P
20M
190 and P
20A
196, suggesting that the polymer chains in the PICsome were more densely packed than those of the unimers. The polymer chains of P
20M
190 and P
20A
196 expanded due to electrostatic repulsion in the pendant ionic groups in 0.1 M NaCl. Therefore, P
20M
190 and P
20A
196 have large
Rg/
Rh ratios. In contrast, the polymer chains in the PICsome were compact and dense in their vesicular membranes.
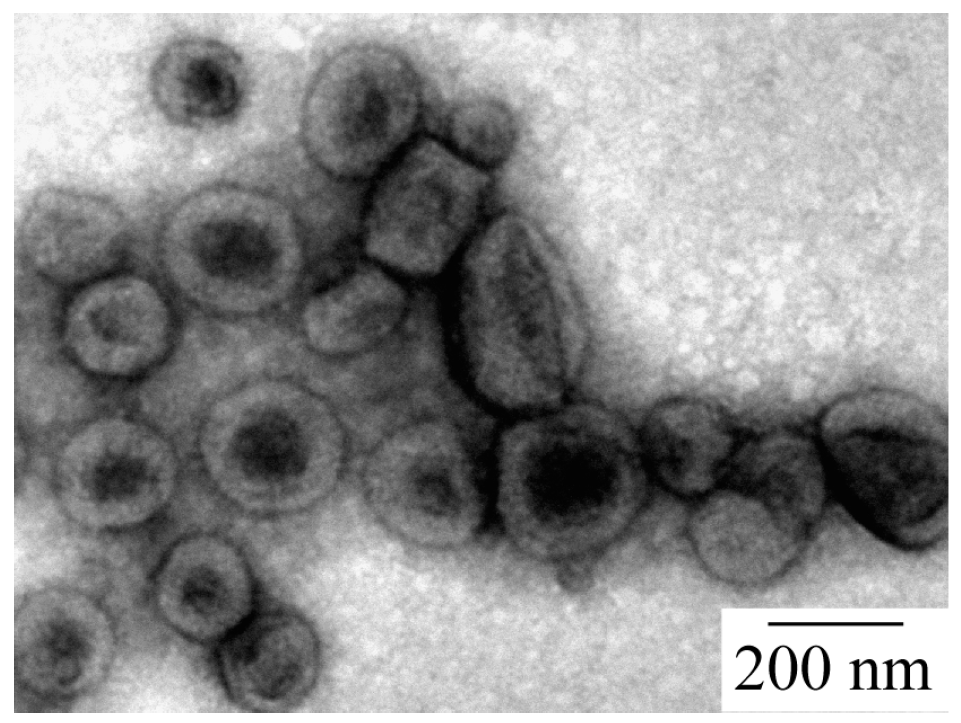
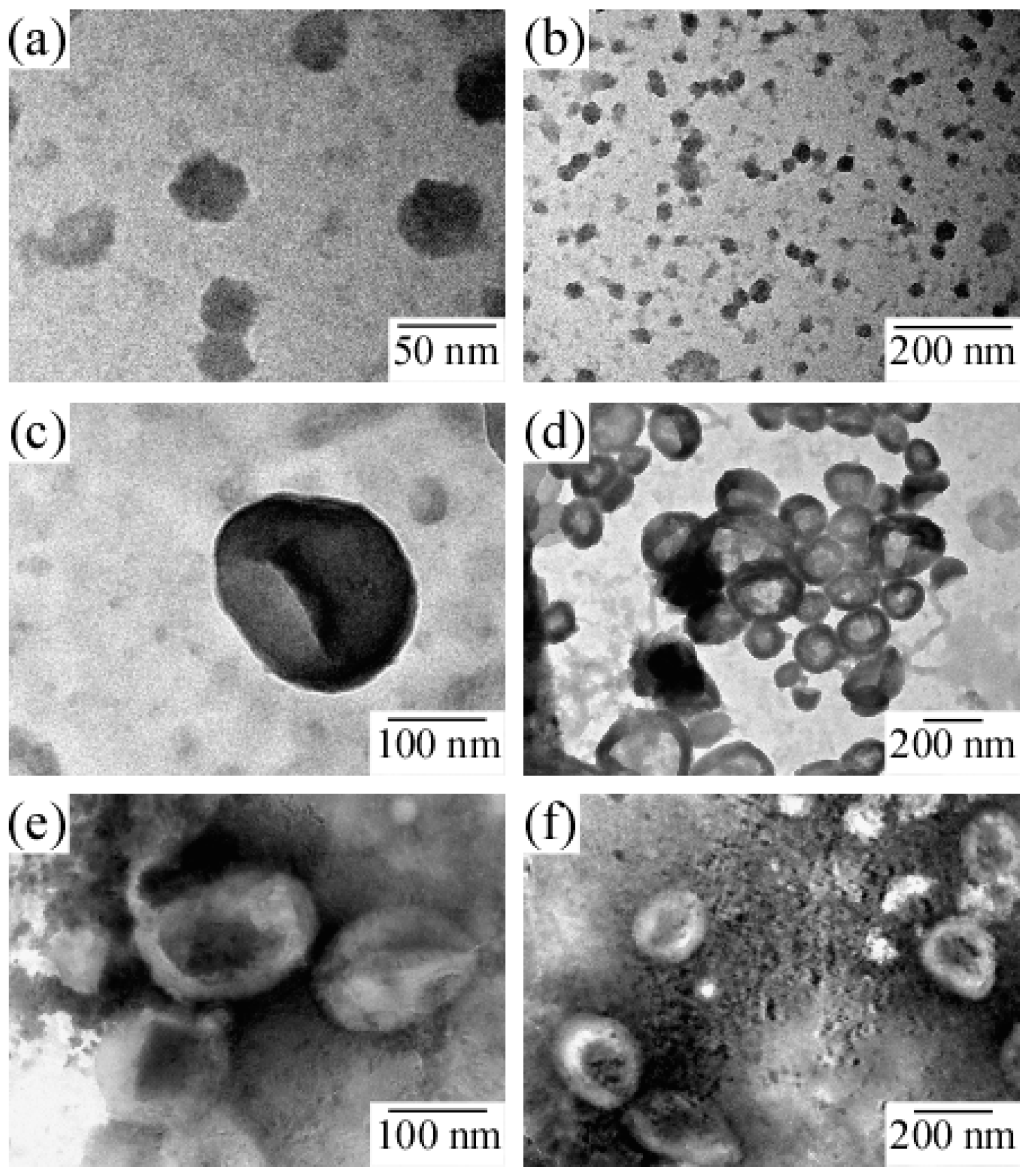
The structure of the PICsome was confirmed by TEM observations, which showed incomplete spherical hollow vesicle structures (
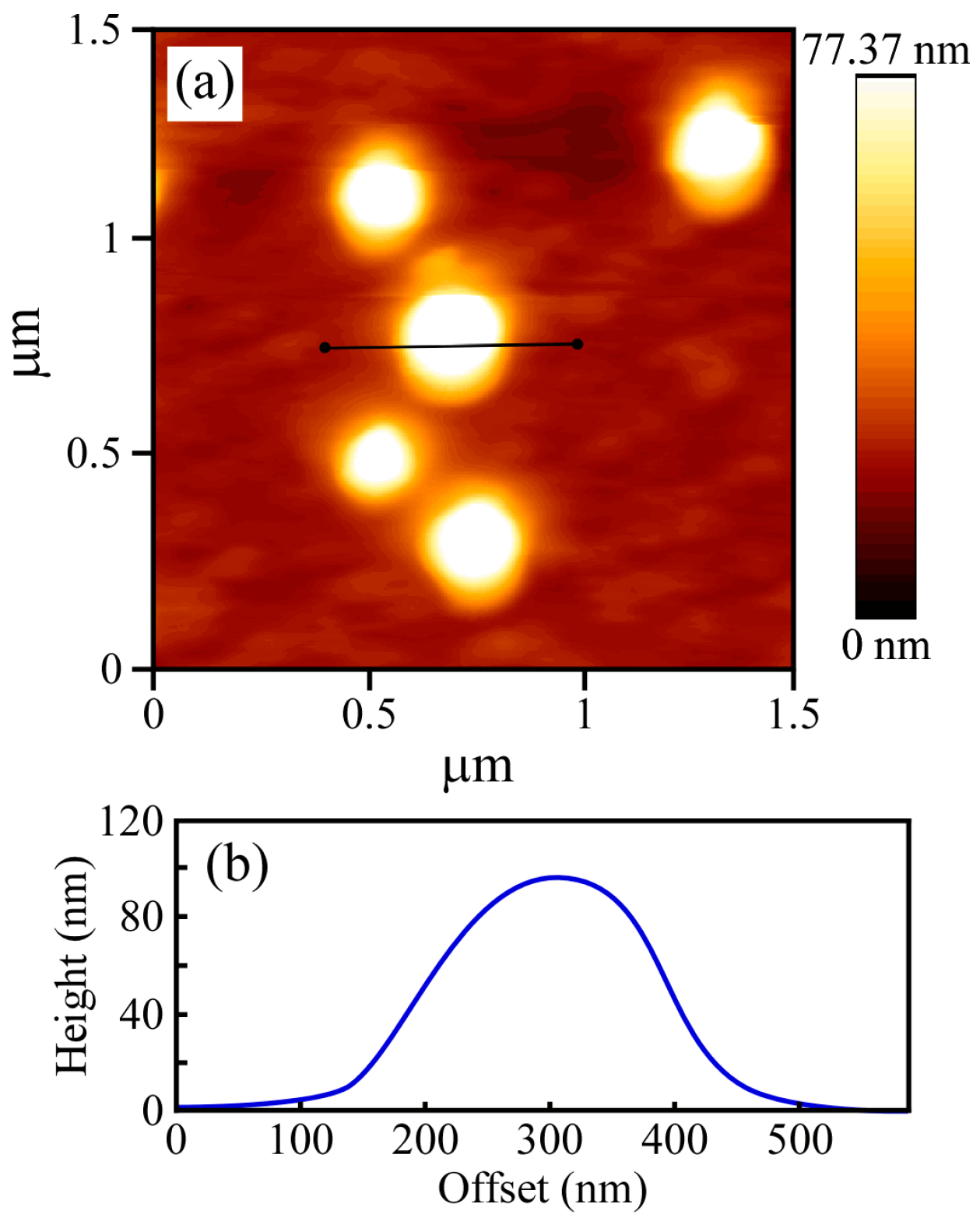
