Enhancement of Carbon Nanotube Particle Distribution in PPS/PEEK/Carbon Nanotube Ternary Composites with Sausage-Like Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Samples
2.3. Characterization Techniques
2.3.1. Contact Angle Examinations
2.3.2. Thermal Characterization
2.3.3. Measurement of Electrical Conductivity
2.3.4. Morphology Examination
3. Results and Discussion
3.1. Theoretical Calculation of Interface Affinity between MWCNT Particles and Blend Components
| Liquid | (N·m−1) | (N·m−1) | (N·m−1) |
|---|---|---|---|
| H2O | 72.8 | 21.8 | 51.0 |
| CH2I2 | 50.8 | 48.5 | 2.3 |
| Components | γ at 20 °C (mN·m−1) | –dγ/dT (mN·m−1) | γ at 360 °C (mN·m−1) | γd at 360 °C (mN·m−1) | γp at 360 °C (mN·m−1) | χp |
|---|---|---|---|---|---|---|
| PPS (25 °C) | 46.99 | 0.070 | 21.08 | 20.92 | 0.16 | 0.0076 |
| PEEK (25 °C) | 48.87 | 0.073 | 21.93 | 17.80 | 4.13 | 0.19 |
| MWCNTs | 27.8 | 0 | 27.8 | 17.6 | 10.2 | 0.37 |
| Component Couple | Interfacial Tension | |
|---|---|---|
| Harmonic Mean Equation (mn·m−1) | Geometric Mean Equation (mn·m−1) | |
