Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers—Synthesis and Medical Applications
Abstract
:1. Introduction
2. Synthesis of Polyglycidol- and Oilyglycidol-Containing Copolymers
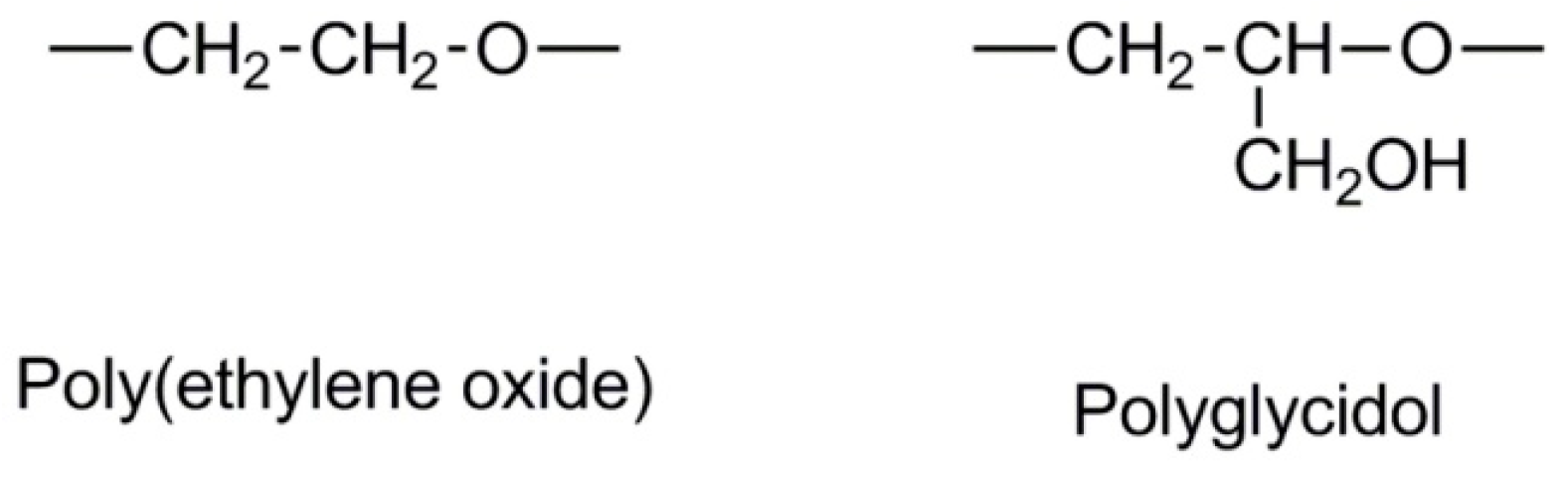
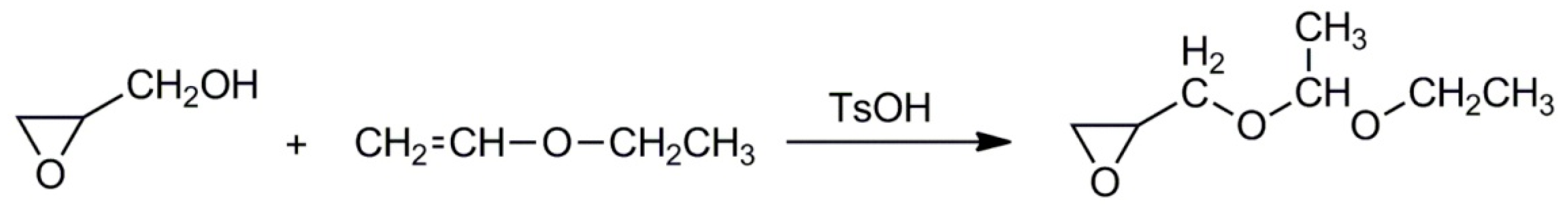
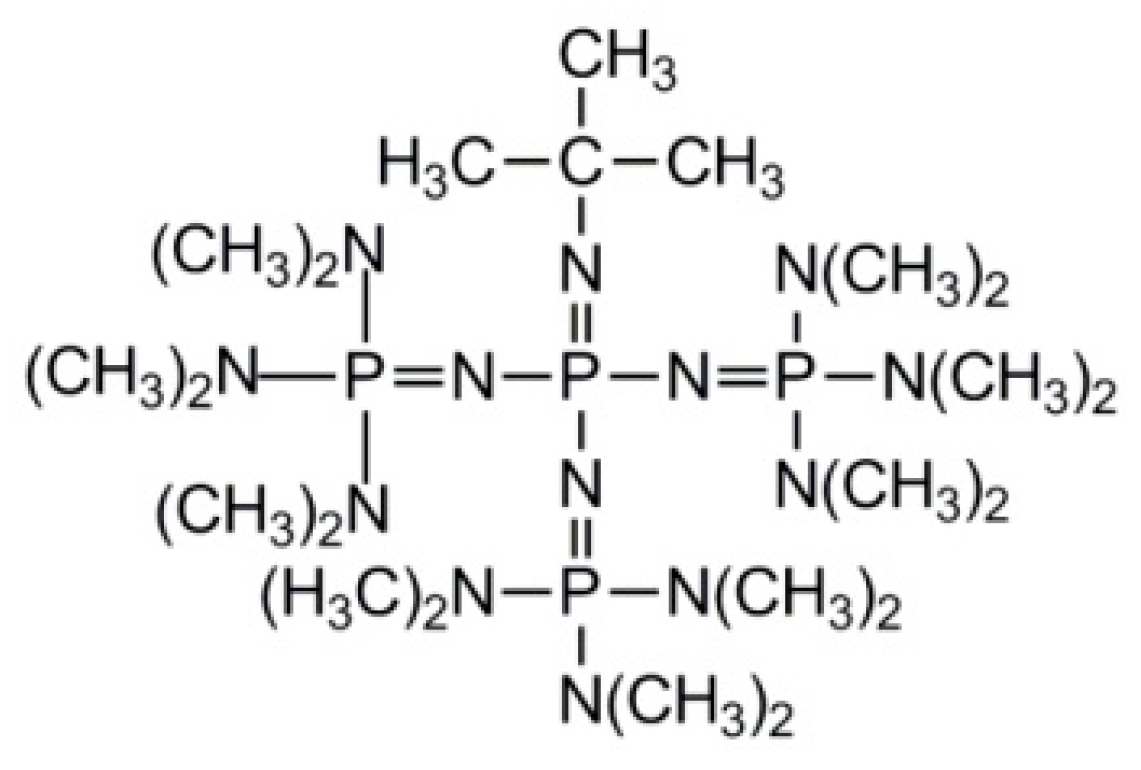
2.1. Monomers
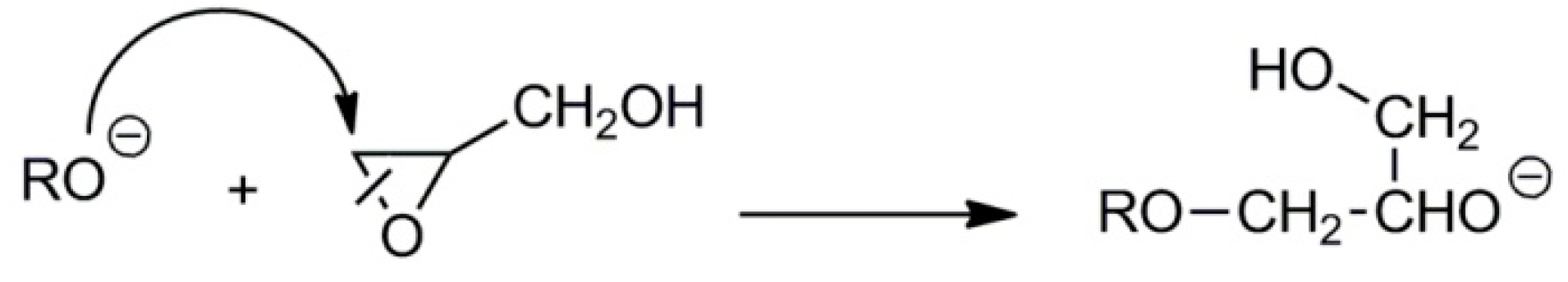
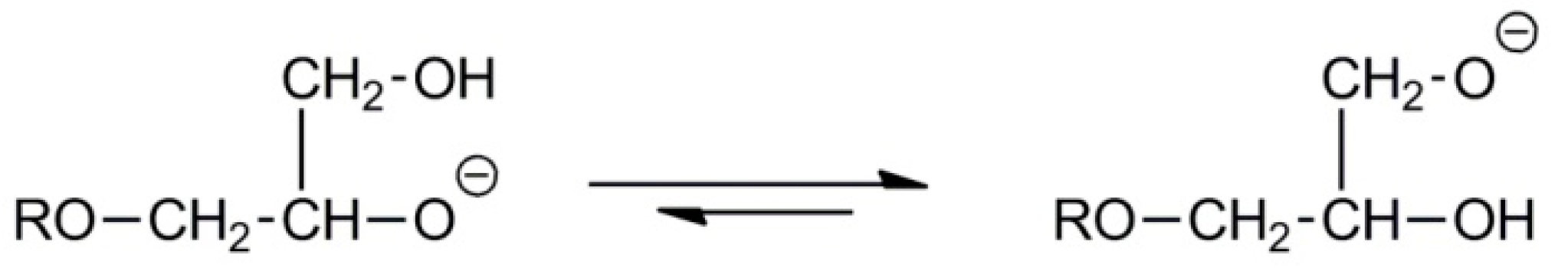
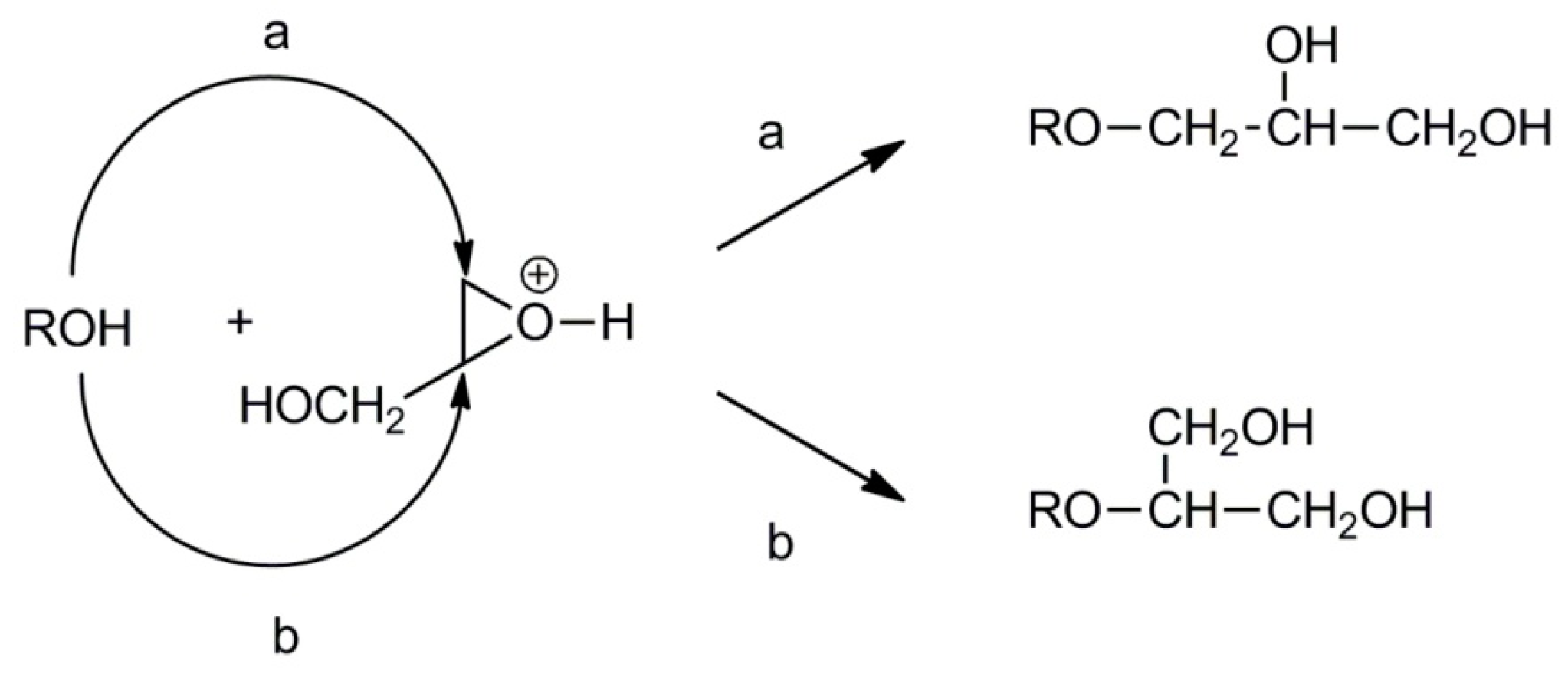
2.2. Synthesis of Linear Polyglycidol
2.3. Synthesis of Star-Like Polyglycidol
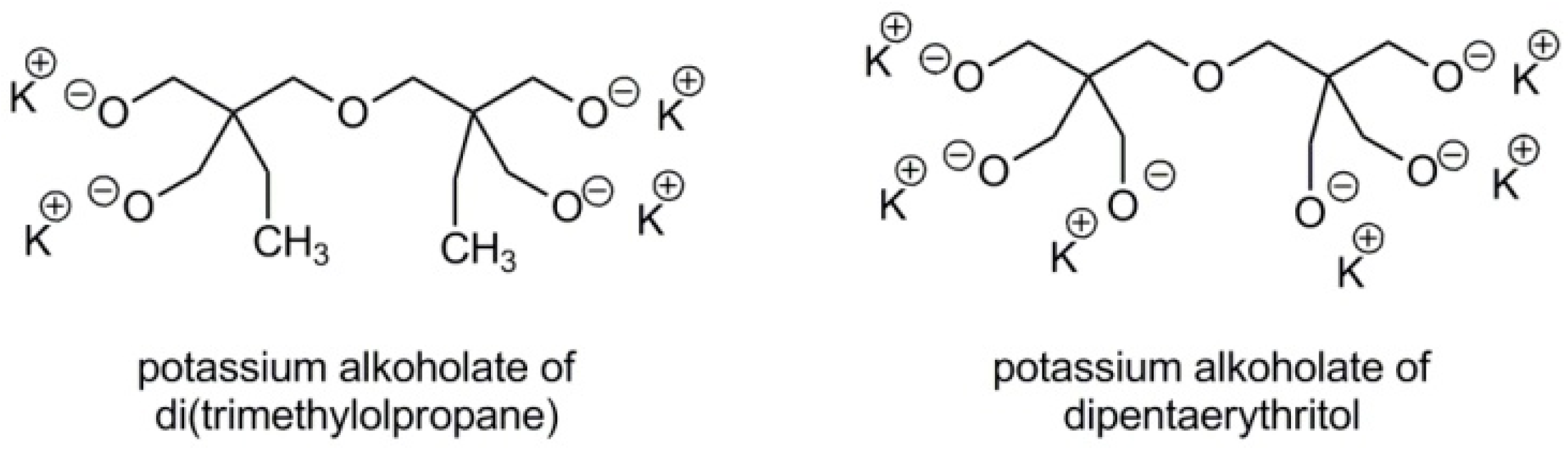
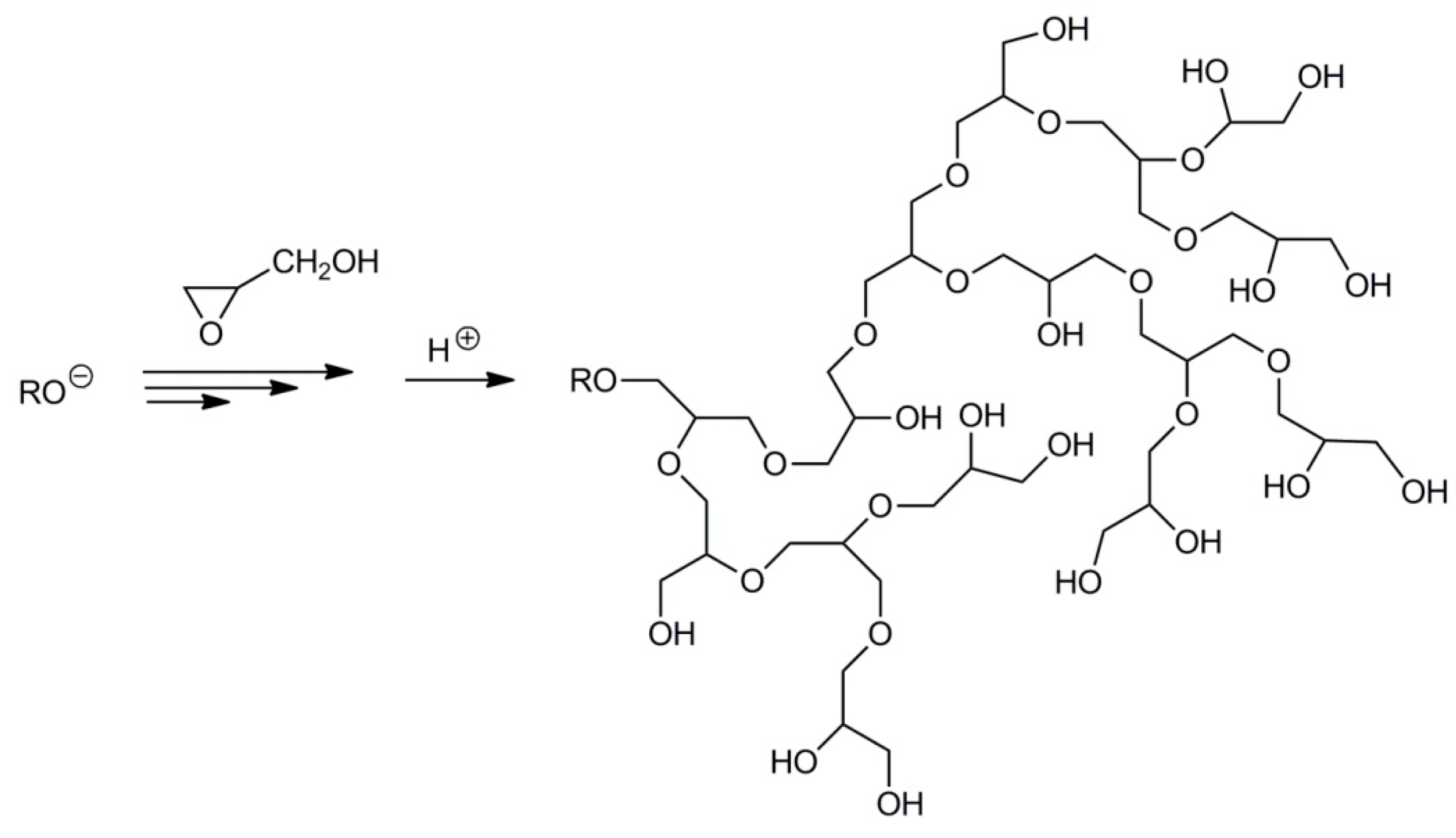
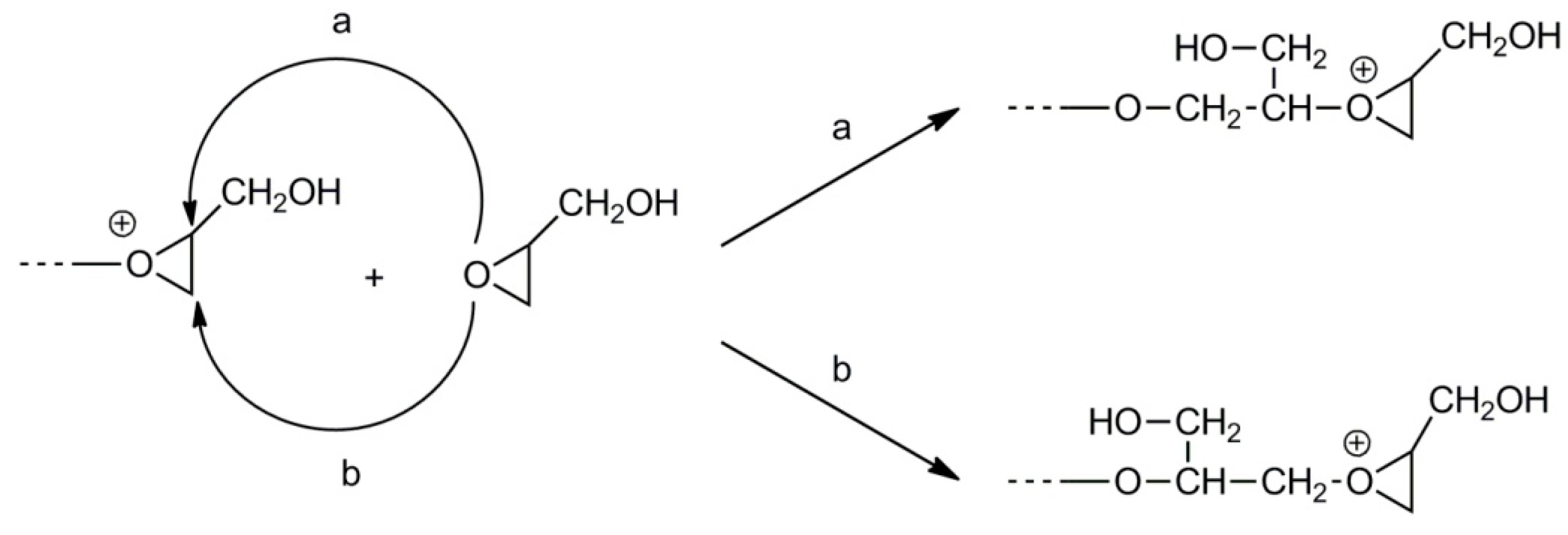
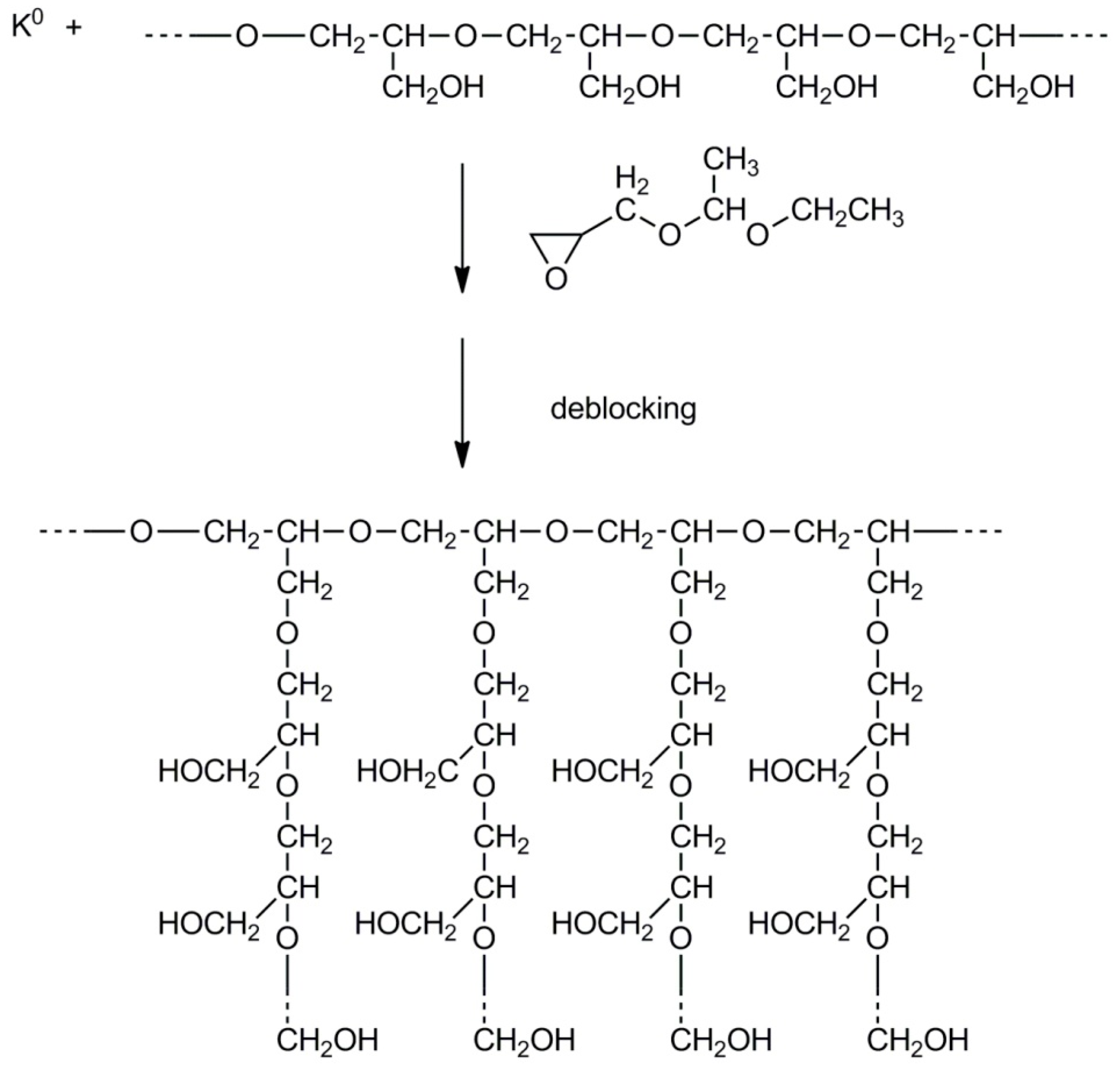
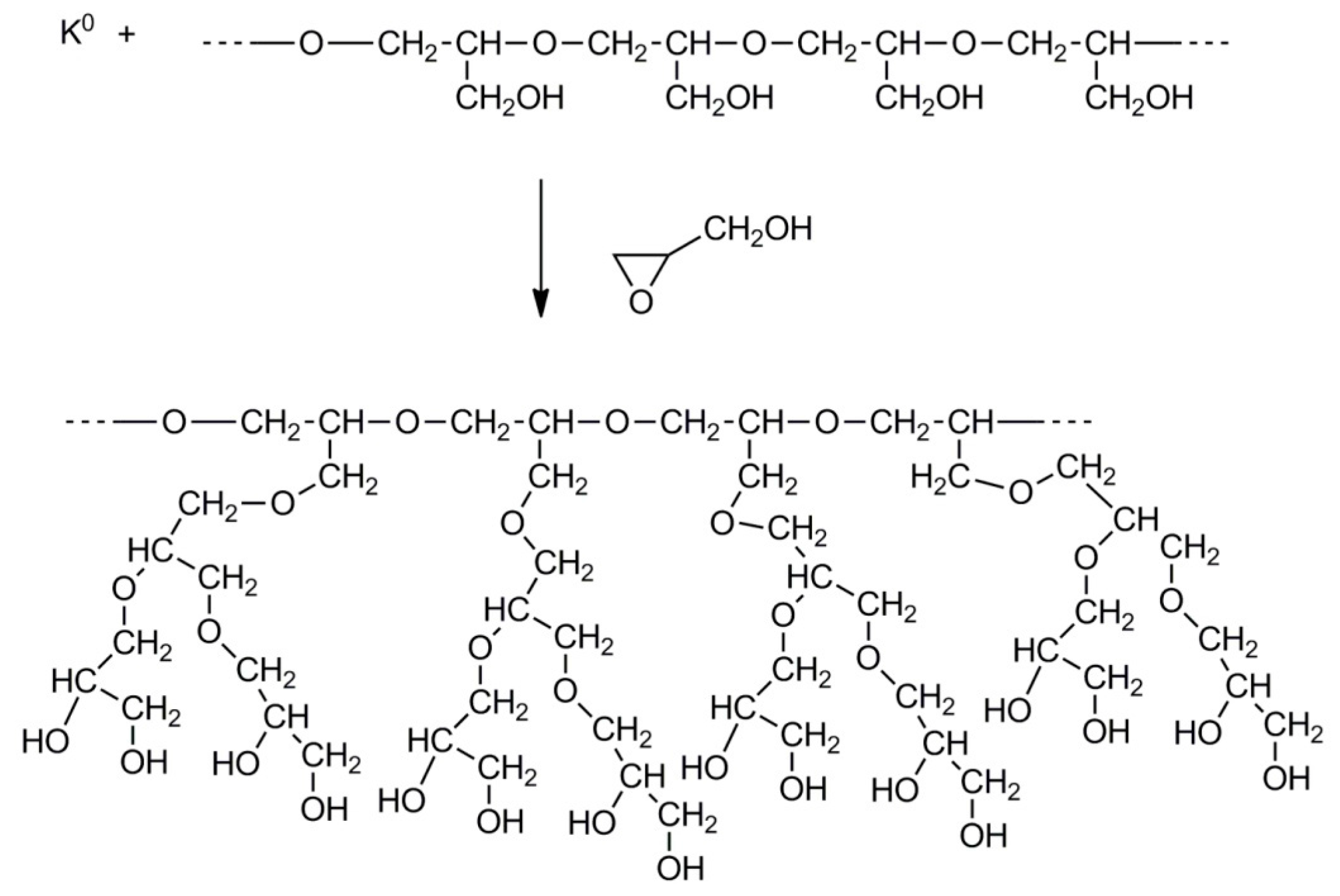
2.4. Synthesis of Branched Polyglycidol
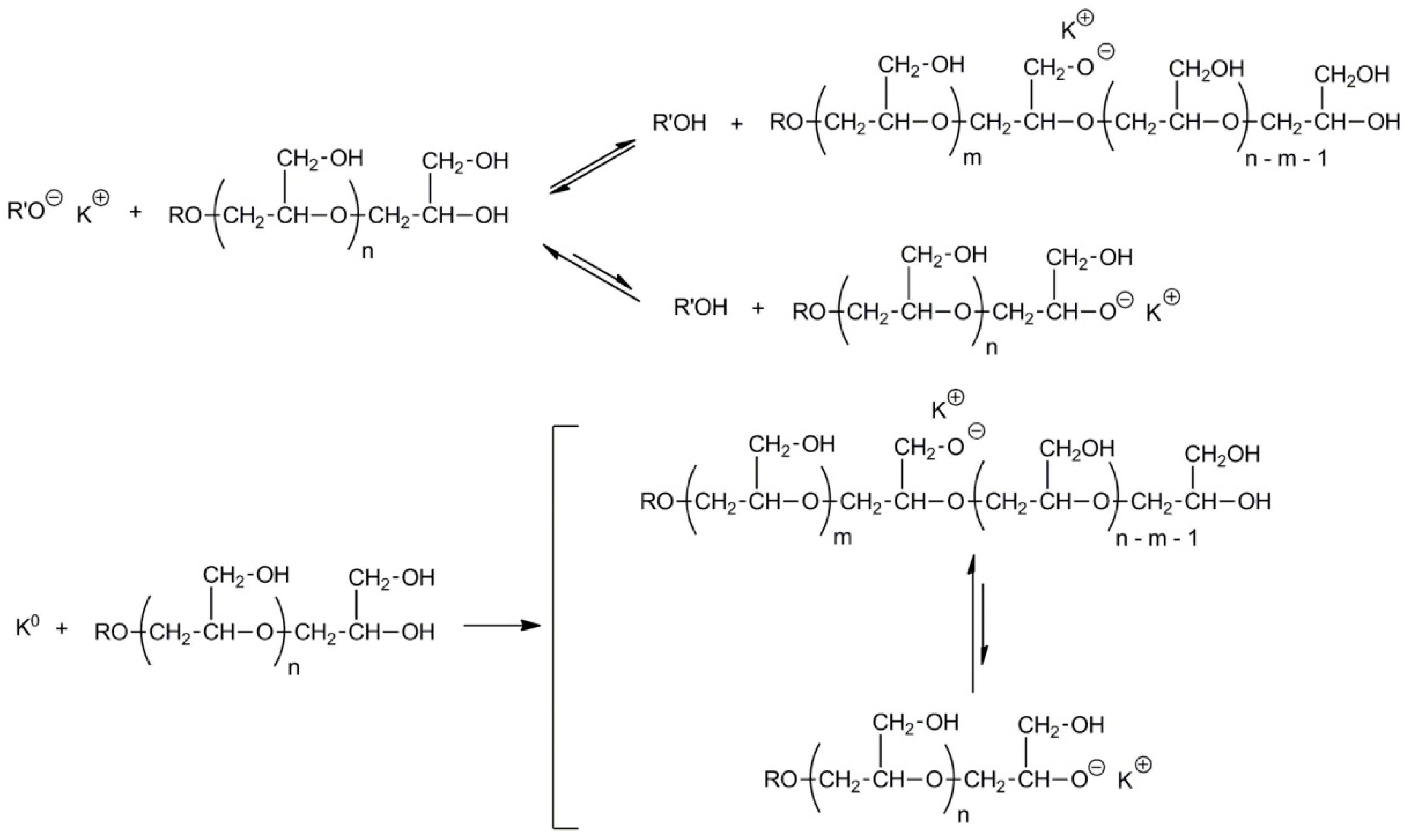
