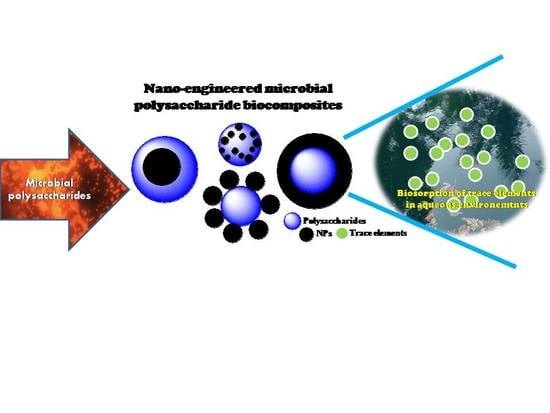
Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments—Scope for Expansion via Nanomaterial Intervention
Abstract
:1. Introduction
2. Mechanism of Biosorption
3. Microbial Polysaccharides—Applications and Relevance in Biosorption
3.1. Four Different Strategies of Microbial EPS Mediated Trace Element Biosorption
3.1.1. Metal Biosorption Following Homogeneous Consortial EPS
3.1.2. Metal Biosorption Following Heterogeneous Consortial EPS
3.1.3. Metal Biosorption by Dead Biomass EPS
3.1.4. Metal Biosorption Using Immobilized EPS
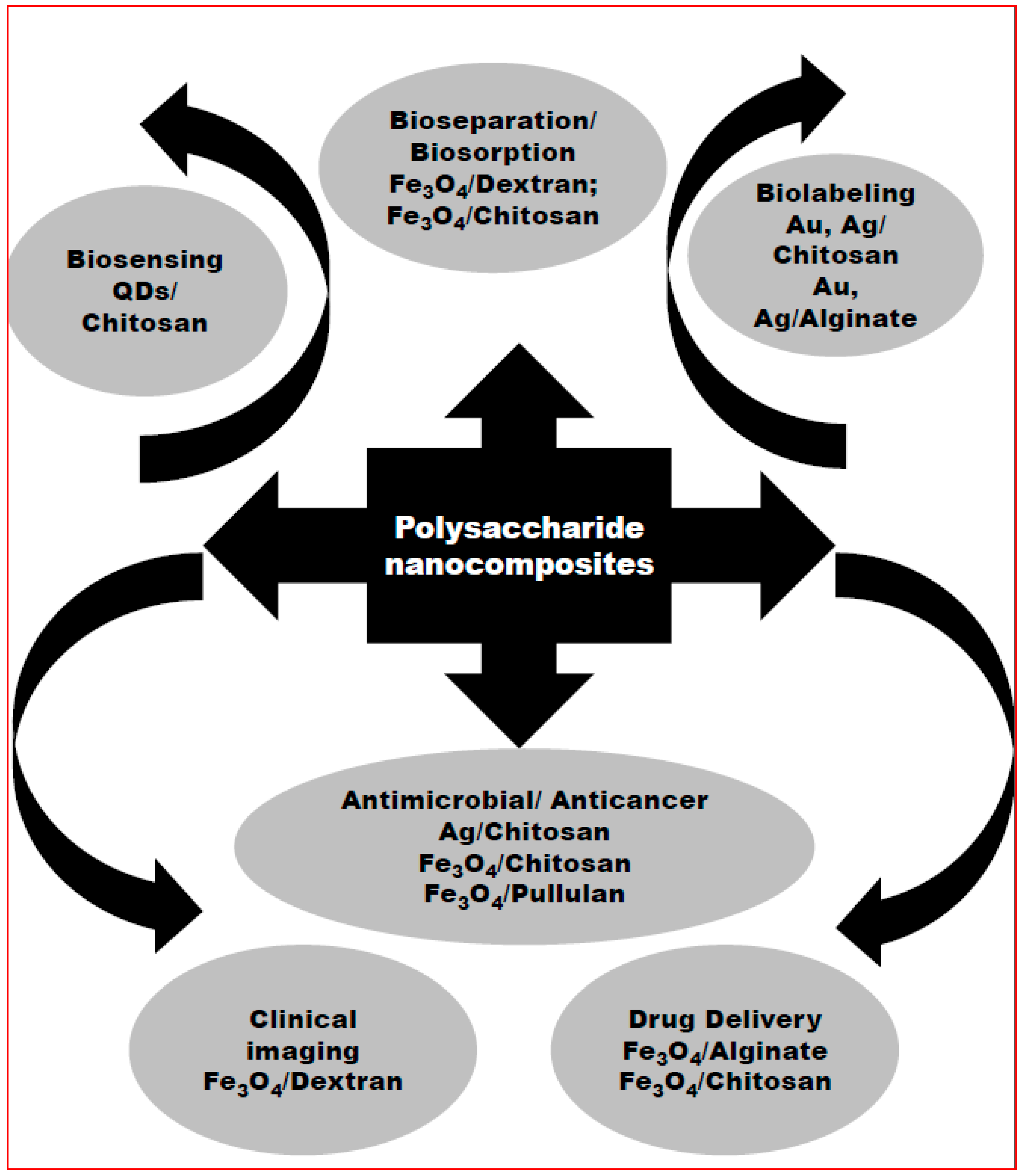
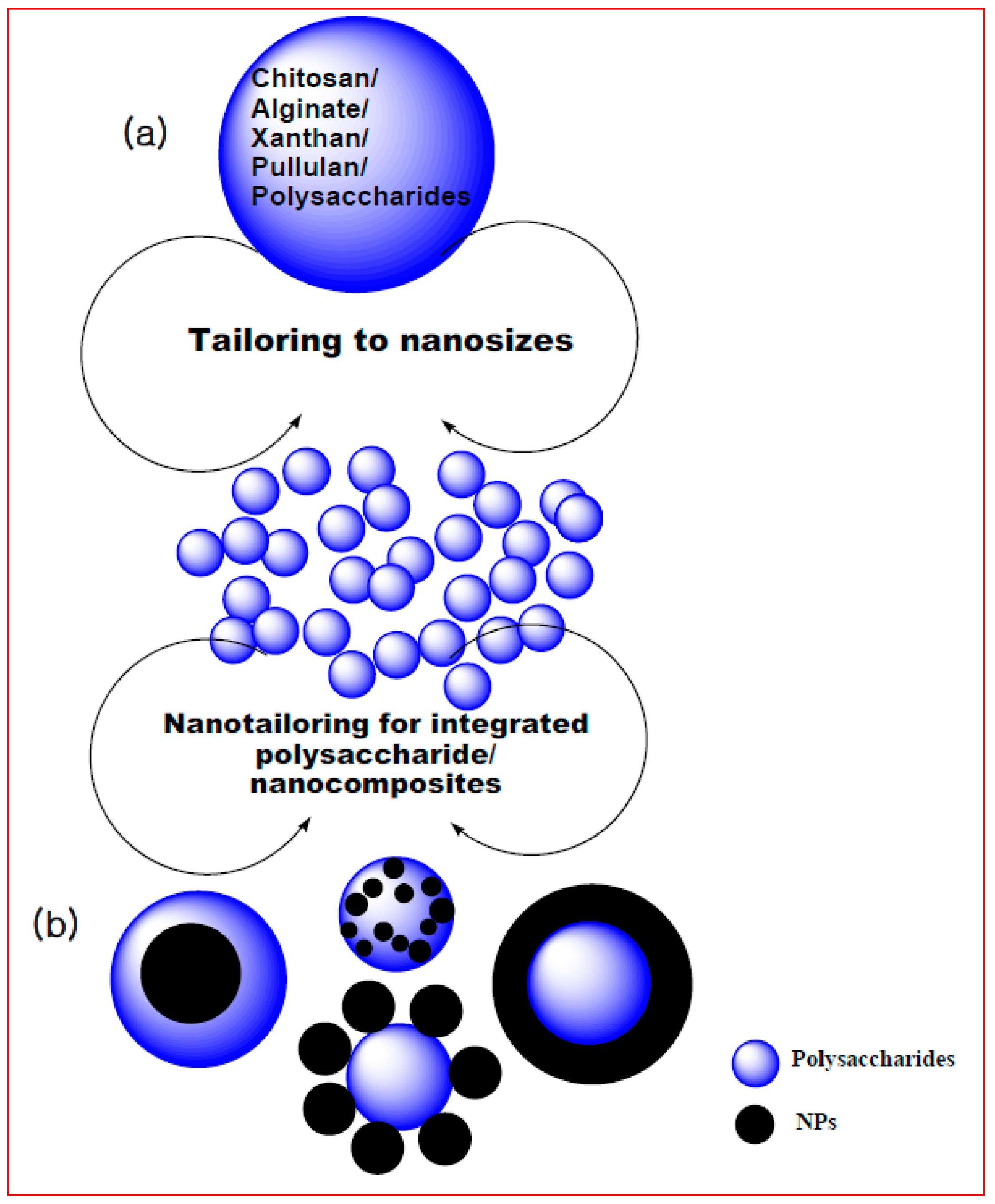
4. Biogenic Polysaccharide Nanomaterial Based Biosorption
5. Future Perspective of Biogenic Polysaccharide Nanocompositesfor Biosorption
Author Contributions
Conflicts of Interest
References
- Vieira, R.H.S.F.; Volesky, B. Biosorption: A solution to pollution. Int. Microbiol. 2000, 3, 17–24. [Google Scholar] [PubMed]
- Vijayabaskar, P.; Babinastarlin, S.; Shankar, T.; Sivakumar, T.; Anandapandian, K.T.K. Quantification and Characterization of Exopolysaccharides from Bacillus subtilis (MTCC 121). Adv. Biol. Res. 2011, 5, 71–76. [Google Scholar]
- Faisal, M.; Hasnain, S. Microbia conversion of Cr (VI) into Cr (III) in industrial effluent. Afr. J. Biotechnol. 2004, 3, 610–617. [Google Scholar]
- Brierly, C.L. Bioremediation of Metal Contaminated Surface and Groundwaters. Geomicrobiol. J. 1990, 8, 201–223. [Google Scholar] [CrossRef]
- Selenska-Pobell, S.; Panak, P.; Miteva, V.; Boudakov, I.; Bernhard, G.; Nitsche, H. Selective accumulation of heavy metals by three indigenous Bacillus strains, B. cereus, B. megaterium and B. sphaericus, from drain waters of a uranium waste pile. Microbiol. Ecol. 1999, 29, 59–67. [Google Scholar] [CrossRef]
- Omar, N.B.; Merroun, M.L.; Gonzales-Munoz, M.T.; Arias, J.M. Brewery yeast as a biosorbent for uranium. J. Appl. Bacteriol. 1996, 81, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Mario, F.D.; Rapana, P.; Lorenzoni, P.; Angelini, R. Copper biosorption by Auricularia polytricha. Lett. Appl. Microbiol. 2003, 37, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.C.M.; Mendonça-Hagler, L.C.S.; Savaidis, I. Metal Biorremediation by Microorganisms. Rev. Microbiol. 1998, 29, 85–92. [Google Scholar]
- Hernadez, A.; Mellado, R.P.; Martinez, J.L. Metal accumulation and vanadium-induced multidrug resistence by environmental isolates of Escherichia coli and Enterobacter cloacae. Appl. Environ. Microbiol. 1998, 1, 4317–4320. [Google Scholar]