| PPS-PEEK | 3.93 | 2.79 |
| PPS-CNTs | 10.02 | 7.95 |
| PEEK-CNTs | 4.16 | 1.35 |
3.2. Effects of Blending Process

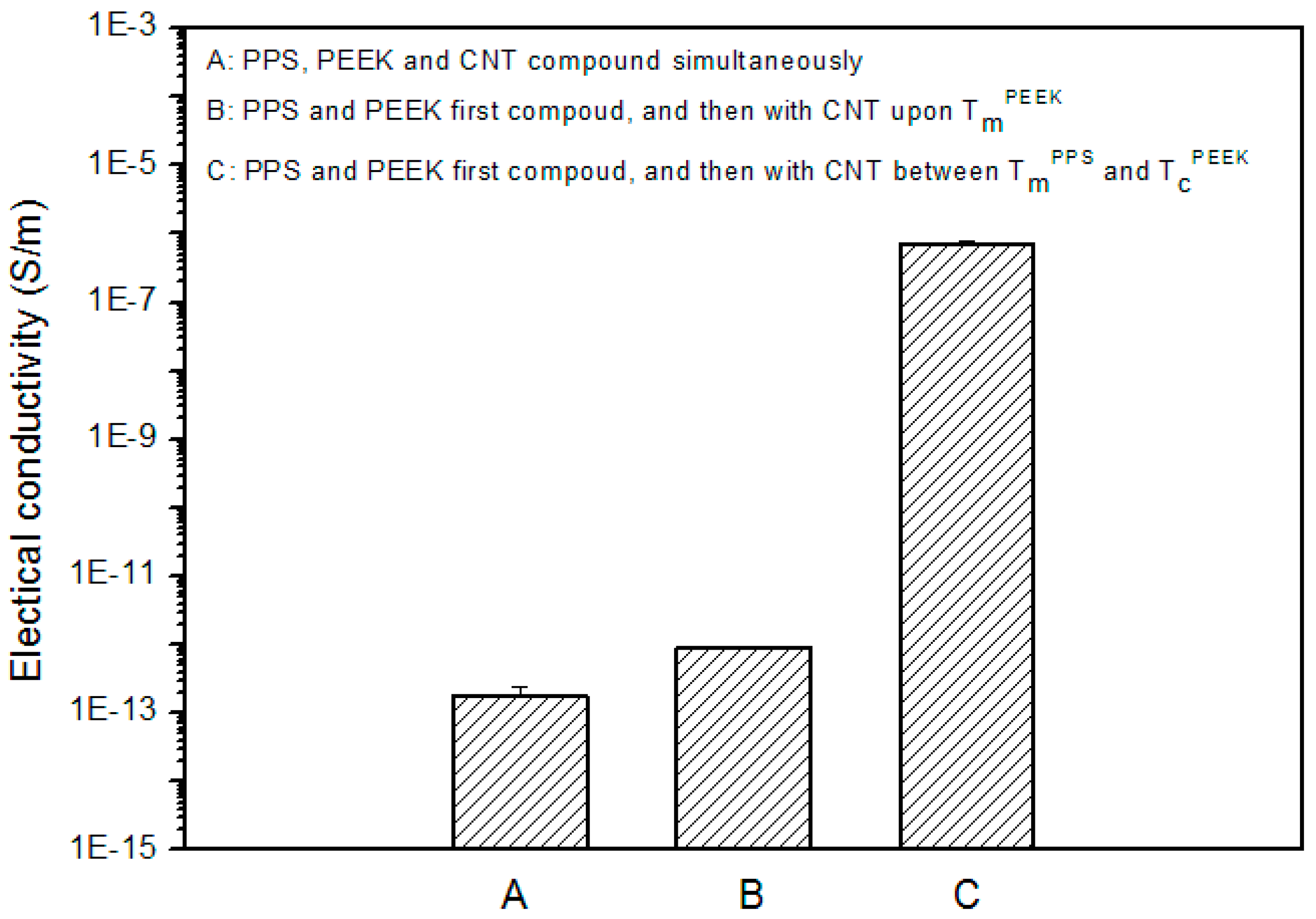
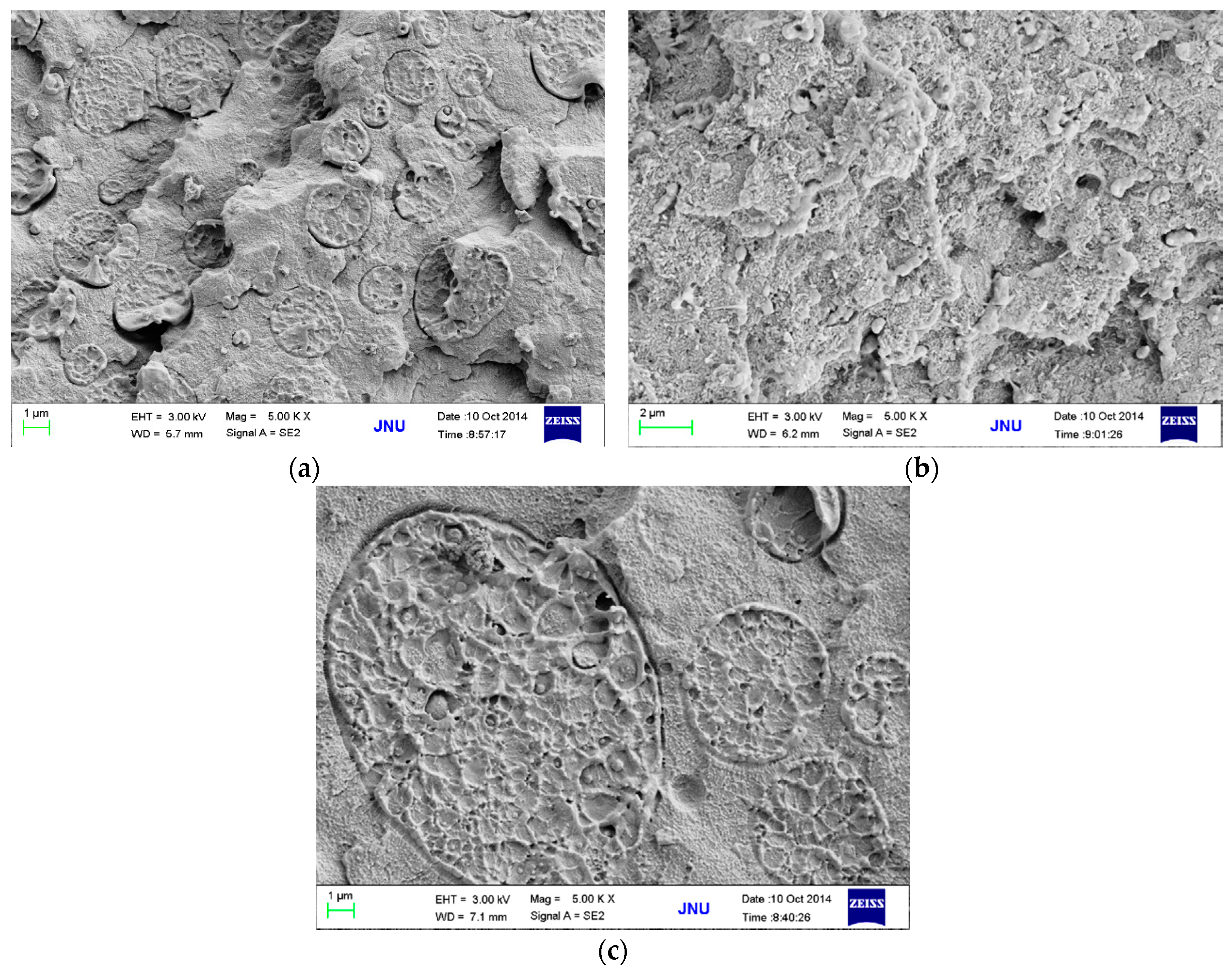
3.3. Effects of MWCNT Content on Electrical Conductivity and Morphology
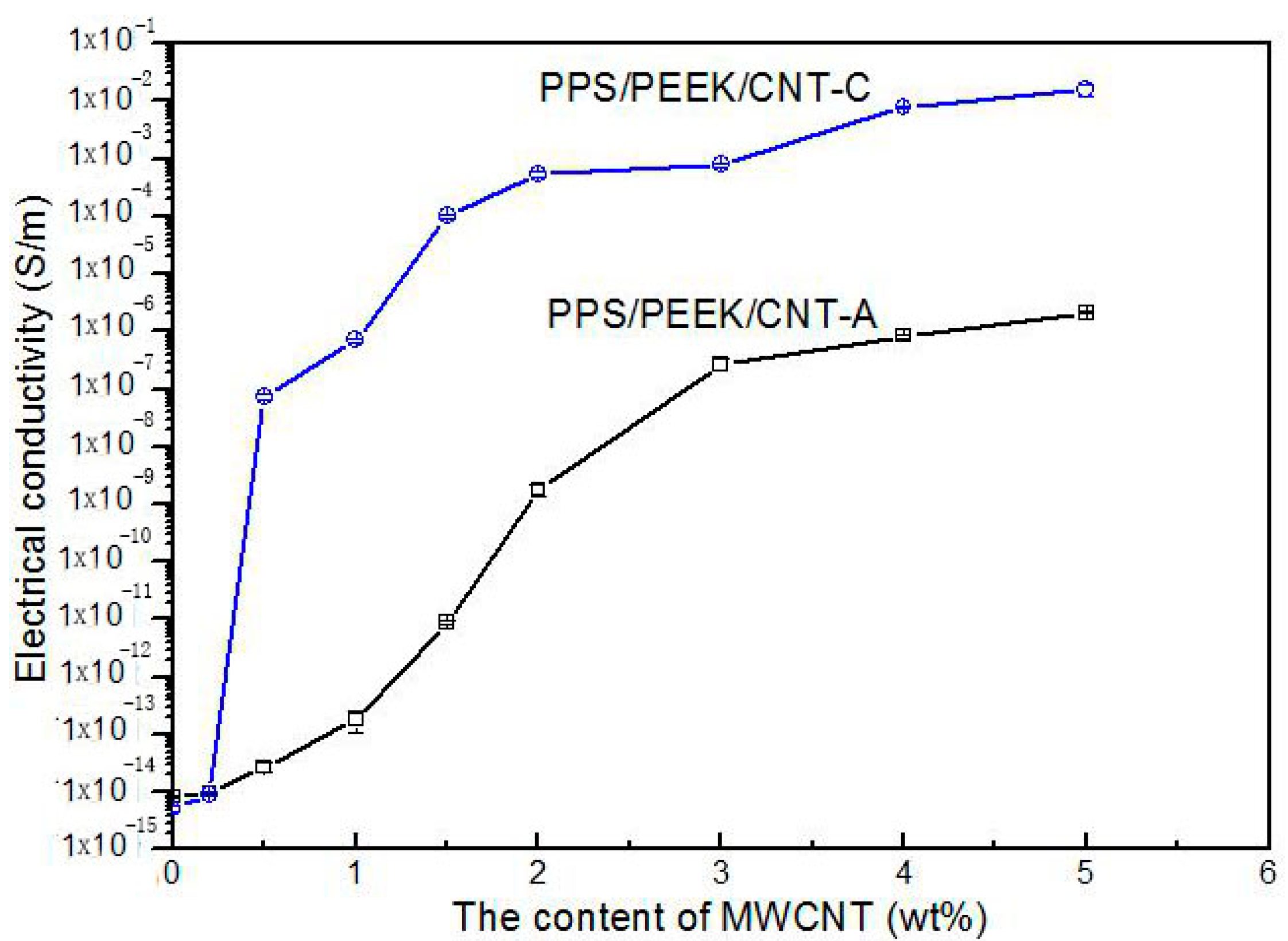

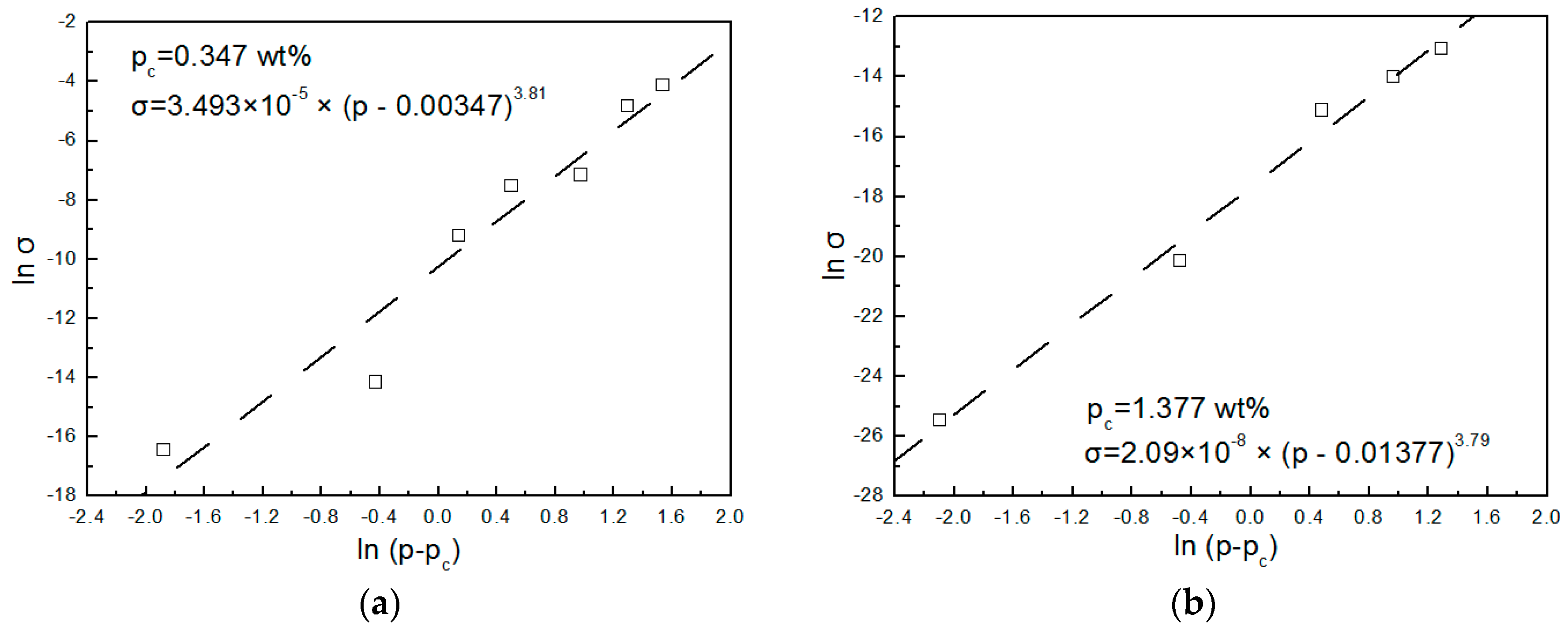
3.4. Stability of Products and Repetition of Processing
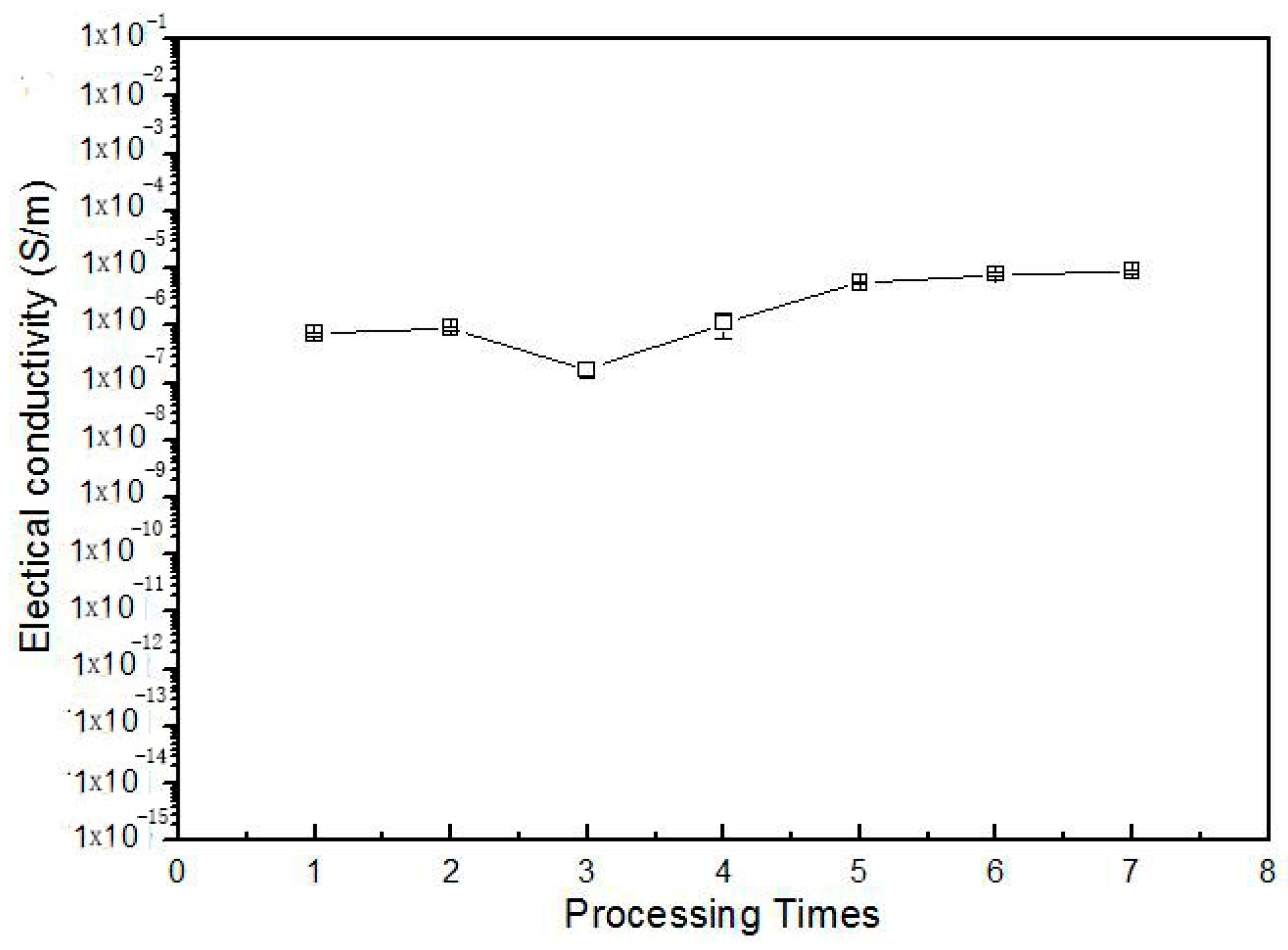
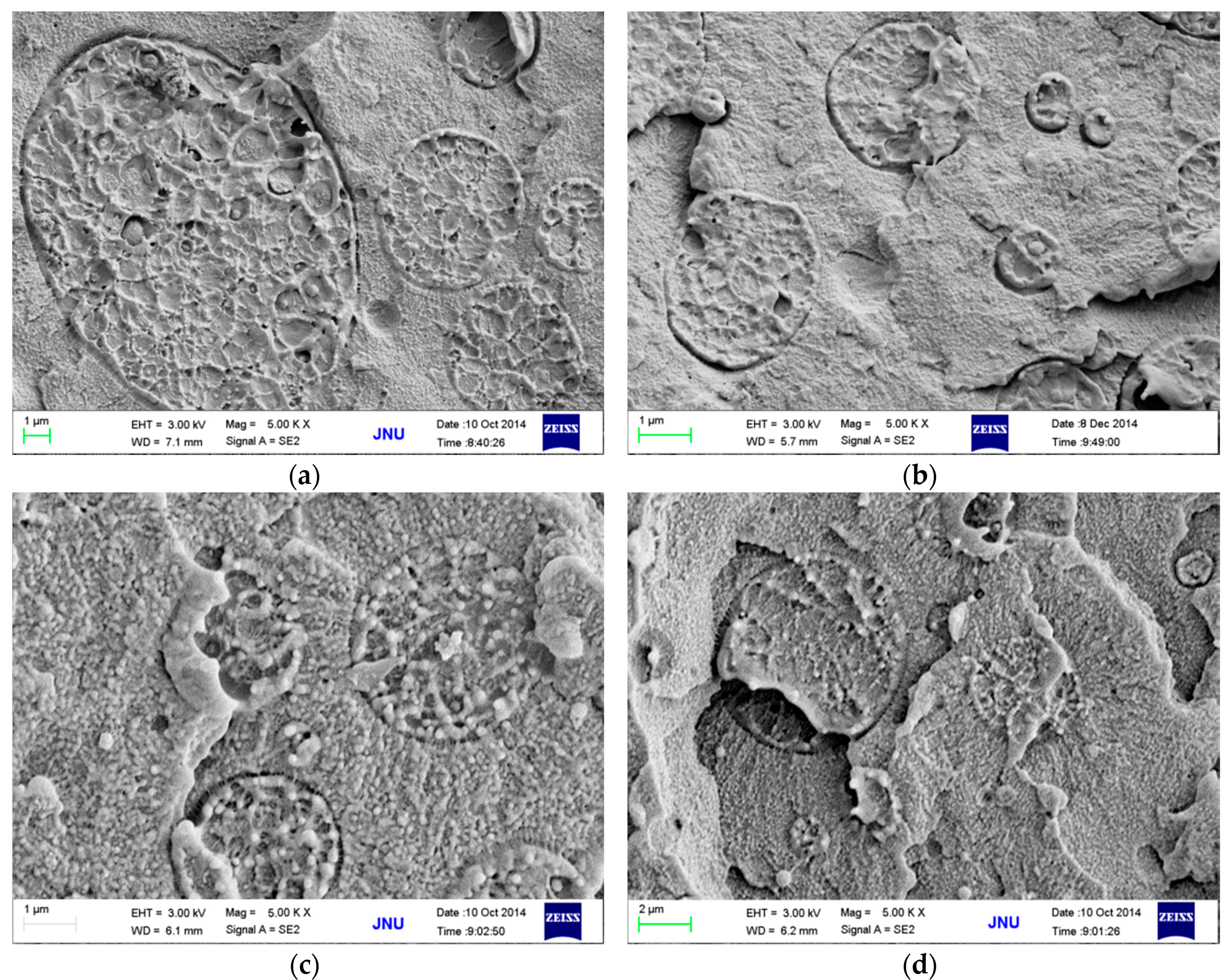
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, J.R.; Mao, C.; Zhu, Y.T.; Jiang, W.; Yang, X.D. Control of carbon nanotubes at the interface of a co-continuous immiscible polymer blend to fabricate conductive composites with ultralow percolation thresholds. Carbon 2014, 73, 267–274. [Google Scholar] [CrossRef]
- Li, Y.J.; Shimizu, H. Conductive PVDF/PA6/CNTs nanocomposites fabricated by dual formation of cocontinuous and nanodispersion structures. Macromolecules 2008, 41, 5339–5344. [Google Scholar] [CrossRef]