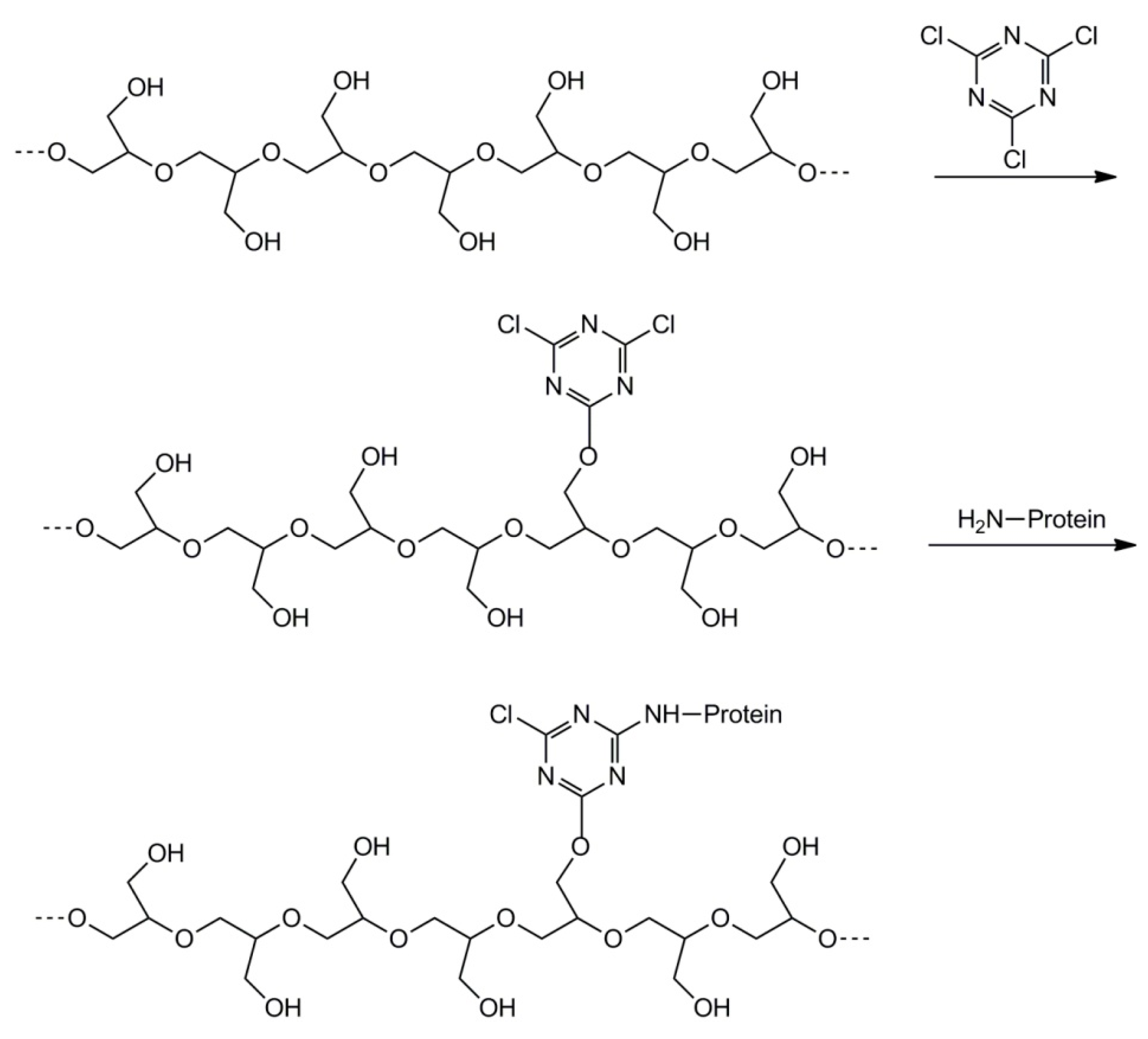
2.5. Synthesis of Polyglycidol with Varied Topology by Grafting from the Linear Polyglycidol
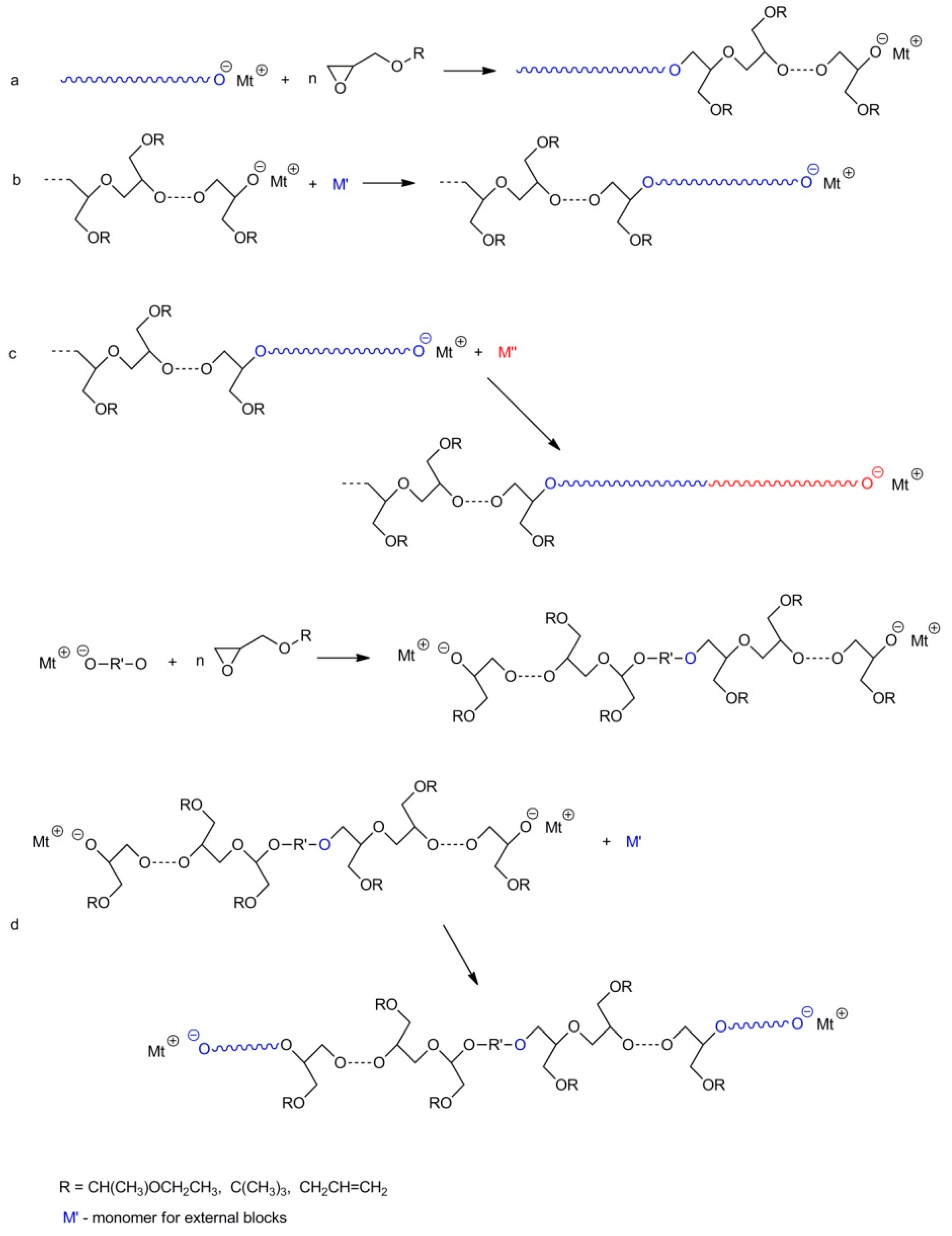
2.6. Synthesis of Polyglycidol-Containing Copolymers
- synthesis of the linear di-block copolymers by polymerization of glycidol with blocked hydroxyl groups in the process initiated with macroinitiator, i.e., with other polymers containing active centers at the ends of their chains;
- synthesis of the linear di-block copolymers using polyglycidol with blocked hydroxyl groups along the chain and active end groups, as an initiator for the polymerization of other comonomers;
- synthesis of the linear tri-block copolymers using di-block copolymers with active end groups (obtained by routes a or b) as macroinitiatiors for the polymerization leading to addition of the third block;