- Wang, J.; Zhan, X.; Ding, D.; Zhou, D. Bioadsorption of lead(II) from aqueous solution by fungal biomass of Aspergillus niger. J. Biotechnol. 2001, 87, 273–277. [Google Scholar]
- Schmuhl, R.; Krieg, H.M.; Keizer, K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetcs and equilibrium studies. Water SA 2001, 27, 1–8. [Google Scholar] [CrossRef]
- Khoo, K.M.; Ting, Y.P. Biosorption of gold by immobilized fungal biomass. Biochem. Eng. J. 2001, 8, 51–59. [Google Scholar] [CrossRef]
- Knorr, D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991, 45, 114–122. [Google Scholar]
- Volesky, B. (Ed.) Biosorption of Heavy Metals; CRC Press: Boca Raton, FL, USA, 1990; p. 318. [Google Scholar]
- Bailiey, S.E.; Trudy, J.O. A Review of Potenially Low-cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Jang, L.K.; Nguyen, D.; Geesy, G.G. An Equilibrium Model for Absorption of Multiple Divalent Metals by Alginate Gel under Acidic Conditions. Water Res. 1999, 33, 2826–2832. [Google Scholar] [CrossRef]
- Monteiro, O.A., Jr.; Airoldi, C. Some Thermodynamic Data on Copper-Chitin and Copper-Chitosan Biopolymer Interactions. J. Colloid Interface Sci. 1999, 212, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Peniche-Covas, C.; Alvarez, L.W.; Argüelle-Monal, W. The Adsorption of Mercuric Ions by Chitosan. J. Appl. Polym. Sci. 1992, 46, 1147–1150. [Google Scholar] [CrossRef]
- Singh, S.; Pradhan, S.; Rai, L.C. Comparative Assessment of Fe3+ and Cu2+ Biosorption by Field and Laboratory-grown Microcystis. Process Biochem. 1998, 33, 495–504. [Google Scholar] [CrossRef]
- Terry, P.A.; Stone, W. Biosorption of Cadmium and Copper Contaminated Water by Scenedesmus abundans. Chemosphere 2002, 47, 249–255. [Google Scholar] [CrossRef]
- Ting, Y.P. Uptake of Cadmium and Zinc by the Alga Chlorella vulgaris: II. Multi-Ion Situation. Biotechnol. Bioeng. 1991, 37, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Udaybhaskar, P.; Iyengar, L.; Abhakara Rao, A.V.S. Hexavalent Chromium Interaction with Chitosan. J. Appl. Polym. Sci. 1990, 39, 739–747. [Google Scholar] [CrossRef]
- Volesky, B. Biosorbent Materials. Biotechnol. Bioeng. Symp. 1986, 16, 121–126. [Google Scholar]
- Gimenez, M.D.; Arino, C.; Esteban, M. Voltammetry of Pb(II), Cd(II) and Zn(II) Ions in the Presence of the Sulphated Polysaccharide λ-Carrageenan. Anal. Chim. Acta 1995, 310, 121–129. [Google Scholar] [CrossRef]
