Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Copolymer PLGA and P(LA-co-GA-co-MMD)
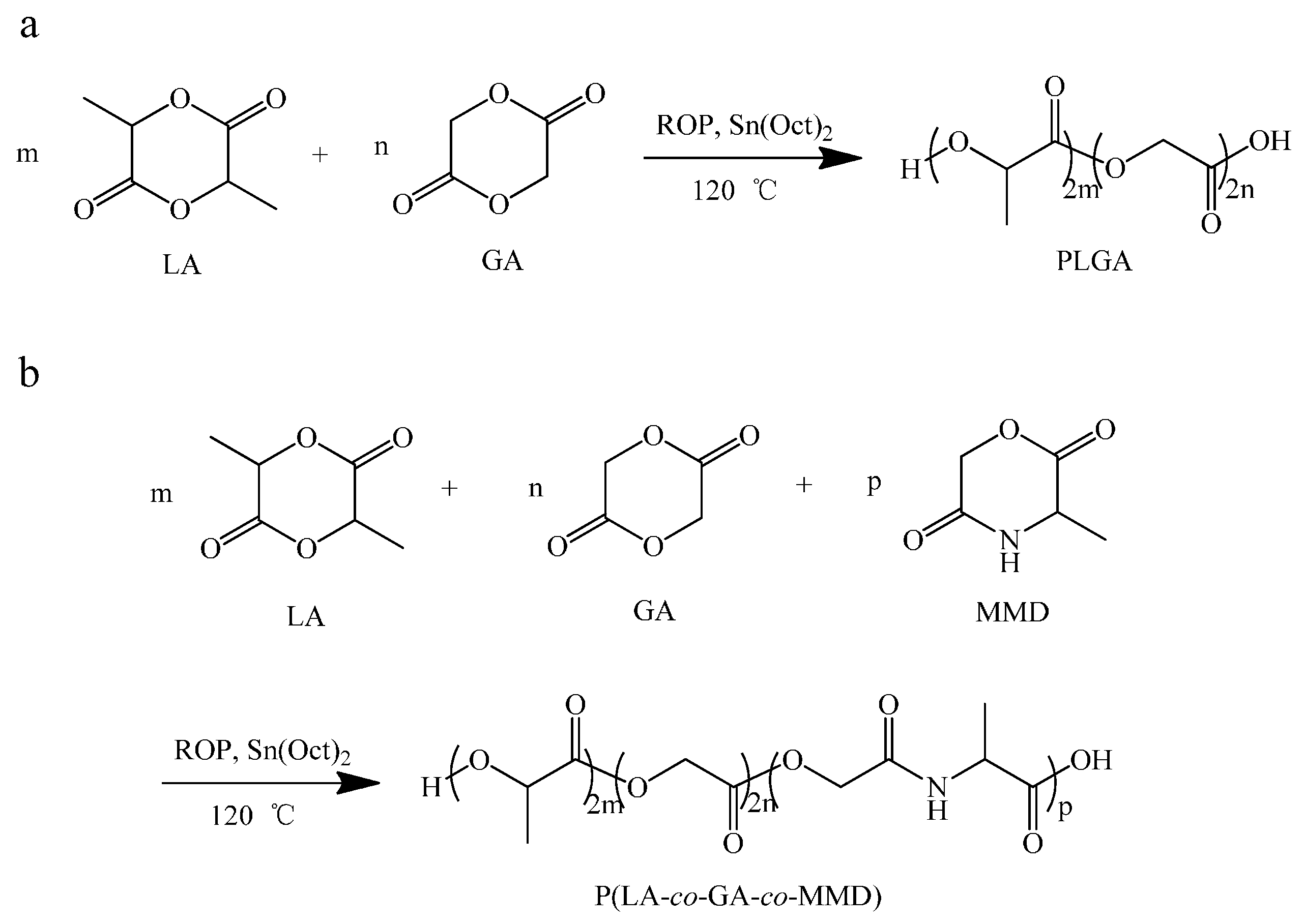
2.2.1. Synthesis of Copolymer PLGA
2.2.2. Synthesis of P(LA-co-GA-co-MMD) Copolymers
| Sample ID | Weight Content a/% | Yield wt % | Mn b/104 | Mw b/104 | PDI b | ||
|---|---|---|---|---|---|---|---|
| LA | GA | MMD | |||||
| PLGA | 70.1 | 29.9 | 0 | 80.3 | 5.19 | 11.89 | 2.29 |
| P(LA-co-GA-co-MMD)1 | 70.3 | 24.2 | 5.5 | 82.1 | 5.74 | 13.35 | 2.33 |