- synthesis of the linear tri-block copolymer by polymerization of glycidol with a blocked hydroxyl group, initiated by a di-functional initiator and using the synthesized di-block macroinitiator for the polymerization of other monomers, resulting in the addition of new blocks at both ends;
- synthesis of the linear tri-block copolymer in a way similar to route d but by using glycidol with blocked hydroxyl groups as a second comonomer;
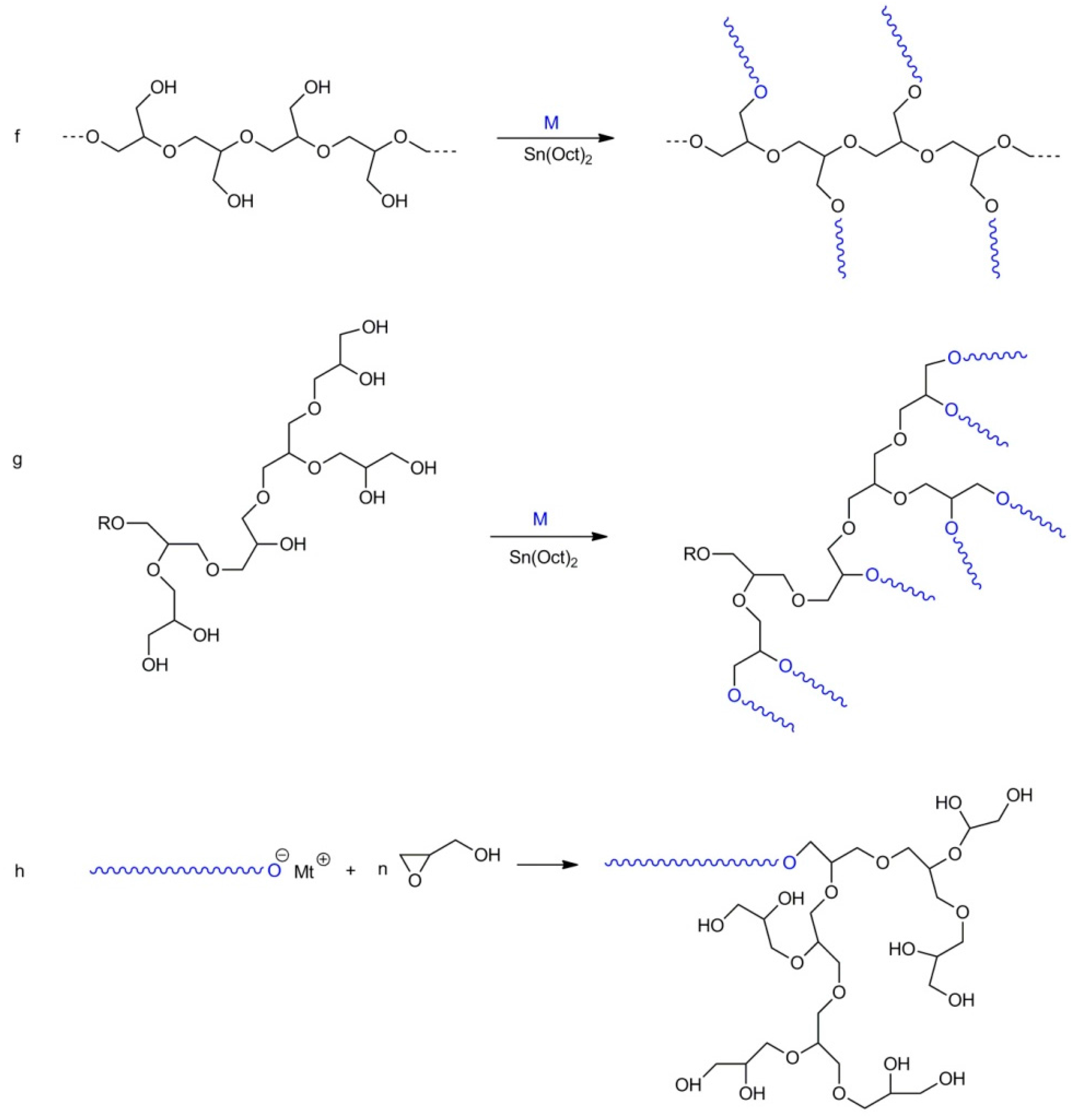
- synthesis of a comb-like copolymer by using polyglycidol (with unprotected hydroxyl groups) as a macroinitiator and catalyst (e.g., Sn(Oct)2) for polymerization of other comonomers;
- synthesis of the branched copolymer in a way similar to route f but using hyperbranched polyglycidol as a macroinitiator;