- Yoshinari, B.; Koichi, M.; Yoshinobu, K. Synthesis of a Chitosan Derivative Recognizing Planar Metal Ion and Its Selective Adsorption Equilibria of Copper(II) over Iron(III). React. Funct. Polym. 1998, 36, 167–172. [Google Scholar]
- Kuyucak, N.; Volesky, B. Biosorbents for recovery of metals from industrial solutions. Biotechnol. Lett. 1988, 10, 137–142. [Google Scholar] [CrossRef]
- Loaecm, M.; Oliver, R.; Guezennec, J. Uptake of lead, cadmium and zinc by a nobel bacterial exopolisaccharide. Water Res. 1997, 31, 1171–1179. [Google Scholar] [CrossRef]
- Naja, G.; Deneux-Mustin, S.; Mustin, C.; Rouiller, J.; Munier-Lamy, C.; Berthelin, J. Potentiometric titration: A dynamic method to study the metal binding-mechanism of microbial biomass. In Biohydrometallurgy and the Environment toward the Mining of the 21st Century; Amils, R., Ballester, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 201–210. [Google Scholar]
- Whitfield, C. Bacterial extracellular polysaccharides. Can. J. Microbiol. 1988, 34, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Czaczyk, K.; Myszka, K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Ahalya, N.; Ramachandra, T.V.; Kanamadi, R.D. Biosorption of heavy metals. Res. J. Chem. Environ. 2003, 7, 71–79. [Google Scholar]
- Wang, J.L.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Sag, Y.; Kutsal, T. The biosorption of copper (II) by C. vulgaris and Z. ramigera. Environ. Technol. 1992, 13, 579–586. [Google Scholar] [CrossRef]
- Javaid, A.; Bajwa, R. Biosorption of electroplating heavy metals by some basidiomycetes. Mycopath 2008, 6, 1–6. [Google Scholar]
- Muraleedharan, T.R.; Venkohachr, C. Mechanism of biosorption of copper (II) by Ganodermalucidium. Biotechnol. Bioeng. 1990, 35, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Veglio, F.; Beolchini, F.; Gasbarro, A. Biosorption of toxic metals: An equilibrium study using free cells of Arthrobacter sp. Process Biochem. 1997, 32, 99–105. [Google Scholar] [CrossRef]
- Crist, R.H.; Oberholser, K.; Shank, N.; Nguyen, M. Nature of bonding between metallic ions and algal cell walls. Environ Sci. Technol. 1981, 15, 1212–1217. [Google Scholar] [CrossRef]
- Greene, B.; McPherson, R.; Darnall, D. Algal sorbents for selective metal ion recovery. In Metals Speciation, Separation and Recovery; Patterson, J.W., Pasino, R., Eds.; Lewis: Chelsea, MI, USA, 1987; pp. 315–338. [Google Scholar]
- Brierley, C.L. Metal immobilization using bacteria. In Microbial Mineral Recovery; Ehrlich, H.L., Brierley, C.L., Eds.; McGraw-Hill: New York, NY, USA, 1990; pp. 303–324. [Google Scholar]