Figure 4). The vesicle structures may shrink during the drying process done prior to TEM observation. The PICsome diameter determined from the TEM images was 171 nm, which is close to the value obtained from the light scattering data. The AFM height image of the PICsome confirmed that the PICsome formed spherical structures that were slightly flattened due to the adsorption and drying process (
Figure 5). The height of the PICsome observed in the AFM image was ca. 100 nm.
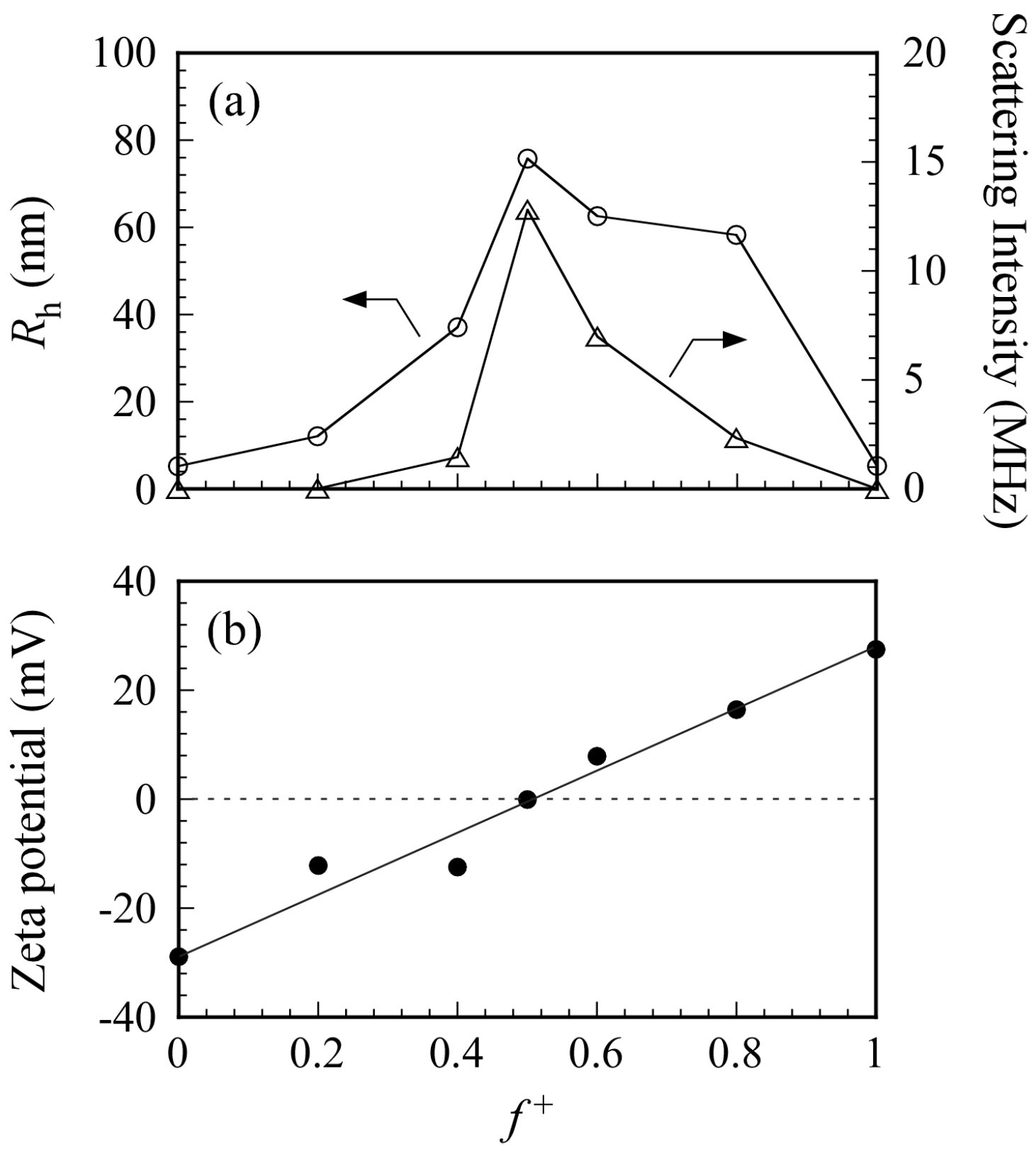
Figure 6a shows the
Rh and light scattering intensity values for PICsomes in 0.1 M NaCl as a function of
f+. Total polymer concentration was kept constant at 0.5 g/L. An increase in
Rh indicates an increase in the size of the PICsome. The maximum
Rh value was observed at
f+ = 0.5. In general, scattering intensity depends on molecular weight of the particles. Therefore, an increase in scattering intensity indicates an increase in