- synthesis of the linear-hyperbranched copolymer in a way similar to route a but using glycidol (i.e., a monomer with unblocked hydroxyl groups) as a second monomer;
- synthesis of the hyperbranched copolymer by polymerization of glycidol from reactive groups in the corona of another hyperbranched copolymer.
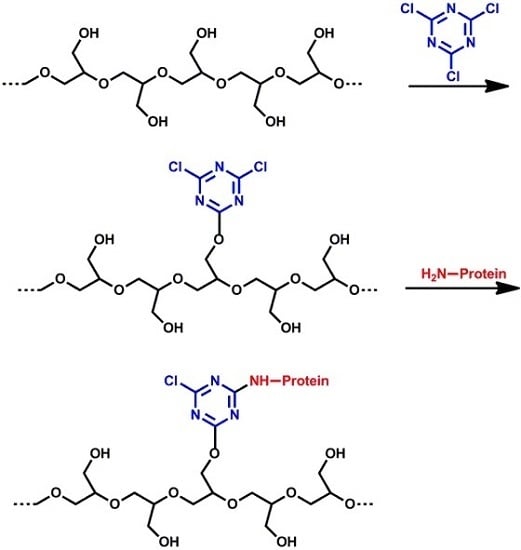
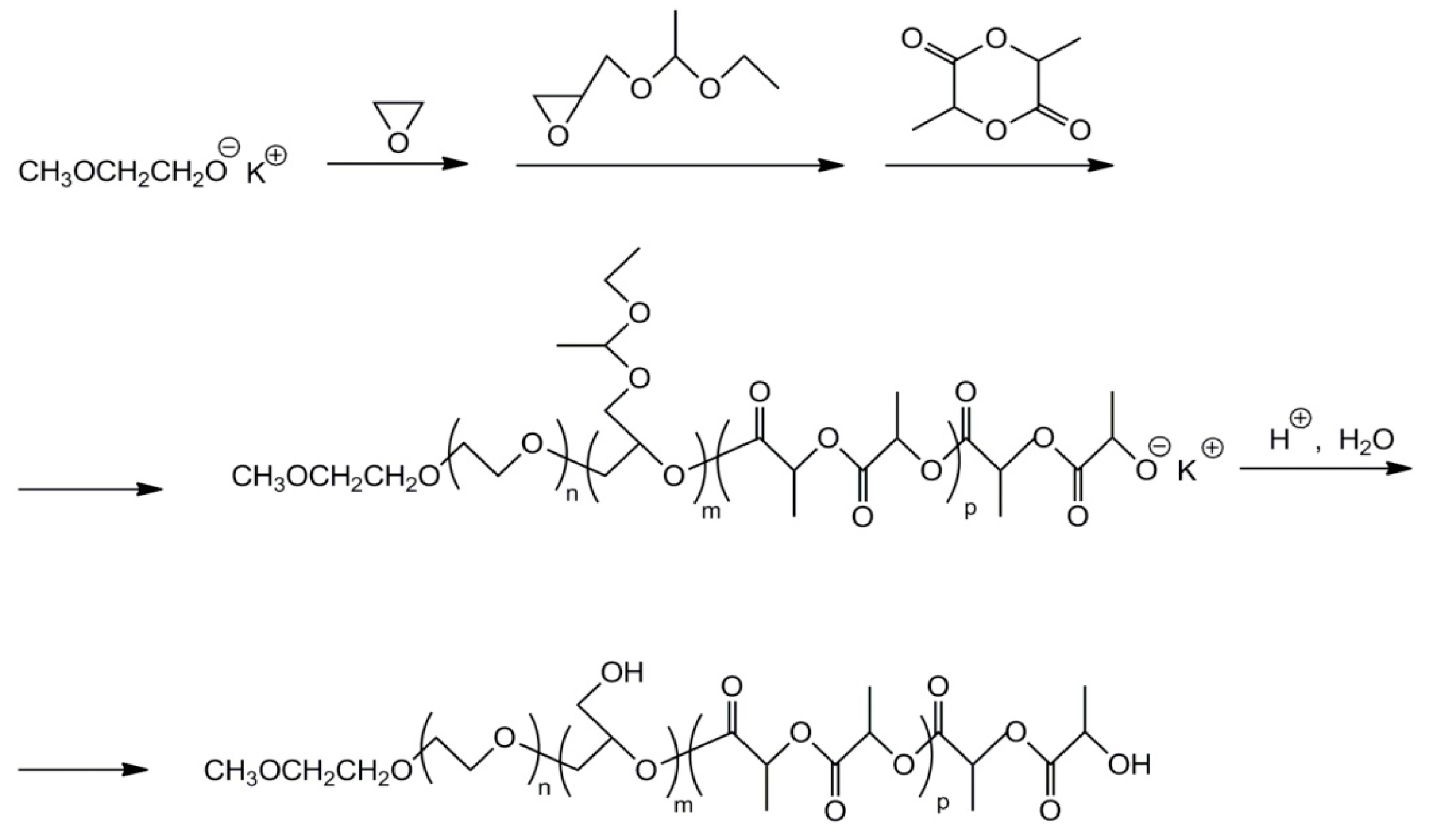
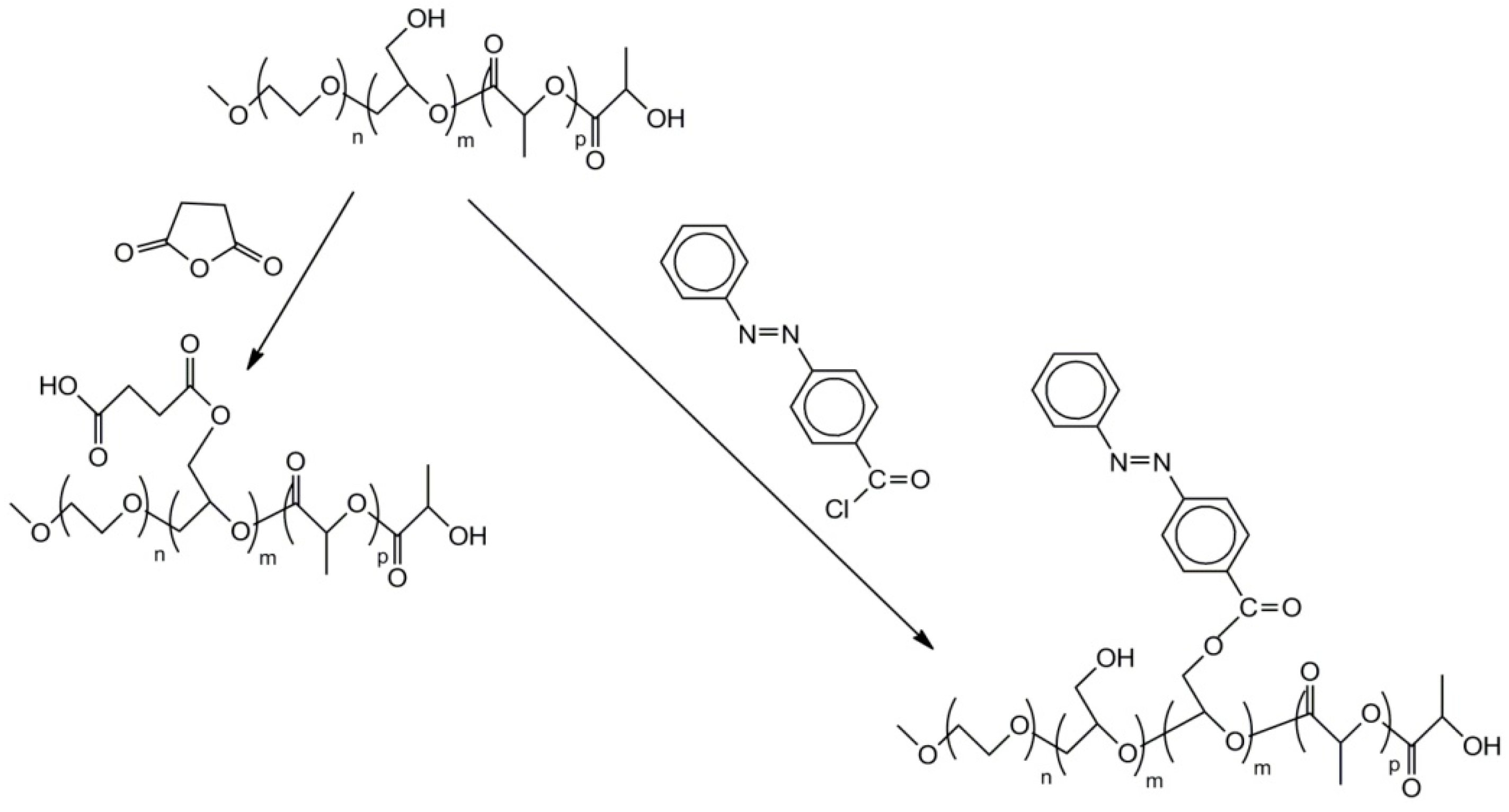
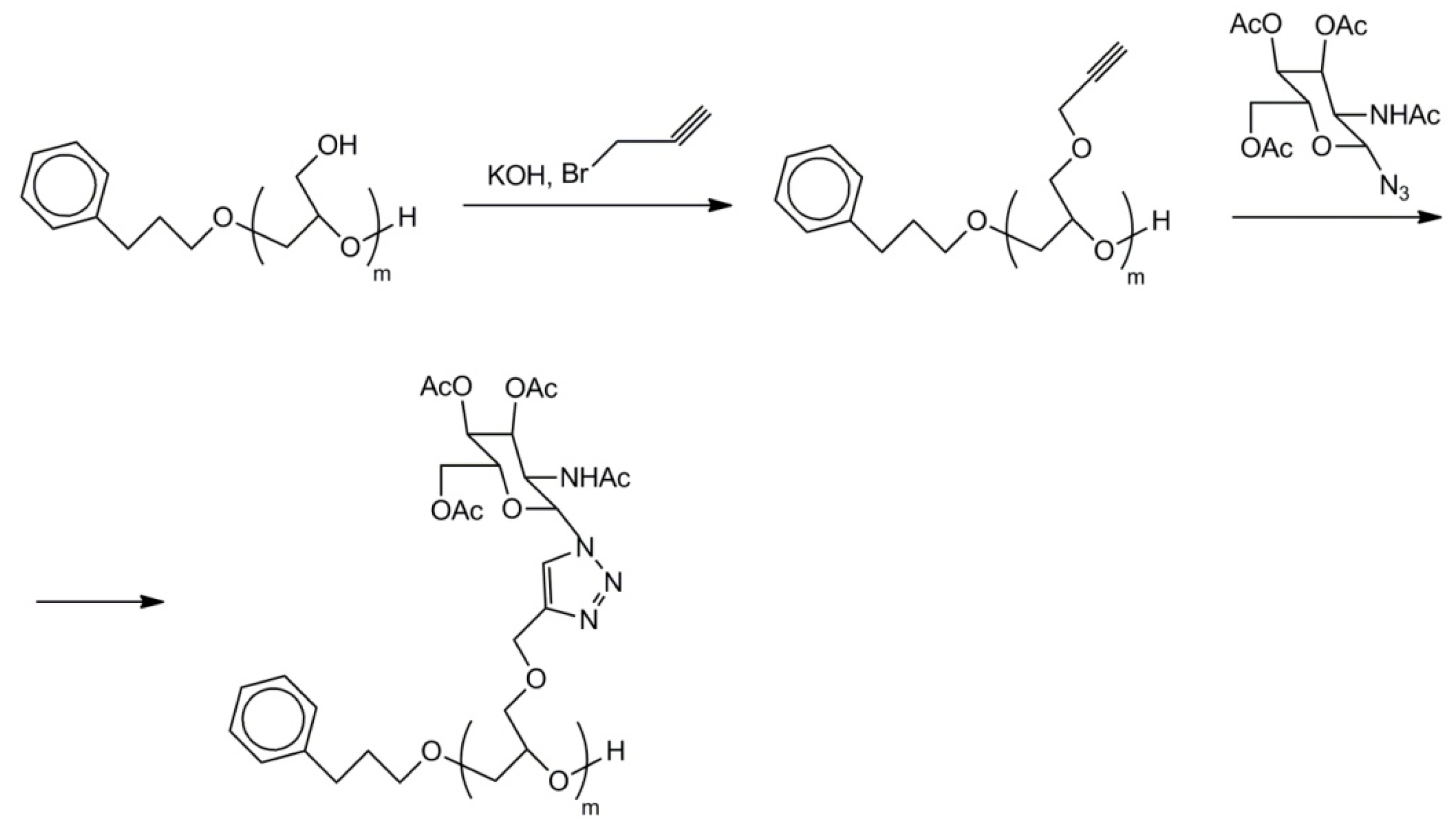
2.7. Functionalized Polyglycidol and Polyglycidol-Containing Copolymers
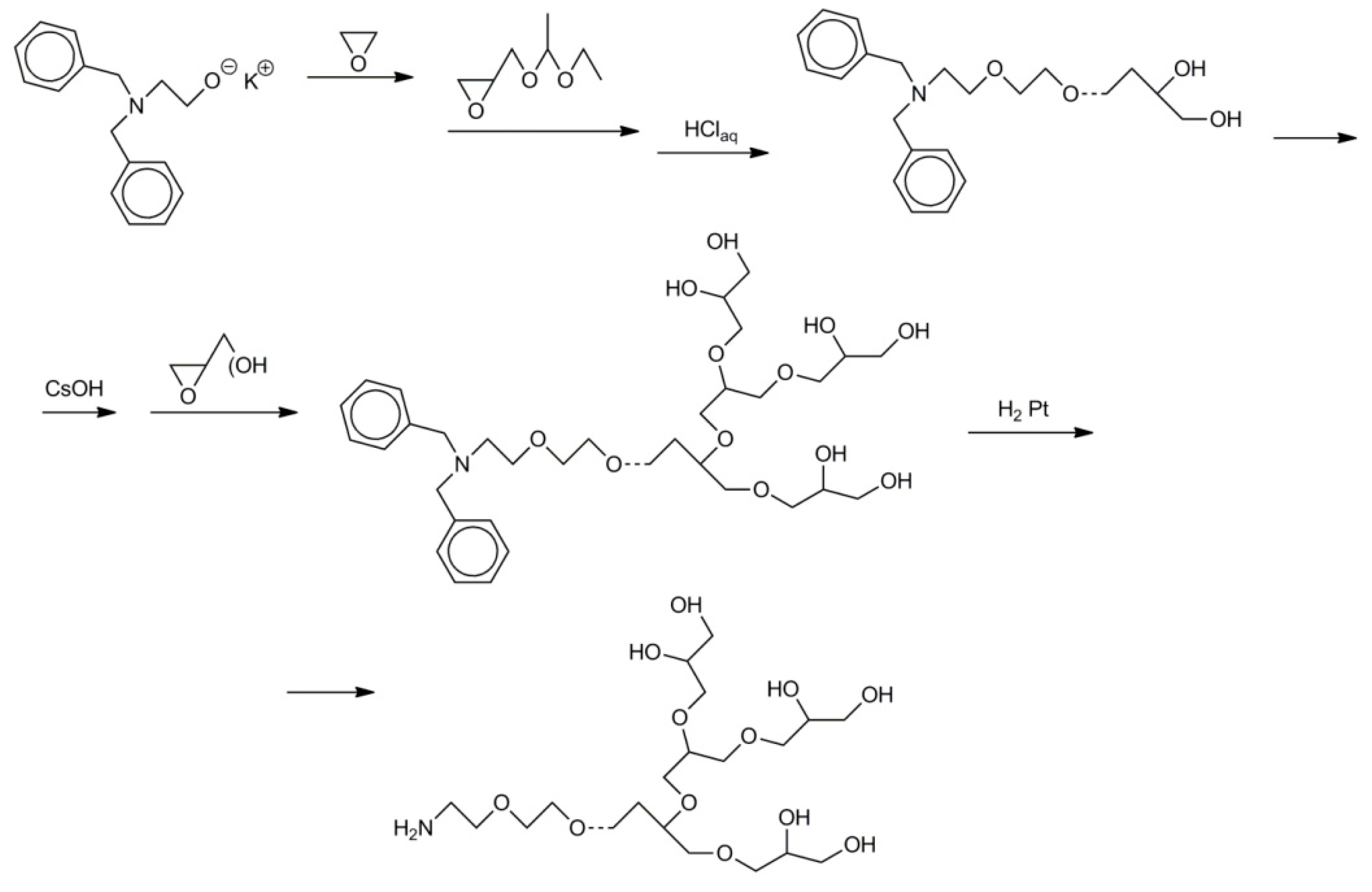
- initiation of the polymerization of ethylene oxide with cesium dibenzyl-2-aminoethanolate;
- using obtained macroinitiator for initiation of the polymerization of 1-ethoxyethylglycidyl ether;