- Mann, H. Biosorption of heavy metals by bacterial biomass. In Biosorption of Heavy Metals; Volesky, B., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 93–137. [Google Scholar]
- Hunt, S. Diversity of biopolymer structure and its potential for ion- binding applications. In Immobilisation of Ions by Bio-Sorption; Eccles, H., Hunt, S., Eds.; Ellis Horwood: Chichester, UK, 1986; pp. 15–45. [Google Scholar]
- Macaskie, L.E.; Dean, A.C.R. Metal-sequestering biochemicals. In Biosorption of Heavy Metals; Volesky, B., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 199–248. [Google Scholar]
- Payne, G.F.; Raghavan, S.R. Chitosan: A soft interconnect for hierarchical assembly of nano-scale components. Soft Matter 2007, 3, 521–527. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. J. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Credou, J.; Berthelot, T.J. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Laurent, T.C. (Ed.) The Chemistry, Biology and Medical Applications of Hyaluronan and Its Derivatives; Wenner-Gren International Series; Portland Press: London, UK, 1998; Volume 1998, p. 341. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Mert, B.; Campanella, O.H.; Reuhs, B. Chemical and rheological properties of bacterial succinoglycan with distinct structural characteristics. Carbohydr. Polym. 2009, 76, 320–324. [Google Scholar] [CrossRef]
- Noel, K.D. Rhizobia. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 261–277. [Google Scholar]
- Vieira, E.; Cestari, A.; Airoldi, C.; Loh, W. Polysaccharide-based hydrogels: Preparation, characterization, and drug interaction behaviour. Biomacromolecules 2008, 9, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Alves, C.M.; Kurtis Kasper, F.; Mikos, A.G.; Reis, R.L. Responsive and in situ-forming chitosan scaffolds for bone tissue engineering applications: An overview of the last decade. J. Mater. Chem. 2010, 20, 1638–1645. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide Mediated Heavy Metal Removal: A Review on Biosynthesis, Mechanism and Remediation Strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, J.-H.; Kim, C.-J.; Oh, D.-K. Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996, 18, 1161–1164. [Google Scholar] [CrossRef]