Nagg for the PICsome, which suggests that stoichiometric charge neutralization in the mixture of the two oppositely charged P
20M
190 and P
20A
196 leads to formation of PICsomes with maximum size and aggregation number. Plots of
Rh (and scattering intensity) vs.
f+ were asymmetric (i.e., the
Rh and scattering intensities for PIC aggregates with
f+ = 0.6 and 0.8 were larger than those with
f+ = 0.4) [
34]. To confirm the structure of PIC aggregates with
f+ = 0.4, 0.6, and 0.8, TEM images were obtained (
Figure 7). Results showed that PIC aggregates with
f+ = 0.4 were micelle-like spherical particles without a hollow core. In contrast, PIC aggregates with
f+ = 0.6 and 0.8 clearly possessed hollow core vesicle structures. The PIC aggregates composed of P
20M
190 and P
20A
196 with excess PMAPTAC blocks tended to form vesicles, presumably because the pendant quaternary amino groups surrounded by three methyl groups in the PMAPTAC blocks were more hydrophobic compared to the pendant sulfonate groups in the PAMPS blocks. When
f+ is larger than 0.5, excess PMAPTAC blocks existed in the aggregate, dehydration of PIC aggregates was promoted, and solubility was less than that at
f+ < 0.5. For aggregates formed from conventional amphiphilic diblock copolymers in water, the greater the hydrophobicity of the aggregate, the more likely diblock copolymers are to form vesicles rather than spherical core-shell micelles [
35]. Therefore, PIC aggregates with
f+ ≥ 0.5 tend to form vesicles.