- deblocking of hydroxyl groups in poly(1-ethoxyethylglycidyl ether), activation of hydroxyl groups with CsOH;
- polymerization of glycidol initiated with …–CH2O−Cs+;
- conversion of the dibenzyl-2-aminoethyl-O–… groups into the NH2CH2CH2O–… groups.
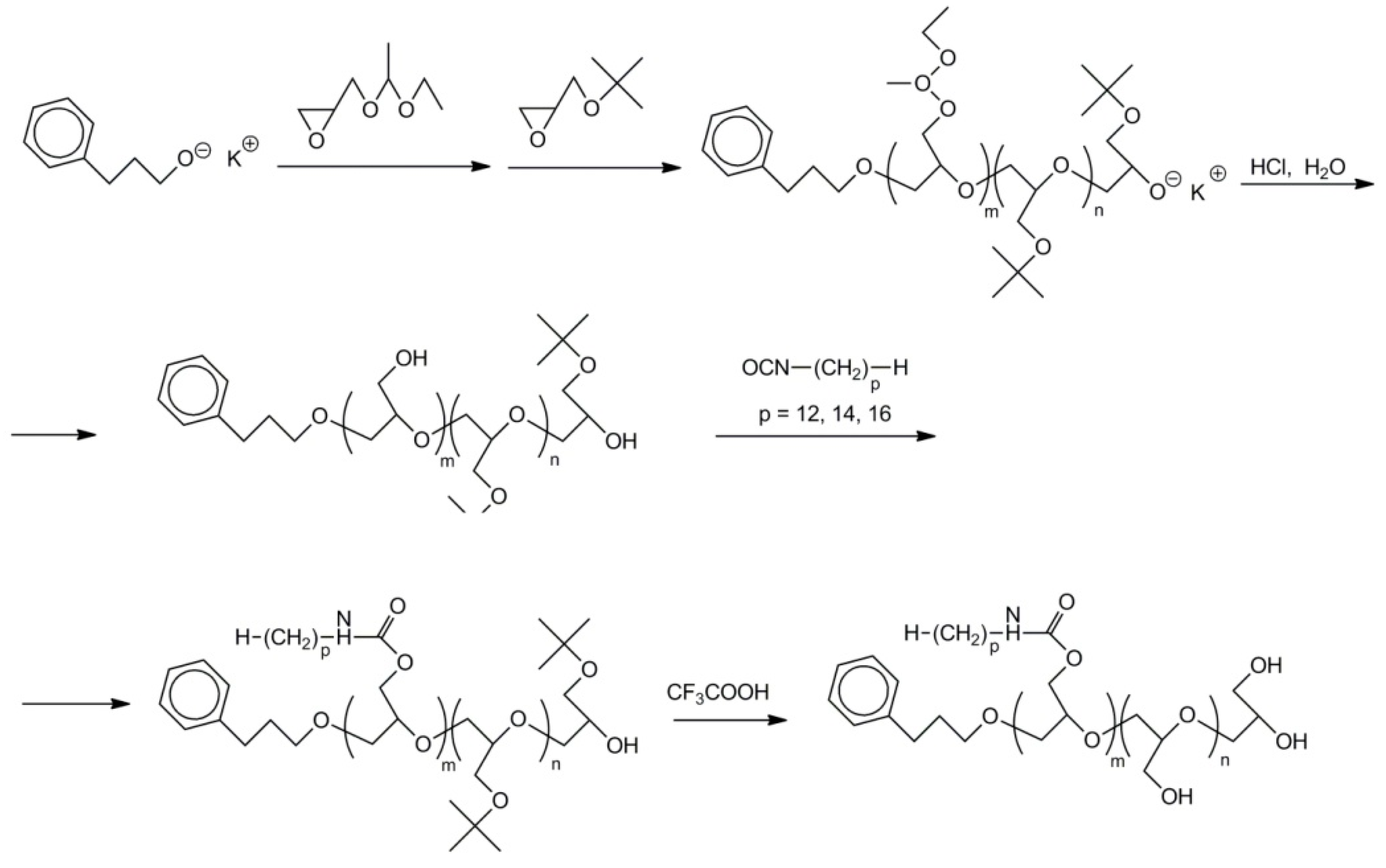
- sequential polymerization of 1-ethoxyethylglycidyl ether and t-butylglycidyl ether initiated with C6H5–(CH2)3O−K+;
- deblocking of hydroxyl groups in poly(1-ethoxyethylglycidol ether) with hydrochloric acid (the concentration of HCl and time of deblocking were optimized);
- labeling of polyglycidol with alkyl chains in reaction between –CH2OH groups of polyglycidol and alkyl chains with isocyanate end-groups;
- deprotection of hydroxyl groups in poly(t-butylglycidyl ether) block using trifluoroacetic acid.