- Marchal, M.; Briandet, R.; Koechler, S.; Kammerer, B.; Bertin, P.N. Effect of arsenite on swimming motility delays surface colonization in Herminiimonas arsenicoxydans. Microbiology 2010, 156, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Weeger, W.; Lievremont, D.; Perret, M.; Lagarde, F.; Hubert, J.-C.; Leroy, M.; Lett, M.-C. Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals 1999, 12, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Marchal, M.; Briandet, R.; Halter, D.; Koechler, S.; DuBow, M.S.; Lett, M.-C.; Bertin, P.N. Subinhibitory arsenite concentrations lead to population dispersal in Thiomonas sp. PLoS ONE 2011, 6, e23181. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Biller, D.V.; Shimmield, T.; Green, D.H. Metal binding properties of the EPS produced by Halomonas sp. TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals 2012, 25, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.; Chen, Z.; Liu, X.; Ma, F. Biosorption of Pb(II) and Zn(II) by extracellular polymeric substance (Eps) of Rhizobium Radiobacter: Equilibrium, kinetics and reuse studies. Arch. Environ. Prot. 2013, 39, 129–140. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of Rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, W.; Pi, S.; Ma, F.; Li, A.; Wu, D.; Xing, J. Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp. J1. Bioresour. Technol. 2015, 196, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhang, M.; Yang, H.; Wang, H.; Xiao, S.; Liu, Y.; Wang, J. Biosorption of Cu2+, Pb2+ and Cr6+ by a novel exopolysaccharide from Arthrobacter ps-5. Carbohydr. Polym. 2014, 101, 50–56. [Google Scholar]
- De Oliveira Franco, L.; Maia, R.d.C.; Porto, A.L.F.; Messias, A.S.; Fukushima, K.; de Campos-Takaki, M. Heavy metal biosorption by chitin and chitosan isolated from Cunninghamella elegans (IFM 46109). Braz. J. Microbiol. 2004, 35, 243–247. [Google Scholar] [CrossRef]
- Liu, Y.; Lam, M.; Fang, H. Adsorption of heavy metals by EPS of activated sludge. Water Sci. Technol. 2001, 43, 59–66. [Google Scholar] [PubMed]
- Gawali Ashruta, A.; Nanoty, V.; Bhalekar, U. Biosorption of heavy metals from aqueous solution using bacterial EPS. Int. J. Life Sci. 2014, 2, 373–377. [Google Scholar]
- De Oliveira Martins, P.S.; de Almeida, N.F.; Leite, S.G.F. Application of a bacterial extracellular polymeric substance in heavy metal adsorption in a co-contaminated aqueous system. Braz. J. Microbiol. 2008, 39, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.J.; Hemambika, B.; Hemapriya, J.; Kannan, V.R. Comparative assessment of heavy metal removal by immobilized and dead bacterial cells: A biosorption approach. Afr. J. Environ. Sci. Technol. 2010, 4, 23–30. [Google Scholar]
- Sultan, S.; Mubashar, K.; Faisal, M. Uptake of toxic Cr (VI) by biomass of exo-polysaccharides producing bacterial strains. Afr. J. Microbiol. Res. 2012, 6, 3329–3336. [Google Scholar]