- Pinnavaia, T.G.; Beall, G.W. Polymer-Clay Nanocomposites; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Okamoto, K.; Sinha Ray, S.; Okamoto, M. New poly(butylene succinate)/layered silicate nanocomposites. II. Effect of organically modified layered silicates on structure, properties, melt rheology, and biodegradability. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Ponomarenko, A.T.; Shevchenko, V.G.; Enikolopyan, N.S. Formation processes and properties of conducting polymer composites. Adv. Polym. Sci. 1990, 96, 125–147. [Google Scholar]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Konishi, Y.; Cakmak, A. Nanoparticle induced network self-assembly in polymer–carbon black composites. Polymer 2006, 47, 5371–5391. [Google Scholar] [CrossRef]
- Xi, Y.; Bin, Y.Z.; Chiang, C.K.; Matsuo, M. Dielectric effects on positive temperature coefficient composites of polyethylene and short carbon fibers. Carbon 2007, 45, 1302–1309. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Bousmina, M. Compatibilization efficiency of organoclay in an immiscible polycarbonate/poly(methyl methacrylate) blend. Macromol. Rapid Commun. 2005, 26, 450–455. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Fu, Q. Front cover: Schematic illustration of biomimetic CaCO3 synthesis based on urease-catalyzed urea hydrolysis exclusively inside a polyelectrolyte capsule microreactor. Confocal transmission microscopy image of polyelectrolyte capsules before and after CaCO3 formation are shown at the bottom. Macromol. Rapid Commun. 2003, 24, 231–235. [Google Scholar]
- Li, Y.J.; Shimizu, H. Co-continuous Polyamide 6 (PA6)/Acrylonitrile-Butadiene-Styrene (ABS) Nanocomposites. Macromol. Rapid Commun. 2005, 26, 710–715. [Google Scholar] [CrossRef]
- Mehrabzadeh, M.; Kamal, M.R. Polymer-clay nanocomposites based on blends of polyamide-6 and polyethylene. Can. J. Chem. Eng. 2002, 80, 1083–1092. [Google Scholar] [CrossRef]
- Gubbels, F.; Blacher, S.; Vanlathem, E.; Jérôme, R.; Deltour, R.; Brouers, F.; Teyssie, P. Design of electrical composites: Determining the role of the morphology on the electrical properties of carbon black filled polymer blends. Macromolecules 1995, 28, 1559–1566. [Google Scholar] [CrossRef]