To confirm PICsome neutralization at
f+ = 0.5, the ζ-potential was measured as a function of
f+ (
Figure 6b). At
f+ = 0, the aqueous solution of P
20A
196 has a negative ζ-potential value of −29 mV because the PAMPS block has pendant anionic sulfonate groups. At
f+ = 1, the aqueous solution of P
20M
190 has a positive ζ-potential value of +27 mV because the PMAPTAC block has pendant cationic quaternary amino groups. The ζ-potential was zero at
f+ = 0.5 because the charges of the PAMPS and PMAPTAC blocks were neutralized. The PICsome was composed of a PIC core and PMPC shells. The pendant phosphorylcholine groups in the PMPC shells contain anionic phosphate and cationic quaternary amine. However, the ζ-potential of PMPC homopolymer was zero (data not shown) because of neutralization of the anion and cation pair within a single polymer chain. Therefore, the ζ-potential of PICsome was zero at
f+ = 0.5.
To confirm that PICsomes with
f+ = 0.5 are at equilibrium or in a kinetically frozen state, excess P
20A
196 was added to the aqueous PICsome solution with
f+ = 0.5 to change the
f+ value. A kinetically frozen state means that the polymer chains cannot break free from the aggregate. The size of a PICsome with
f+ = 0.5 in the kinetically frozen state should not be affected by the addition of excess P
20A
196. The size of a PICsome in the equilibrium state may decrease upon addition of excess P
20A
196.
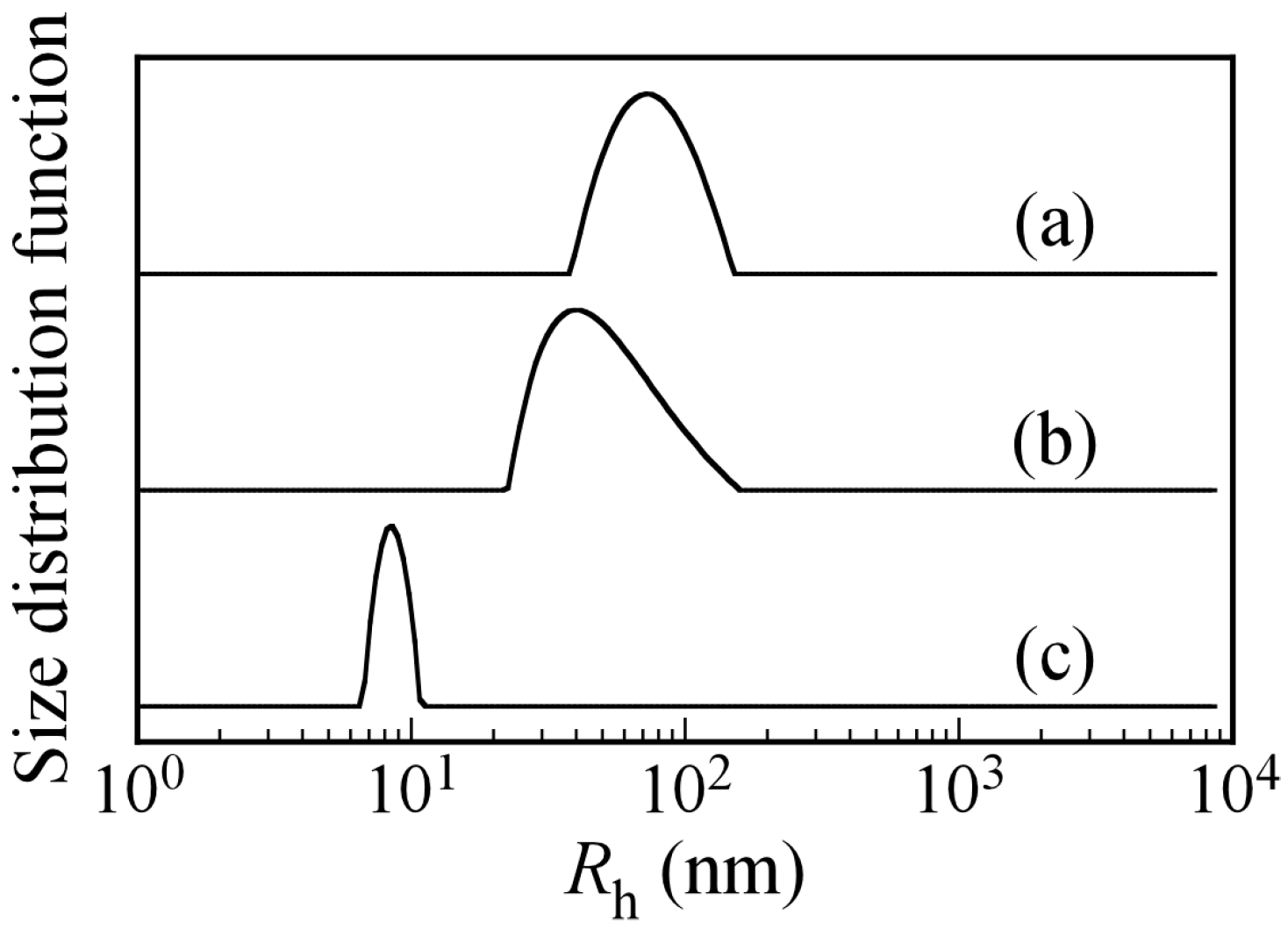
Figure 8 shows
Rh distributions for PICsomes with