- Vandevivere, P.; Kirchman, D.L. Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl. Environ. Microbiol. 1993, 59, 3280–3286. [Google Scholar] [PubMed]
- Kemp, M.M.; Linhardt, R.J. Heparin-based nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Simkovic, I. Unexplored possibilities of all-polysaccharide composites. Carbohydr. Polym. 2013, 95, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramström, O.; Yan, M. Glyconanomaterials: Synthesis, characterization, and ligand presentation. Adv. Mater. 2010, 22, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Hassiba, M.; Naima, A.; Yahia, K.; Zahra, S. Study of lead adsorption from aqueous solutions on agar beads with EPS produced from Paenibacillus polymyxa. Chem. Eng. Trans. 2014, 38, 31–36. [Google Scholar]
- Srivastava, A.; Srivastava, O.N.; Talapatra, S.; Vajtai, R.; Ajayan, P.M. Carbon nanotube filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fallgren, P.H.; Morris, J.M.; Chen, Q. Removal of bacteria and viruses from waters using layered double hydroxide nanocomposites. Sci. Technol. Adv. Mater. 2007, 8, 67–70. [Google Scholar] [CrossRef]
- Tahaikt, M.; Habbani, R.E.; Haddou, A.A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride removal from groundwater by nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.; Medina, M.; Valencia, F.; Tapia, J. Removal of inorganic mercury from polluted water using structured nanoparticles. J. Environ. Eng. 2006, 132, 342–349. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 5, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Batalha, I.L.; Hussain, A.; Roque, A.C.A. Gum Arabic coated magnetic nanoparticles with affinity ligands specific for antibodies. J. Mol. Recognit. 2010, 23, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Hosseini, S.H.; Seidi, F.; Soleyman, R. Magnetic removal of crystal violet from aqueous solutions using polysaccharide-based magnetic nanocomposite hydrogels. Polym Int. 2013, 62, 1038–1044. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2009, 26, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Khan, R.; Solanki, P.R.; Pandey, P.; Alam, J.; Ahmad, S.; Malhotra, B.D. Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens. Bioelectron. 2008, 24, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Fluid, M.A.G. Iron Nanoparticle-Labeled Mesenchymal Stem Cells for Tracking Cell Homing to Tumors. Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004–2013.