3. Applications of Polyglycidol and Polyglycidol-Containing Polymers in Medicine
3.1. Biocompatibility
3.2. Polyglycidol-Based Drug Carriers
3.3. Applications of Polyglycidol and Polyglycidol Derivatives in Diagnostics-Based Drug Carriers
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Monfardini, C.; Veronese, F.M. Stabilization of substances in circulation. Bioconj. Chem. 1998, 9, 418–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Ulrich, S.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lin, C.-W.; Mansour, R.; Gu, F. Improving biocompatibility by surface modification techniques on implantable bioelectronics. Biosens. Bioelectron. 2013, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nagasaki, Y. Impacts of PEGylation on the gene and oligonucleotide delivery system. J. Appl. Polym. Sci. 2014, 40293. [Google Scholar] [CrossRef]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable, coatings for long-circulating nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, M.T.; Fattal, E.; Desmaele, D.; Besnard, M.; Noël, J.P.; Gomis, J.M.; Appel d’Angelo, J.; Couvreur, P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Control. Release 1999, 60, 121–128. [Google Scholar] [CrossRef]
- Peracchia, M.T.; Harnisch, S.; Pinto-Alphandary, H.; Gulik, A.; Dedieu, J.C.; Desmaële, D.; d’Angelo, J.; Müller, R.H.; Couvreur, P. Visualization of in vitro protein-rejecting properties of PEGylated stealth’ polycyanoacrylate nanoparticles. Biomaterials 1999, 20, 1269–1275. [Google Scholar] [CrossRef]
- O’Connor, S.M.; DeAnglis, A.P.; Gehrke, S.H.; Retzinger, G.S. Adsorption of plasma proteins on to poly(ethylene oxide)/poly(propylene oxide) triblock copolymer films: A focus on fibrinogen. Biotechnol. Appl. Biochem. 2000, 31, 185–196. [Google Scholar] [CrossRef] [PubMed]
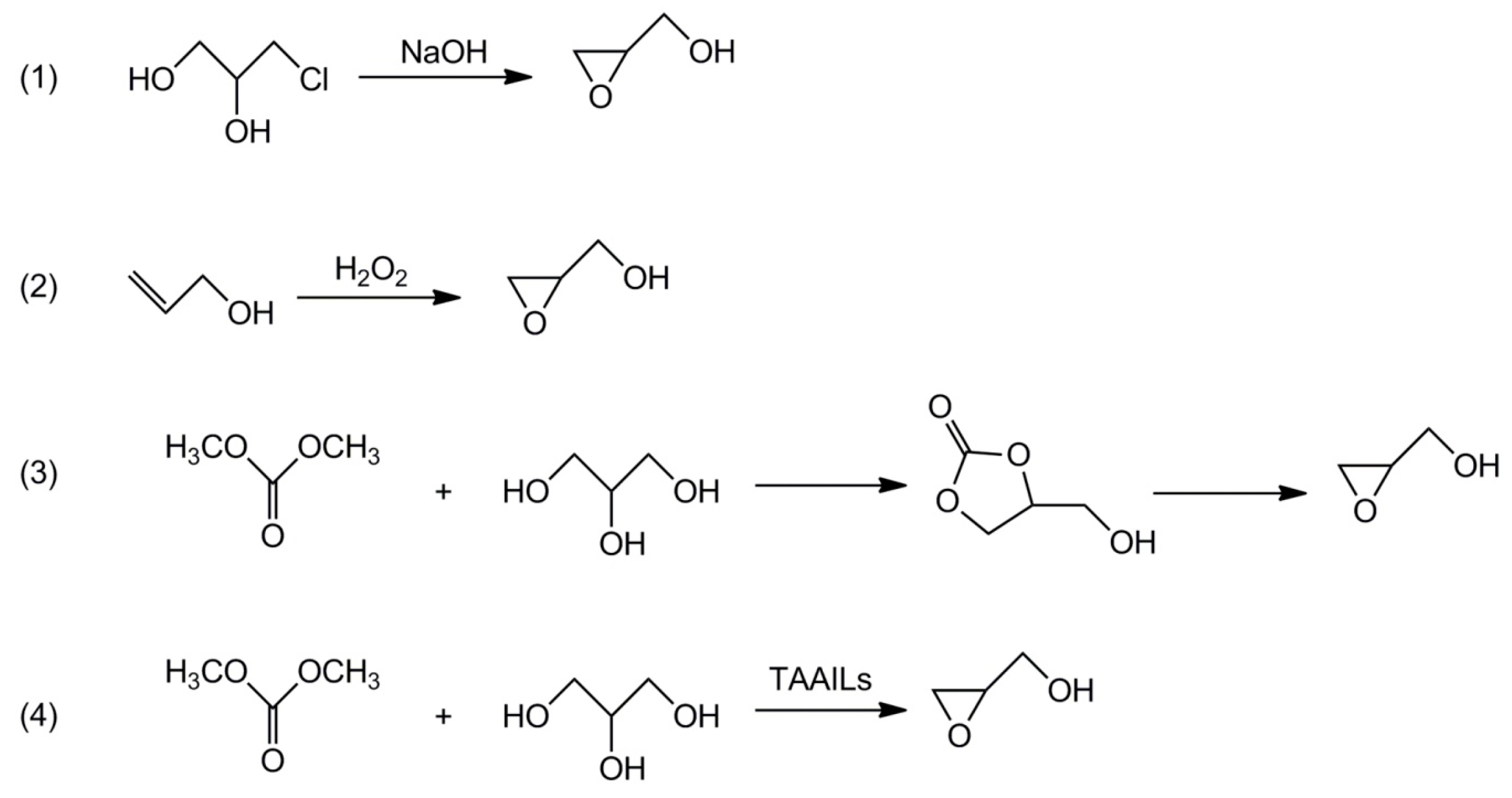
- Hanson, R.M. The synthetic methodology of nonracemic glycidol and related 2,3-epoxy alcohols. Chem. Rev. 1991, 91, 437–475. [Google Scholar] [CrossRef]
- Sulimov, A.V.; Danov, S.M.; Ovcharova, A.V.; Ovcharov, A.A.; Flid, V.R. Regularities of glycidol synthesis by the liquid-phase epoxidation of allyl alcohol with hydrogen peroxide. Russ. Chem. Bull. Int. Ed. 2014, 63, 2647–2651. [Google Scholar] [CrossRef]
- Malkemus, J.D.; Currier, V.A.; Bell, J.R., Jr. Method for preparing glycidol. US 2,856,413, 14 October 1958. [Google Scholar]
- Kim, S.C.; Kim, Y.H.; Lee, H.; Yoon, D.Y.; Song, B.K. Lipase-catalyzed synthesis of glycerol carbonate from renewable glycerol and dimethyl carbonate through transesterification. J. Mol. Catal. B Enzym. 2007, 49, 75–78. [Google Scholar] [CrossRef]
- Naik, P.U.; Petitjean, L.; Refes, K.; Picquet, M.; Plasseraud, L. Imidazolium-2-carboxylate as an efficient, expeditious and eco-friendly organocatalyst for glycerol carbonate synthesis. Adv. Synth. Catal. 2009, 351, 1753–1756. [Google Scholar] [CrossRef]
- Choi, J.S.; Simanjuntaka, F.S.H.; Oh, J.Y.; Lee, K.I.; Lee, S.D.; Cheong, M.; Kim, H.S.; Lee, H. Ionic-liquid-catalyzed decarboxylation of glycerol carbonate to glycidol. J. Catal. 2013, 297, 248–255. [Google Scholar] [CrossRef]
- Bolívar-Diaz, C.L.; Calvino-Casilda, V.; Rubio-Marcos, F.; Fernández, J.F.; Bañares, M.A. New concepts for process intensification in the conversion of glycerol carbonate to glycidol. Appl. Catal. B Environ. 2013, 129, 575–579. [Google Scholar] [CrossRef]
- Lu, P.; Wang, H.; Hu, K. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over the extruded CaO-based catalyst. Chem. Eng. J. 2013, 228, 147–154. [Google Scholar] [CrossRef]
- Munshi, M.K.; Biradar, P.S.; Gade, S.M.; Vilas, H.; Rane, V.H.; Kelkar, A.A. Efficient synthesis of glycerol carbonate/glycidol using 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) based ionic liquids as catalyst. RSC Adv. 2014, 4, 17124–17128. [Google Scholar] [CrossRef]
- Zhou, Y.; Ouyang, F.; Song, Z.-B.; Yang, Z.; Tao, D.-J. Facile one-pot synthesis of glycidol from glycerol and dimethyl carbonate catalyzed by tetraethylammonium amino acid ionic liquids. Catal. Commun. 2015, 66, 25–29. [Google Scholar] [CrossRef]
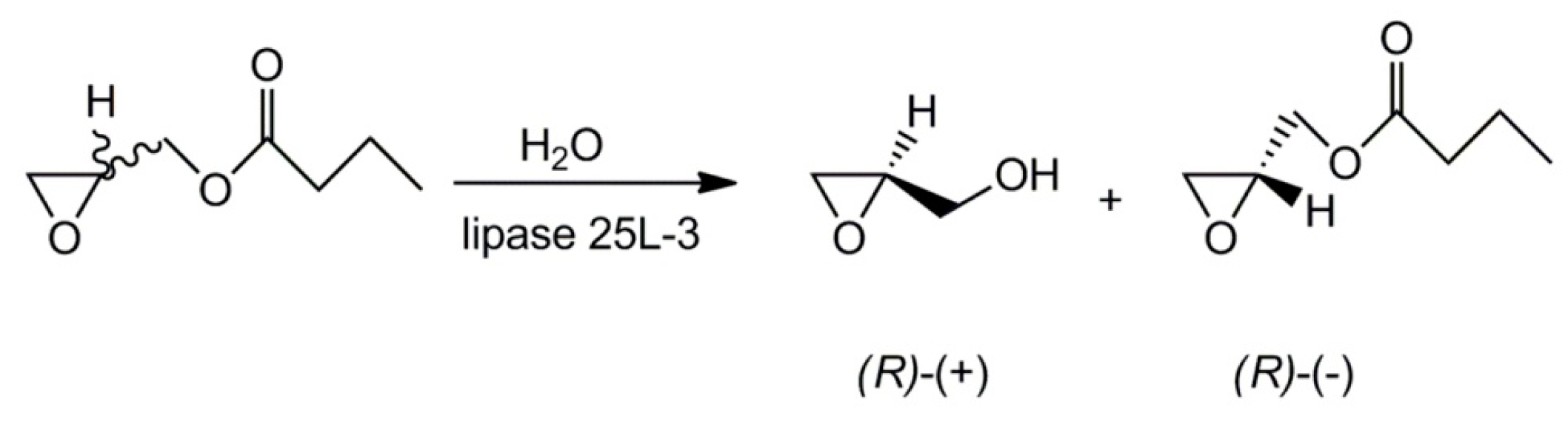
- Palomo, J.M.; Segura, R.L.; Mateo, C.; Terreni, M.; Guisana, J.M.; Fernández-Lafuente, R. Synthesis of enantiomerically pure glycidol via a fully enantioselective lipase-catalyzed resolution. Tetrahedron Asymmetry 2005, 16, 869–874. [Google Scholar] [CrossRef]
- Fitton, A.O.; Hill, J.; Jane, D.E.; Millar, R. Synthesis of simple oxetanes carrying reactive 2-substituents. Synthesis 1987, 12, 1140–1142. [Google Scholar] [CrossRef]
- Taton, D.; Leborgne, A.; Sepulchre, M.; Spassky, N. Synthesis of chiral and racemic functional polymers from glycidol and thioglycidol. Macromol. Chem. Phys. 1994, 95, 139–148. [Google Scholar] [CrossRef]
- Dimitrov, P.; Hasan, E.; Rangelov, S.; Trzebicka, B.; Dworak, A.; Tsvetanov, C.B. High molecular weight functionalized poly(ethylene oxide). Polymer 2002, 43, 7171–7178. [Google Scholar] [CrossRef]
- Hans, M.; Gasteier, P.; Keul, H.; Moeller, M. Ring-opening polymerization of ε-caprolactone by means of mono and multifunctional initiators: Comparison of chemical and enzymatic catalysis. Macromolecules 2006, 39, 3184–3193. [Google Scholar] [CrossRef]
- Rangelov, S.; Trzebicka, B.; Jamroz-Piegza, M.; Dworak, A. Hydrodynamic behavior of high molar mass linear polyglycidol in dilute aqueous solution. J. Phys. Chem. B 2007, 111, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Keul, H.; Moeller, M. Chain transfer reactions limit the molecular weight of polyglycidol prepared via alkali metal based initiating systems. Polymer 2009, 50, 1103–1108. [Google Scholar] [CrossRef]
- Gervais, M.; Brocas, A.-L.; Cendejas, G.; Deffieux, A.; Carlotti, S. Linear high molar mass polyglycidol and its direct α-azido functionalization. Macromol. Symp. 2011, 308, 101–111. [Google Scholar] [CrossRef]
- Sosnowski, S. Selective cleavage of acetal bonds in copolymers with polylactide block. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6978–6982. [Google Scholar] [CrossRef]
- Gervais, M.; Labbé, A.; Carlotti, S.; Deffieux, A. Direct Synthesis of R-azido, ω-hydroxypolyethers by monomer-activated anionic polymerization. Macromolecules 2009, 42, 2395–2400. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Haamann, D.; Keul, H.; Klee, D.; Möller, M. Functionalization of linear and star-shaped polyglycidols with vinyl sulfonate groups and their reaction with different amines and alcohols. Macromolecules 2010, 43, 6295–6301. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Müllhaupt, R. Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Wilms, D.; Wurm, F.; Nieberle, J.; Böhm, P.; Kemmer-Jonas, U.; Frey, H. Hyperbranched polyglycerols with elevated molecular weights: A facile two-step synthesis protocol based on polyglycerol macroinitiators. Macromolecules 2009, 42, 3230–3236. [Google Scholar] [CrossRef]
- Wilms, D.; Stiriba, S.-E.; Frey, H. Hyperbranched polyglycerols: From the controlled synthesis of biocompatible polyether polyols to multipurpose applications. Acc. Chem. Res. 2010, 43, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Schömer, M.; Schüll, C.; Frey, H. Hyperbranched aliphatic polyether polyols. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 995–1019. [Google Scholar] [CrossRef]
- Tokar, R.; Kubisa, P.; Penczek, S. Cationic polymerization of glycidol: Coexistence of the activated monomer and active chain end mechanism. Macromolecules 1994, 27, 320–322. [Google Scholar] [CrossRef]
- Siebert, M.; Keul, H.; Moller, M. Synthesis of well-defined polystyrene-block-polyglycidol (PS-b-PG) block co-polymers by anionic polymerization. Des. Monomers. Polym. 2010, 13, 547–563. [Google Scholar] [CrossRef]
- Siebert, M.; Henke, A.; Eckert, T.; Richtering, W.; Keul, H.; Moller, M. Polystyrene-block-polyglycidol micelles cross-linked with titanium tetraisopropoxide. Laser light and small-angle X-ray scattering studies on their formation in solution. Langmuir 2010, 26, 16791–16800. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Quirk, R.P. Anionic Polymerizations Principles and Practical Applications; Marcel Dekker: New York, NY, USA, 1996; p. 688. [Google Scholar]
- Ah Toy, A.; Reinicke, S.; Müller, A.H.E.; Schmalz, H. One-pot synthesis of polyglycidol-containing block copolymers with alkyllithium initiators using the phosphazene base t-BuP4. Macromolecules 2007, 40, 5241–5244. [Google Scholar] [CrossRef]
- Esswein, B.; Möller, M. Polymerization of ethylene oxide with alkyllithium compounds and the phosphazene base “tBu-P-4”. Angew. Chem. Int. Ed. Engl. 1996, 5, 625. [Google Scholar] [CrossRef]
- Esswein, B.; Molenberg, A.; Möller, M. Use of polyiminophosphazene bases for ring-opening polymerizations. Macromol. Symp. 1996, 107, 331–340. [Google Scholar] [CrossRef]
- Haladjova, E.; Dishovsky, N.; Meier, W.; Tsvetanov, C.B.; Novakov, C.P. Synthesis of poly(styrene-co-diene)-block-polyglycidol. Self-association and stabilization of aggregates. Soft Matter 2011, 7, 9459–9469. [Google Scholar] [CrossRef]
- Gadzinowski, M.; Sosnowski, S. Biodegradable/biocompatible ABC triblock copolymer bearing hydroxyl groups in the middle block. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3750–3760. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Ye, X.; Ma, C.; Zhang, G.; Pispas, S. Synthesis and properties of amphiphilic and biodegradable poly(ε-caprolactone-co-glycidol) copolymers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 846–853. [Google Scholar] [CrossRef]
- Halacheva, S.; Rangelov, S.; Tsvetanov, C.; Vasil, M.; Garamus, V.M. Aqueous solution properties of polyglycidol-based analogues of pluronic copolymers. Influence of the poly(propylene oxide) block molar mass. Macromolecules 2010, 43, 772–781. [Google Scholar] [CrossRef]
- Goodwin, A.; Bobade, S.; Kang, N.-G.; Baskaran, D.; Hongb, K.; Mays, J. Poly(styrene-graft-hyperbranched polyglycidol): Synthesis and solution behavior of a hyperbranched polyelectrolyte. RSC Adv. 2015, 5, 5611–5616. [Google Scholar] [CrossRef]
- Petchsuk, A.; Buchatip, S.; Supmak, W.; Opaprakasit, M.; Opaprakasit, P. Preparation and properties of multi-branched poly(d-lactide) derived from polyglycidol and its stereocomplex blends. Express Polym. Lett. 2014, 8, 779–789. [Google Scholar] [CrossRef]
- Wurm, F.; Hofmann, A.M.; Thomas, A.; Dingels, C.; Frey, H. αωn-Heterotelechelic hyperbranched polyethers solubilize carbon nanotubes. Macromol. Chem. Phys. 2010, 211, 932–939. [Google Scholar] [CrossRef]
- Dworak, A.; Panchev, I.; Trzebicka, B.; Walach, W. Poly(α-t-butoxy-ω-styrylo-glycidol): A new reactive surfactant. Polym. Bull. 1998, 40, 461–468. [Google Scholar] [CrossRef]
- Basinska, T.; Slomkowski, S.; Dworak, A.; Panchev, I.; Chehimi, M.M. Synthesis and characterization of poly(styrene/α-t-butoxy-ω-vinylbenzylpolyglycidol) microspheres. Colloid. Polym. Sci. 2001, 279, 916–924. [Google Scholar] [CrossRef]