f+ = 0.5 and PIC aggregates with
f+ = 0.4 and 0.2 formed by the addition of P
20A
196 to the PICsome with
f+ = 0.5. The
Rh value of the PICsome with
f+ = 0.5 was 78.0 nm at
Cp = 0.5 g/L. When a P
20A
196 solution at
Cp = 0.5 g/L was added to the PICsome solution, which changed the
f+ to 0.4 and 0.2, the
Rh values of the PIC aggregates decreased to 49 and 7.9 nm, respectively. This observation suggested that PICsomes formed by mixing oppositely charged diblock copolymers existed in an equilibrium state in water. Thus, small pairs of the oppositely charged diblock copolymers may dissociate from and associate with the PICsome [
36]. NaCl concentrations in the aqueous solution are very important for stability of PICsomes because they were formed by electrostatic interactions. We measured the
Rh values of PICsomes in various NaCl concentrations. When NaCl concentration was 0.5 M, the
Rh value was 77.8 nm, which is close to the value (
Rh = 78.0 nm) in 0.1 M NaCl aqueous solutions. Therefore, at least below 0.5 M of NaCl concentration, PICsomes were stable.
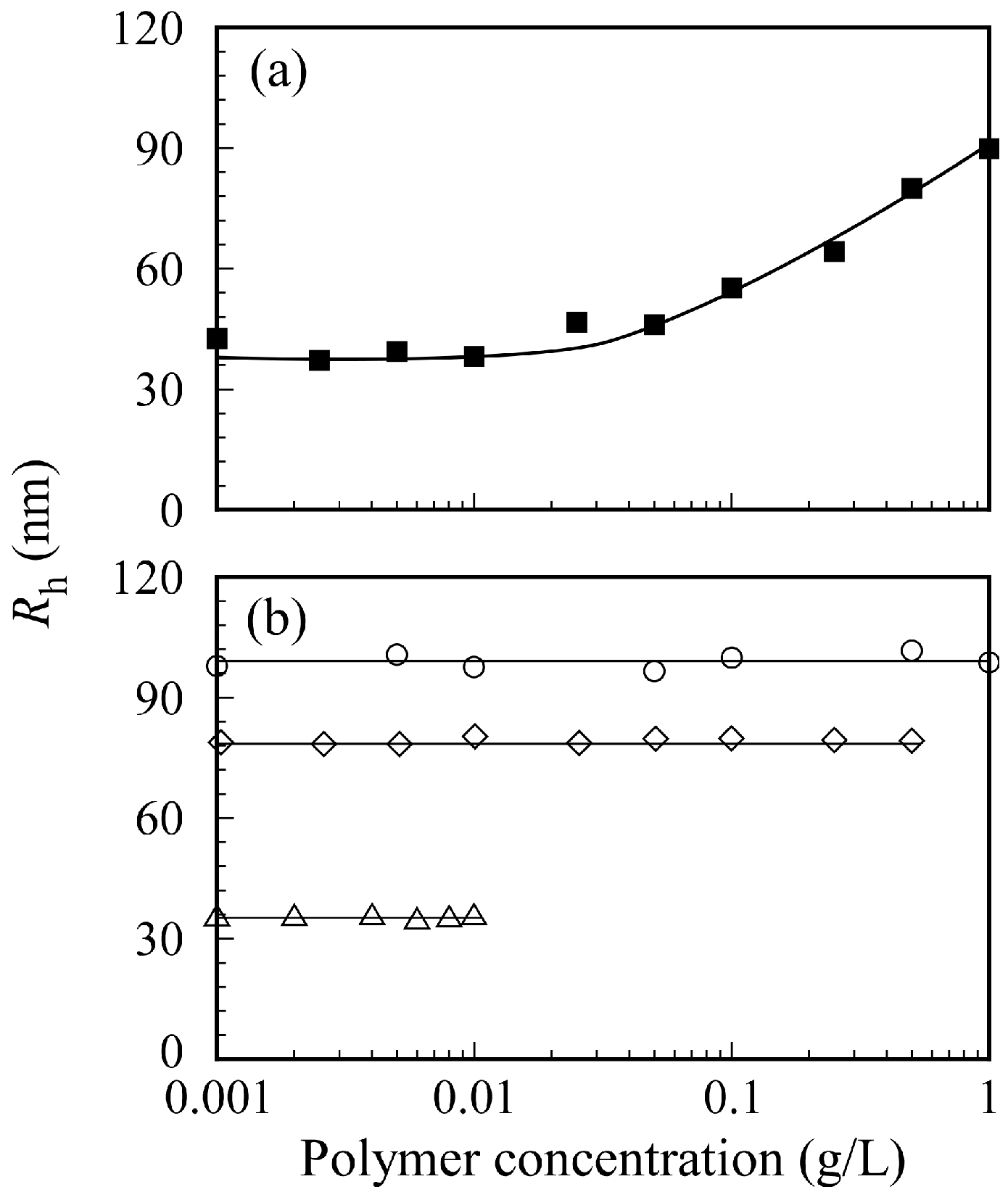
The relation between PICsome size and
Cp in 0.1 M NaCl is shown in
Figure 9. The sample solutions were prepared by two different methods. The first method involved preparing separate aqueous P
20M
190 and P
20A
196 solutions with a target
Cp from 0.001 to 1 g/L before mixing a pair of two oppositely charged diblock copolymers. Then, the two aqueous P
20M
190 and P
20A
196 solutions with the same