- Su, H.; Liu, Y.; Wang, D.; Wu, C.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Amphiphilicstarlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 2013, 34, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, J.; Ouyang, W.; Li, D.; Li, L.; Li, L.; Tian, J. Magnetic mediated hyperthermia for cancer treatment: Research progress and clinical trials. Chin. Phys. B 2013, 22, 108104. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; Román, J.S.; Goya, G.; Hernández, R.; Mijangos, C. Magnetic core–shell chitosan nanoparticles: Rheological characterization and hyperthermia application. Carbohydr. Polym. 2014, 102, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Letourneur, D.; Chauvierre, C. Polysaccharide Nanosystems for Future Progress in Cardiovascular Pathologies. Theranostics 2014, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, Y.; Zhu, Y.Z.; Lu, J.; Maguire, J.A.; Hosmane, N.S. Boron drug delivery via encapsulated magnetic nanocomposites: A new approach for BNCT in cancer treatment. J. Nanomater. 2010, 2010, 409320. [Google Scholar] [CrossRef]
- Lim, E.K.; Sajomsang, W.; Choi, Y.; Jang, E.; Lee, H.; Kang, B.; et al. Chitosan-based intelligent theragnosis nanocomposites enable pH-sensitive drug release with MR-guided imaging for cancer therapy. Nanoscale Res. Lett. 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Sabine, M.; Gunter, K.; Wolfgang, P.; Horst, B. Algae-Silica Hybrid Materials for Biosorption of Heavy Metals. J. Water Resour. Prot. 2010, 2, 115–122. [Google Scholar]
- Gandhi, M.R.; Viswanathan, N.; Meenakshi, S. Preparation and application of alumina/chitosan biocomposite. Int. J. Biol. Macromol. 2010, 47, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Fierro, S.; del Pilar Sanchez-Saavedra, M.; Copalcua, C. Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour. Technol. 2008, 99, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kang, K.-H.; Kim, D.G.; Kim, S.-K. Production of polysaccharide-based bioflocculant for the synthesis of silver nanoparticles by Streptomyces sp. Int. J. Biol. Macromol. 2015, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, S.; Branford White, C.J.; Ning, X.; Nie, H.; Zhu, L. Studies on electrospun nylon-6/chitosan complex nanofiber interactions. Electrochim. Acta 2009, 54, 5739–5745. [Google Scholar] [CrossRef]
- Nirmala, R.; Navamathavan, R.; Kang, H.-S.; El-Newehy, M.H.; Kim, H.Y. Preparation of polyamide-6/chitosan composite nanofibers by a single solvent system via electrospinning for biomedical applications. Colloids Surf. B 2011, 83, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Navamathavan, R.; El-Newehy, M.H.; Kim, H.Y. Preparation and electrical characterization of polyamide-6/chitosan composite nanofibers via electrospinning. Mater. Lett. 2011, 65, 493–496. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Zhang, Y.; Branford White, C.J.; Xue, Y.; Nie, H.; Zhu, L. Elaboration, characterization and study of a novel affinity membrane made from electrospun hybrid chitosan/nylon-6 nanofibers for papain purification. J. Mater. Sci. 2010, 45, 2296–2304. [Google Scholar] [CrossRef]