- Göldel, A.; Marmur, A.; Kasaliwal, G.R.; Pötschke, P.; Heinrich, G. Shape-dependent localization of carbon nanotubes and carbon black in an immiscible polymer blend during melt mixing. Macromolecules 2011, 44, 6094–6102. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Zhang, R.C.; Xu, Y.; Lu, Z.; Min, M.; Gao, Y.; Huang, Y.; Lu, A. Investigation on the crystallization behavior of poly(ether ether ketone)/poly(phenylene sulfide) blends. J. Appl. Polym. Sci. 2008, 108, 1829–1836. [Google Scholar] [CrossRef]
- Deng, S.; Lin, Z.; Cao, L.; Xian, J.; Liu, C. PPS/recycled PEEK/carbon nanotube composites: Structure, properties and compatibility. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Static and dynamic wetting measurements of single carbon nanotubes. Phys. Rev. Lett. 2004, 92, 186103. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.H.; Cohen, S.R.; Kenig, S.; Wagner, H.D. Interfacial fracture energy measurements for multi-walled carbon nanotubes pulled from a polymer matrix. Compos. Sci. Technol. 2004, 64, 2283–2289. [Google Scholar] [CrossRef]
- Yan, D.; Li, X.; Ma, H.L.; Tang, X.Z.; Zhang, Z.; Yu, Z.Z. Effect of compounding sequence on localization of carbon nanotubes and electrical properties of ternary nanocomposites. Compos. A Appl. Sci. Manuf. 2013, 49, 35–41. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wang, Z.; Sun, N.; Li, H. Enhancement of electrical conductivity by changing phase morphology for composites consisting of polylactide and poly(ε-caprolactone) filled with acid-oxidized multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.Y.; Yan, D.; Jiang, Z.; Cao, Y.K.; Yu, Z.Z.; Yavari, F.; Koratkar, N. Enhanced electrical conductivity in polystyrene nanocomposites at ultra-low graphene content. ACS Appl. Mater. Interfaces 2011, 3, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.K.; Khatua, B.B. Development of electrical conductivity with minimum possible percolation threshold in multi-wall carbon nanotube/polystyrene composites. Carbon 2011, 49, 4571–4579. [Google Scholar] [CrossRef]