Cp were mixed to form a PICsome solution (
Figure 9a). The second method involved mixing pairs of oppositely charged diblock copolymer solutions at
Cp = 1, 0.5, and 0.01 g/L to form PICsomes. Subsequently, the aqueous PICsome solutions were diluted with 0.1 M NaCl to adjust
Cp to the target value (
Figure 9b). These PICsome solutions prepared via these two
Cp adjustment methods were measured using DLS to determine
Rh. The
Rh values for PICsomes depended on the value of
Cp of the P
20M
190 and P
20A
196 aqueous solutions before mixing to form the PICsome. When each P
20M
190 and P
20A
196 solution was prepared at
Cp = 1 g/L, the
Rh value for the PICsome was ca. 100 nm. In contrast, when each aqueous P
20M
190 and P
20A
196 solution was prepared at
Cp = 0.01 g/L, the
Rh value for the PICsome was ca. 38 nm. The size of the PICsomes could be controlled by adjusting the
Cp values of oppositely charged diblock copolymer solutions before mixing. When 0.1 M NaCl PICsome solutions were diluted with 0.1 M NaCl, the
Rh values for the PICsome remained nearly constant, independent of
Cp. These findings suggest that the structure of the PICsome, once prepared, is stable against dilution. In general, sonication and extrusion techniques are used to control the size of vesicles [
37]. However, the easily adjustable size of the stable PICsome system described here indicates that the size of vesicles and polymersomes can be easily controlled by adjusting the
Cp before mixing a pair of oppositely charged diblock copolymers.