- Wang, Y.; El-Deen, A.G.; Li, P.; Oh, B.H.L.; Guo, Z.; Khin, M.M.; Vikhe, Y.S.; Wang, J.; Hu, R.G.; Boom, R.M.; et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [PubMed]
- The Royal Society and the Royal Academy of Engineers. Nanoscience and Nanotechnologies: Opportunities and Uncertainties; The Royal Society and the Royal Academy of Engineers: London, UK, 2004; pp. 6–9. [Google Scholar]
| Strain | Metal Specificity | Biopolymers Involved |
|---|---|---|
| Escherichia coli K-12 | Sr2+, Ce3+, Pr2+, , Sc3+, La3+, Co2+, Hg2+, Pb2+, Cu2+ | N-acetylglucosamineNacetylmuramic acid crosslinked sugar residues, Lipopolysaccharide (LPS), EPS |
| Pseudomonas aeruginosa | La3+ | LPS |
| Acinetobacter lwoffii RAG-1 TF1-35 | apoemulsan | |
| Thiobacillus ferrooxidans TF1-35 | LPS | |
| Bacillus licheniformis | Cr3+ | γ-glutamyl capsular polymer |
| Enterobacter aerogenes | Cd2+ | EPS |
| Zooglea ramigera | , Cu2+, Cd2+ | Zooglan |
| Xanthomonas campestris | Cu2+ | Xanthan |
| Cunninghamella elegans | Cu2+, Pb2+ | Chitosan |
| Chlorella vulgaris | Cu2+, Pb2+ | EPS |
| Polysaccharide | Source | Ionic Nature | Active Functional Group |
|---|---|---|---|
| Gellan | Sphingomonas elodea | Anionic | OH |
| Dextran | Leuconostoc mesenteroides, Lactobacillus sps and Steptococcus mutans | Neutral | OH |
| Pullulan | Aureobasidium pullulans | Neutral | OH |
| Cellulose | Aerobacter, Acetobacter, Achromobacter, Agrobacterium, Alcaligenes, Azotobacter, Pseudomonas, Rhizobium and Sarcina | Neutral | OH |
| Hyaluronic acid | Streptococcal sps and Bacillus subtilis | Anionic | OH |
| Curdlan | Alcaligenes faecalis var myxogenes 10C3, Agrobacterium sps | Cationic | OH, COO− |
| Alginate | Pseudomonas sps and Azotobacter vinelandii | Anionic | OH, COO− |
| Chitosan | Cunninghamella elegans, Fungal cells walls | Cationic | OH, COO− |
| Xanthan | Xanthomonas campestris | Anionic | OH |
| Zooglan | Zoogloea ramigera | Anionic | OH |
| Succinoglycan | Agrobacterium sps, Rhizobium sps, Rhizobium meliloti, Alcaligenes faecalis | Anionic | OH |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, M.; Wu, H.-F.; Gopal, J.; Sivanesan, I.; Chun, S. Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments—Scope for Expansion via Nanomaterial Intervention. Polymers 2017, 9, 721. https://doi.org/10.3390/polym9120721
Muthu M, Wu H-F, Gopal J, Sivanesan I, Chun S. Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments—Scope for Expansion via Nanomaterial Intervention. Polymers. 2017; 9(12):721. https://doi.org/10.3390/polym9120721
Chicago/Turabian StyleMuthu, Manikandan, Hui-Fen Wu, Judy Gopal, Iyyakkannu Sivanesan, and Sechul Chun. 2017. "Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments—Scope for Expansion via Nanomaterial Intervention" Polymers 9, no. 12: 721. https://doi.org/10.3390/polym9120721
APA StyleMuthu, M., Wu, H.-F., Gopal, J., Sivanesan, I., & Chun, S. (2017). Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments—Scope for Expansion via Nanomaterial Intervention. Polymers, 9(12), 721. https://doi.org/10.3390/polym9120721