- Lux, F. Models proposed to explain the electrical conductivity of mixtures made of conductive and insulating materials. J. Mater. Sci. 1993, 28, 285–301. [Google Scholar] [CrossRef]
- Laredo, E.; Prutsky, N.; Bello, A.; Grimau, M.; Castillo, R.V.; Müller, A.J.; Dubois, P. Miscibility in poly(l-lactide)-b-poly(ε-upvarepsilon-caprolactone) double crystalline diblock copolymers. Eur. Phys. J. E 2007, 23, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Tailor & Francis: London, UK; Washington, DC, USA, 1994. [Google Scholar]
- Bhagat, N.A.; Shrivastava, N.K.; Suin, S.; Maiti, S.; Khatua, B.B. Development of electrical conductivity in PP/HDPE/MWCNT nanocomposite by melt mixing at very low loading of MWCNT. Polym. Compos. 2013, 34, 787–798. [Google Scholar] [CrossRef]
- Calberg, C.; Blacher, S.; Gubbels, F.; Brouers, F.; Deltour, R.; Jérôme, R. Electrical and dielectric properties of carbon black filled co-continuous two-phase polymer blends. J. Phys. D Appl. Phys. 1999, 32, 1517. [Google Scholar] [CrossRef]
- Logakis, E.; Pollatos, E.; Pandis, C.; Peoglos, V.; Zuburtikudis, I.; Delides, C.G.; Pissis, P. Structure–property relationships in isotactic polypropylene/multi-walled carbon nanotubes nanocomposites. Compos. Sci. Technol. 2010, 70, 328–335. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Deng, S.; Lin, Z. Enhancement of Carbon Nanotube Particle Distribution in PPS/PEEK/Carbon Nanotube Ternary Composites with Sausage-Like Structure. Polymers 2016, 8, 50. https://doi.org/10.3390/polym8020050
Cao L, Deng S, Lin Z. Enhancement of Carbon Nanotube Particle Distribution in PPS/PEEK/Carbon Nanotube Ternary Composites with Sausage-Like Structure. Polymers. 2016; 8(2):50. https://doi.org/10.3390/polym8020050
Chicago/Turabian StyleCao, Lin, Shuling Deng, and Zhidan Lin. 2016. "Enhancement of Carbon Nanotube Particle Distribution in PPS/PEEK/Carbon Nanotube Ternary Composites with Sausage-Like Structure" Polymers 8, no. 2: 50. https://doi.org/10.3390/polym8020050
APA StyleCao, L., Deng, S., & Lin, Z. (2016). Enhancement of Carbon Nanotube Particle Distribution in PPS/PEEK/Carbon Nanotube Ternary Composites with Sausage-Like Structure. Polymers, 8(2), 50. https://doi.org/10.3390/polym8020050