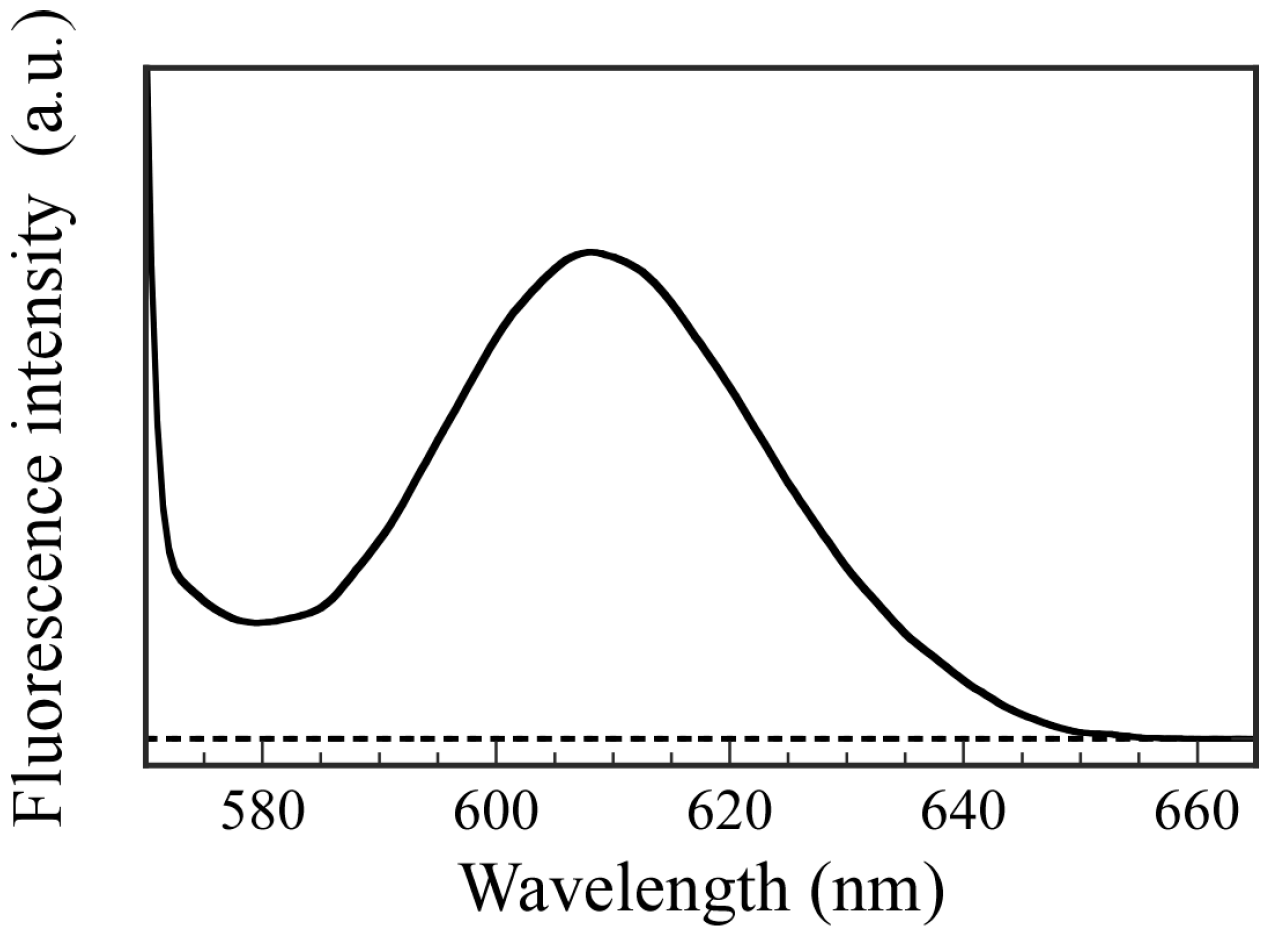
To confirm the ability to incorporate hydrophilic guest molecules into the interior aqueous phase of PICsomes, fluorescence experiments were performed using Texas red-labeled Dex as a fluorescence probe. The hydrophilic Dex molecule contains no charged groups. The P
20M
190 and P
20A
196 were dissolved in Dex-containing PBS buffer solutions, and then these solutions were mixed to form PICsomes. The Dex molecules that could not be incorporated into the PICsomes were removed by dialysis against fresh PBS buffer for 18 h. Fluorescence spectra were obtained for the solution inside the dialyzer after dialysis (
Figure 10). Fluorescence emission with a maximum wavelength at 610 nm for Dex was observed, which indicates that the Dex molecules were incorporated into the hollow core of PICsome. In contrast, a blank solution in the absence of PICsomes produced no fluorescence from Dex because the small Dex molecules were removed when using a dialysis membrane with a pore size of 100 nm. These results demonstrate that PICsomes can incorporate Dex guest molecules into the hollow core. The weight of the Dex incorporated into the PICsomes was calculated using a calibration curve, and was 0.00315 mg. The LE and LC values determined using the encapsulated Dex weight were 78.8% and 1.58%, respectively.