- Slomkowski, S.; Gadzinowski, M.; Sosnowski, S.; De Vita, C.; Pucci, A.; Ciardelli, F.; Jakubowski, W.; Matyjaszewski, K. Biodegradable nano- and microparticles with controlled surface properties. Macromol. Symp. 2005, 226, 239–252. [Google Scholar] [CrossRef]
- Backes, M.; Messager, L.; Mourran, A.; Keul, H.; Moeller, M. Synthesis and thermal properties of well-defined amphiphilic block copolymers based on polyglycidol. Macromolecules 2010, 43, 3238–3248. [Google Scholar] [CrossRef]
- Erberich, M.; Keul, H.; Mo1ller, M. Polyglycidols with two orthogonal protective groups: Preparation, selective deprotection, and functionalization. Macromolecules 2007, 40, 3070–3079. [Google Scholar] [CrossRef]
- Basinska, T. Hydrophilic core-shell microspheres: A suitable support for controlled attachment of proteins and biomedical diagnostics. Macromol. Biosci. 2005, 12, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Matrab, T.; Chehimi, M.M.; Pinson, J.; Slomkowsi, S.; Basinska, T. Growth of polymer brushes by atom transfer radical polymerization on glassy carbon modified by electro-grafted initiators based on aryl diazonium salts. Surf. Interface Anal. 2006, 38, 565–568. [Google Scholar] [CrossRef]
- Basinska, T.; Krolik, S.; Slomkowski, S. Hydrophilic microspheres containing α-tert butoxy-omega-vinylbenzyl-polyglycidol for immunodiagnostics: Synthesis, properties and biomedical applications. Macromol. Symp. 2009, 281, 96–105. [Google Scholar] [CrossRef]
- Gam-Derouich, S.; Gosecka, M.; Lepinay, S.; Turmine, M.; Carbonnier, B.; Basinska, T.; Slomkowski, S.; Millot, M.C.; Othmane, A.; Ben Hassen-Chehimi, D.; et al. Highly hydrophilic surfaces from polyglycidol grafts with dual antifouling and specific protein recognition properties. Langmuir 2011, 27, 9285–9294. [Google Scholar] [CrossRef] [PubMed]
- Dworak, A.; Slomkowski, S.; Basinska, T.; Gosecka, M.; Walach, W.; Trzebicka, B. Polyglycidol—How is it synthesized and what is it used for? Polimery 2013, 58, 641–649. [Google Scholar] [CrossRef]
- Gosecka, M.; Slomkowski, S.; Basinska, T. Interactions of serum proteins and alkaline phosphatase with poly(styrene/α-tert-butoxy-ω-vinylbenzyl-polyglycidol) microspheres with various surface concentrations of polyglycidol. Polym. Adv. Technol. 2014, 25, 1264–1272. [Google Scholar] [CrossRef]
- Klajnert, B.; Walach, W.; Bryszewska, M.; Dworak, A.; Shcharbin, D. Cytotoxicity, haematotoxicity and genotoxicity of high molecular mass arborescent polyoxyethylene polymers with polyglycidol-block-containing shells. Cell Biol. Int. 2006, 30, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Royappa, A.T.; Tundel, S.; Tsukamoto, K.; Sharma, V. Biocompatibility of polyglycidol with human peripheral blood mononuclear cells. J. Appl. Polym. Sci. 2009, 111, 2275–2278. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Janzen, J.; Levin, E.; Devine, D.V.; Brooks, D.E. Biocompatibility testing of branched and linear polyglycidol. Biomacromolecules 2006, 7, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. PNAS 2010, 107, 19679–19684. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Wu, Z.; Zhang, X.; Wang, Z.; Li, C. Synthesis and physicochemical characterization of a novel amphiphilic polylactic acid-hyperbranched polyglycerol conjugate for protein delivery. J. Control. Release 2009, 140, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Zhang, X.; Cheng, C.; Wang, Z.; Li, C. Encapsulation of BSA in polylactic acid–hyperbranched polyglycerol conjugate nanoparticles: Preparation, characterization, and release kinetics. Polym. Bull. 2010, 65, 787–805. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wu, Z.; Gao, X.; Shu, S.; Wang, Z.; Li, C. Β-cyclodextrin grafting hyperbranched polyglycerols as carriers for nasal insulin delivery. Carbohydr. Polym. 2011, 84, 1419–1425. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Yang, X.; Luo, P.; Wu, Z.; Zhang, X. The supramolecular hydrogel based on hyperbranched polyglycerol and dextran as a scaffold for living cells and drug delivery. RSC Adv. 2015, 5, 86730–86739. [Google Scholar] [CrossRef]
- Steinhilber, D.; Witting, M.; Zhang, X.; Staegemann, M.; Paulus, F.; Friess, W.; Kuchler, S.; Haag, R. Surfactant free preparation of biodegradable dendritic polyglycerol nanogels by inverse nanoprecipitation for encapsulation and release of pharmaceutical biomacromolecules. J. Control. Release 2013, 169, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderon, M.; Friess, W.; Hedtrich, S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomedicine 2015, 11, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Gnanamani, M.; Ganguli, M.; Ghosh, T.; Brooks, D.E.; Maiti, S.; Kizhakkedathu, J.N. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials 2006, 27, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Psarra, A.M.; Tsiourvas, D.; Paleos, C.M. Synthesis and evaluation of functional hyperbranched polyether polyols as prospected gene carriers. Int. J. Pharm. 2008, 356, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Calderon, M.; Schulz, A.; Andreou, I.; Weber, M.; Haag, R. Dendritic polyglycerols with oligoamine shells show low toxicity and high siRNA transfection efficiency in vitro. Bioconj. Chem. 2010, 21, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Bauer, H.; Tschiche, A.; Staedtler, A.M.; Mohr, A.; Calderon, M.; Parmar, V.S.; Hoeke, L.; Sharbati, S.; Einspanier, R.; et al. Glycine-terminated dendritic amphiphiles for nonviral gene delivery. Biomacromolecules 2012, 13, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Tschiche, A.; Staedtler, A.M.; Malhotra, S.; Bauer, H.; Böttcher, C.; Sharbati, S.; Calderón, M.; Koch, M.; Zollner, T.M.; Barnard, A.; et al. Polyglycerol-based amphiphilic dendrons as potential siRNA carriers for in vivo applications. J. Mater. Chem. B 2014, 2, 2153–2167. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, L.; Wu, J.; Tu, C.; Su, Y.; Zhu, B.; Gu, H.; Yan, D.; Zhu, X. A supramolecular approach to the preparation of charge-tunable dendritic polycations for efficient gene delivery. Chem. Commun. 2011, 47, 5473–5475. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Z.; Cai, M.; Zhang, X.; Zhong, Z.; Zhuo, R. Phenylboronic-acid-modified amphiphilic polyether as a neutral gene vector. Macromol. Biosci. 2012, 12, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sun, Y.X.; Yi, W.J.; Yang, J.; Liu, C.W.; Cheng, H.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. A linear-dendritic cationic vector for efficient DNA grasp and delivery. Acta Biomater. 2012, 8, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Nakae, Y.; Qin, H.; Ito, T.; Kimura, T.; Kojima, H.; Chan, L.; Komatsu, N. Polyglycerol-functionalized nanodiamond as a platform for gene delivery: Derivatization, characterization, and hybridization with DNA. Beilstein J. Org. Chem. 2014, 10, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, X.; Lei, Q.; Zhuo, R.; Feng, J.; Zhang, X. Host-guest interaction-based self-engineering of nano-sized vesicles for co-delivery of genes and anticancer drugs. ACS Appl. Mater. Interfaces 2015, 7, 22084–22094. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Quadir, M.A.; Barnard, A.; Smith, D.K.; Haag, R. Controlled release of DNA from photoresponsive hyperbranched polyglycerols with oligoamine shells. Macromol. Biosci. 2011, 11, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.; Graeser, R.; Kratz, F.; Haag, R. Development of enzymatically cleavable prodrugs derived from dendritic polyglycerol. Bioorg. Med. Chem. Lett. 2009, 19, 3725–3728. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.; Welker, P.; Licha, K.; Fichtner, I.; Graeser, R.; Haag, R.; Kratz, F. Development of efficient acid cleavable multifunctional prodrugs derived from dendritic polyglycerol with a poly(ethylene glycol) shell. J. Control. Release 2011, 151, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Saito, K.; Lee, H.R.; Lee, M.J.; Shibasaki, Y.; Oishi, Y.; Kim, B.S. Hyperbranched double hydrophilic block copolymer micelles of poly(ethylene oxide) and polyglycerol for pH-responsive drug delivery. Biomacromolecules 2012, 13, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.F.; Kruger, H.R.; Kampmeier, F.; Weissbach, T.; Licha, K.; Kratz, F.; Haag, R.; Calderon, M.; Barth, S. Targeted delivery of dendritic polyglycerol-doxorubicin conjugates by scFV-SNAP fusion protein suppresses EGFR+ cancer cell growth. Biomacromolecules 2013, 14, 2510–2520. [Google Scholar] [CrossRef] [PubMed]
- Kruger, H.R.; Schutz, I.; Justies, A.; Licha, K.; Welker, P.; Haucke, V.; Calderon, M. Imaging of doxorubicin release from theranostic macromolecular prodrugs via fluorescence resonance energy transfer. J. Control. Release 2014, 194, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, Y.H.; Akasaka, T.; Abe, S.; Komatsu, N.; Watari, F.; Chen, X. Polyglycerol-coated nanodiamond as a macrophage-evading platform for selective drug delivery in cancer cells. Biomaterials 2014, 35, 5393–5406. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Letchford, K.; Heller, M.; Liggins, R.; Guan, D.; Kizhakkedathu, J.N.; Brooks, D.E.; Jackson, J.K.; Burt, H.M. Synthesis and characterization of carboxylic acid conjugated, hydrophobically derivatized, hyperbranched polyglycerols as nanoparticulate drug carriers for cisplatin. Biomacromolecules 2011, 12, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, Y.-H.; Qin, H.; Abe, S.; Akasaka, T.; Chano, T.; Watari, F.; Kimura, T.; Komatsu, N.; Chen, X. Platinum on nanodiamond: A promising prodrug conjugated with stealth polyglycerol, targeting peptide and acid-responsive antitumor drug. Adv. Funct. Mater. 2014, 24, 5348–5357. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Wang, R.; Zong, B.Y.; Tan, J.Y.; Kang, E.T. Methotrexate-conjugated and hyperbranched polyglycerol-grafted Fe3O4 magnetic nanoparticles for targeted anticancer effects. Eur. J. Pharm. Sci. 2013, 48, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Herves, A.; Wurfel, P.; Wegner, N.; Khandare, J.; Licha, K.; Haag, R.; Welker, P.; Calderon, M. Dendritic polyglycerol sulfate as a novel platform for paclitaxel delivery: Pitfalls of ester linkage. Nanoscale 2015, 7, 3923–3932. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Mugabe, C.; Burt, H.M.; Brooks, D.E. Unimolecular micelles based on hydrophobically derivatized hyperbranched polyglycerols: Ligand binding properties. Biomacromolecules 2008, 9, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Liggins, R.T.; Guan, D.; Manisali, I.; Chafeeva, I.; Brooks, D.E.; Heller, M.; Jackson, J.K.; Burt, H.M. Development and in vitro characterization of paclitaxel and docetaxel loaded into hydrophobically derivatized hyperbranched polyglycerols. Int. J. Pharm. 2011, 404, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Wu, Z.; Gao, X.; Cheng, C.; Wang, Z.; Li, C. A hydrotropic β-cyclodextrin grafted hyperbranched polyglycerol co-polymer for hydrophobic drug delivery. Acta Biomater. 2011, 7, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, X.; Li, H.; Ma, D.; Xue, W. Complex aggregates formed with a hyperbranched polyglycerol derivative for drug delivery. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Richter, A.; Wiedekind, A.; Krause, M.; Kissel, T.; Haag, R.; Olbrich, C. Non-ionic dendritic glycerol-based amphiphiles: Novel excipients for the solubilization of poorly water-soluble anticancer drug Sagopilone. Eur. J. Pharm. Sci. 2010, 40, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.; Shukla, A.; Alves Rodrigues, P.C.; Rehage, H.; Haag, R. Water-soluble dendritic core-shell-type architectures based on polyglycerol for solubilization of hydrophobic drugs. Chemistry 2007, 13, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Zhang, X.; Wang, Y.; Sun, L.; Li, C. Amphiphilic polylactic acid-hyperbranched polyglycerol nanoparticles as a controlled release system for poorly water-soluble drugs: Physicochemical characterization. J. Pharm. Pharmacol. 2011, 63, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Jin, X.; Li, L.; Lv, F.; Liu, T. OX26 modified hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) nanoparticles: Synthesis, characterization and evaluation of its brain delivery ability. J. Mater. Sci. Mater. Med. 2012, 23, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Jászberényi, Z.; Moriggi, L.; Schmidt, P.; Weidensteiner, C.; Kneuer, R.; Merbach, A.E.; Helm, L.; Tóth, E. Physicochemical and MRI characterization of Gd3+-loaded polyamidoamine and hyperbranched dendrimers. J. Biol. Inorg. Chem. 2007, 12, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, N.; Fattahi, H.; Laurent, S.; Burtea, C.; Elst, L.V.; Muller, R.N. Polyglycerol-grafted superparamagnetic iron oxide nanoparticles: Highly efficient MRI contrast agent for liver and kidney imaging and potential scaffold for cellular and molecular imaging. Contrast Media Mol. Imaging 2012, 7, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Ernenwein, D.; Shkumatov, A.; Clay, N.E.; Lee, J.Y.; Melhem, M.; Misra, S.; Zimmerman, S.C.; Kong, H. Hydrophilic packaging of iron oxide nanoclusters for highly sensitive imaging. Biomaterials 2015, 69, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, Y.; Graeser, R.; Warnecke, A.; Kratz, F.; Hauff, P.; Licha, K.; Haag, R. Development of pH-responsive core–shell nanocarriers for delivery of therapeutic and diagnostic agents. Bioorg. Med. Chem. Lett. 2009, 19, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Licha, K.; Welker, P.; Weinhart, M.; Wegner, N.; Kern, S.; Reichert, S.; Gemeinhardt, I.; Weissbach, C.; Ebert, B.; Haag, R.; et al. Fluorescence imaging with multifunctional polyglycerol sulfates: Novel polymeric near-IR probes targeting inflammation. Bioconj. Chem. 2011, 22, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Neoh, K.G.; En-Tang Kang, E.T.; Shuter, B. Multifunctional polyglycerol-grafted Fe3O4@SiO2 nanoparticles for targeting ovarian cancer cells. Biomaterials 2011, 32, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, K.; Soema, P.; Gelder, N.; Misri, R.; McPhee, K.; Baker, J.H.E.; Reinsberg, S.A.; Brooks, D.E.; Häfeli, U.O. Hyperbranched polyglycerols as trimodal imaging agents: Design, biocompatibility, and tumor uptake. Bioconj. Chem. 2012, 23, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, K.; Gelder, N.; Gershkovich, P.; Sivak, O.; Wasan, K.M.; Kainthan, R.K.; Brooks, D.E.; Hafeli, U.O. Long-circulating non-toxic blood pool imaging agent based on hyperbranched polyglycerols. Int. J. Pharm. 2012, 422, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Vonnemann, J.; Beziere, N.; Böttcher, C.; Riese, S.B.; Kuehne, C.; Dernedde, J.; Licha, K.; von Schacky, C.; Kosanke, Y.; Kimm, M.; et al. Polyglycerolsulfate functionalized gold nanorods as optoacoustic signal nanoamplifiers for in vivo bioimaging of rheumatoid arthritis. Theranostics 2014, 4, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.N.; Soares, D.A.W.; de Queiroz, A.A.A. Low potential stable glucose detection at dendrimers modified polyaniline nanotubes. Mater. Res. 2010, 13, 5–10. [Google Scholar] [CrossRef]
- Basinska, T.; Wisniewska, M.; Chmiela, M. Principle of a new immunoassay based on electrophoretic mobility of poly(styrene/α-tert-butoxy-ω-vinylbenzyl-polyglycidol) microspheres: Application for the determination of Helicobacter pylori IgG in blood serum. Macromol. Biosci. 2005, 5, 70–77. [Google Scholar] [CrossRef] [PubMed]




| Encapsulator bioactive compound | Carrier | Method of encapsulation | Reference |
|---|---|---|---|
| Proteins | |||
| BSA | Polylactide-hyperbranched polyglycidol nanoparticles | Nanoprecipitation | [68,69] |
| Insulin | β-cyclodextrin labeled polyglycidol nanoparticles | Coprecipitation | [70] |
| Insulin | β-cyclodextrin labeled polyglycidol hydrogel | Gelation | [71] |
| Asparaginase | Nanogels from dendritic polyglycidols with azide and -p-propargyloxy-benzacetale | Inverse nanoprecipitation | [72] |
| transglutaminase 1 | Poly(N-isopropylacrylamide)-polyglycerol-based nanogels | Nanogel swelling in protein solution | [73] |
| Nucleic acids | |||
| DNA | Quarternized a poly(glycidol-co-ethylene oxide) | Nanoprecipitation | [74] |
| pDNA | Quarternized branched poly(glycidol-co-2,3-epoxypropyldiethyl-amine) and DNA polyplexes | Incubation of polymer and nucleic acid solution | [75] |
| siRNA | Polyplexes of corona aminated hyperbranched polyglycidol and siRNA | Incubation of polymer and nucleic acid solution | [76] |
| siRNA | Polyplexes of hyperbranched polyglycidol modified with glycine and siRNA | Incubation of polymer and nucleic acid solution | [77] |
| siRNA | Polyplexes of amphiphilic copolymer containing hyperbranched polyglycidol end-capped with amines and siRNA | Incubation of polymer and nucleic acid solution | [78] |
| pDNA | Polyplexes of adamantane-modified hyperbranched polyglycerol end-capped with cationic β-cyclodextrin and pDNA | Incubation of polymer and nucleic acid solution | [79] |
| pDNA | Polyplexes of polyglycidol-pluronic-poly-glycidol with 2-(N,N-dimethylaminomethyl)-5-aminomethyl phenylboronic acid groups and pDNA | Incubation of polymer and nucleic acid solution | [80] |
| pDNA | Polyplexes of poly(ethylene oxide)-(branched polyglycidol) with endgroups capped with tris(2-aminoethyl)amine) | Incubation of polymer and nucleic acid solution | [81] |
| pDNA | nanodiamond particles with immobilized polyglycidol modified with grafted poly(arg-co-lys-co-his) | Incubation of nanocarrier and nucleic acid solution | [82] |
| pDNA and doxorubicin hydrochloride | Vesicles composed of cyclodextrin labeled with branched polyglycidol end-capped with tris(2-aminoethyl)amine) and bearing aliphatic chains | Nanoprecipitation | [83] |
| DNA Lamin A/C) and 5′-CTGGACTTCCAGAAGAACATT-3− | Polyplexes of hyperbranched polyglycidol modified by addition of oligoamines via the photo-cleavable linkages | Incubation of polymer and nucleic acid solution | [84] |
| Anticancer drugs | |||
| Doxorubicin | Hyperbranched polyglycidol | Covalent immobilization via the enzymatically cleavable linkage | [85] |
| Doxorubicin | Hyperbranched polyglycidol with grafted poly(ethylene oxide) | Covalent immobilization via the enzymatically cleavable linkage | [86] |
| Doxorubicin | Poly(ethylene oxide)-[hyperbranched polyglycidol] | Covalent immobilization via pH sensitive hydrazone linkage | [87] |
| Doxorubicin | Hyperbranched polyglycidol with grafted poly(ethylene oxide) and targeting antibodies agains epidermal growth factor | Covalent immobilization via pH sensitive hydrazone linkage | [88] |
| Doxorubicin, indodicarbocyanine dye | Hyperbranched polyglycidol | Covalent immobilization of drug and dye via cleavable linkage. Monitoring of drug release by fluorescence | [89] |
| Doxorubicin | Nanodiamond particles with grafted hyperbranched polyglycidol end-capped with RGD tripeptide | Covalent immobilization via pH sensitive hydrazone linkage | [90] |
| Cisplatin | Hyperbranched polyglycidol modified with succinic anhydride | Covalent immobilization via carboxyl groups | [91] |
| Cisplatin | Nanodiamond particles with grafted hyperbranched polyglycidol end-capped with RGD tripeptide and COOH groups | Covalent immobilization via carboxyl groups | [92] |
| Methotrexate | Fe3O4 nanoparticles with immobilized hyperbranched polyglycidol | Covalent immobilization via pH sensitive hydrazone linkage | [93] |
| Paclitaxel | Hyperbranched polyglycidol with sulfate and amine end groups | Covalent immobilization via cleavable ester linkage | [94] |
| Paclitaxel | Hyperbranched polyglycidol with attached poly(ethylene oxide) and alkyl chains | Solubilization of hydrophobic drug | [95,96] |
| Paclitaxel | Polymeric micelles of hyperbranched polyglycidol labeled with β-cyclodextrin | Nanoprecipitation | [97] |
| Docetaxel | Hyperbranched polyglycidol with attached poly(ethylene oxide) and alkyl chains | Solubilization of hydrophobic drug | [96] |
| Docetaxel | Hyperbranched polyglycidol with attached alkyl chains | Nanoprecipitation | [98] |
| Sagopilone | Hyperbranched polyglycidol with attached alkyl chains | Entrapment during micellization | [99] |
| Miscellaneous | |||
| Nimodipine | Hyperbranched polyglycidol with biphenyl groups in the core | Solubilization of hydrophobic drug | [100] |
| Quercetin | Polylactide-(hyperbranched polyglycidol) | Nanoprecipitation | [101] |
| Endomorphins | Hyperbrached polyglycidol modified by addition of poly(lactide-co-glycolide) chains | Water-in oil-in water double emulsification | [102] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosecki, M.; Gadzinowski, M.; Gosecka, M.; Basinska, T.; Slomkowski, S. Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers—Synthesis and Medical Applications. Polymers 2016, 8, 227. https://doi.org/10.3390/polym8060227
Gosecki M, Gadzinowski M, Gosecka M, Basinska T, Slomkowski S. Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers—Synthesis and Medical Applications. Polymers. 2016; 8(6):227. https://doi.org/10.3390/polym8060227
Chicago/Turabian StyleGosecki, Mateusz, Mariusz Gadzinowski, Monika Gosecka, Teresa Basinska, and Stanislaw Slomkowski. 2016. "Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers—Synthesis and Medical Applications" Polymers 8, no. 6: 227. https://doi.org/10.3390/polym8060227
APA StyleGosecki, M., Gadzinowski, M., Gosecka, M., Basinska, T., & Slomkowski, S. (2016). Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers—Synthesis and Medical Applications. Polymers, 8(6), 227. https://doi.org/10.3390/polym8060227