| P(LA-co-GA-co-MMD)2 | 66.1 | 20.9 | 13.0 | 78.4 | 4.67 | 10.88 | 2.33 |
| P(LA-co-GA-co-MMD)3 | 60.9 | 21.7 | 17.4 | 76.8 | 4.08 | 9.44 | 2.32 |
2.3. Preparation of Spinning Solution
2.4. Fabrication of the Electrospun Scaffolds
2.5. Characterization
2.6. In Vitro Cell Culture Experiment
2.7. Cell Seeding and Morphology Observation
2.8. MTT Assay
2.9. Subcutaneous Implantation
3. Results and Discussion
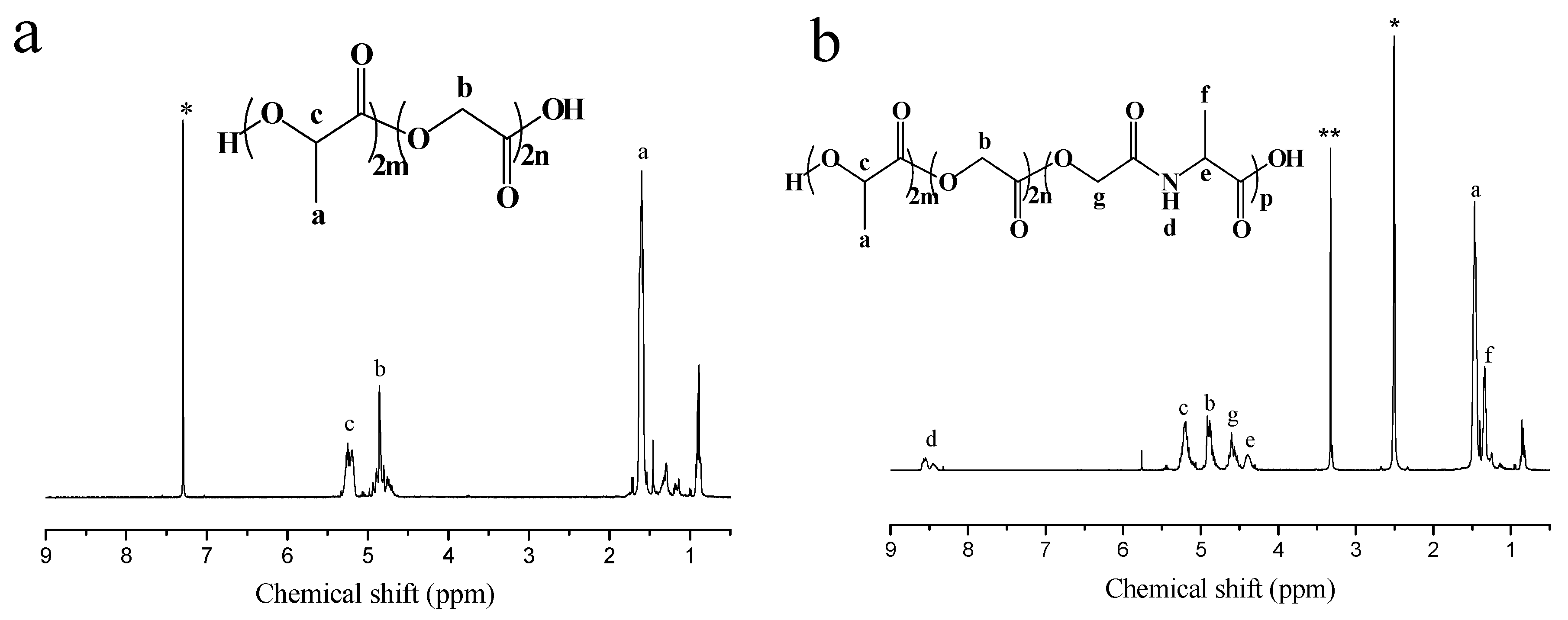
3.1. Synthesis of P(LA-co-GA-co-MMD) Copolymers

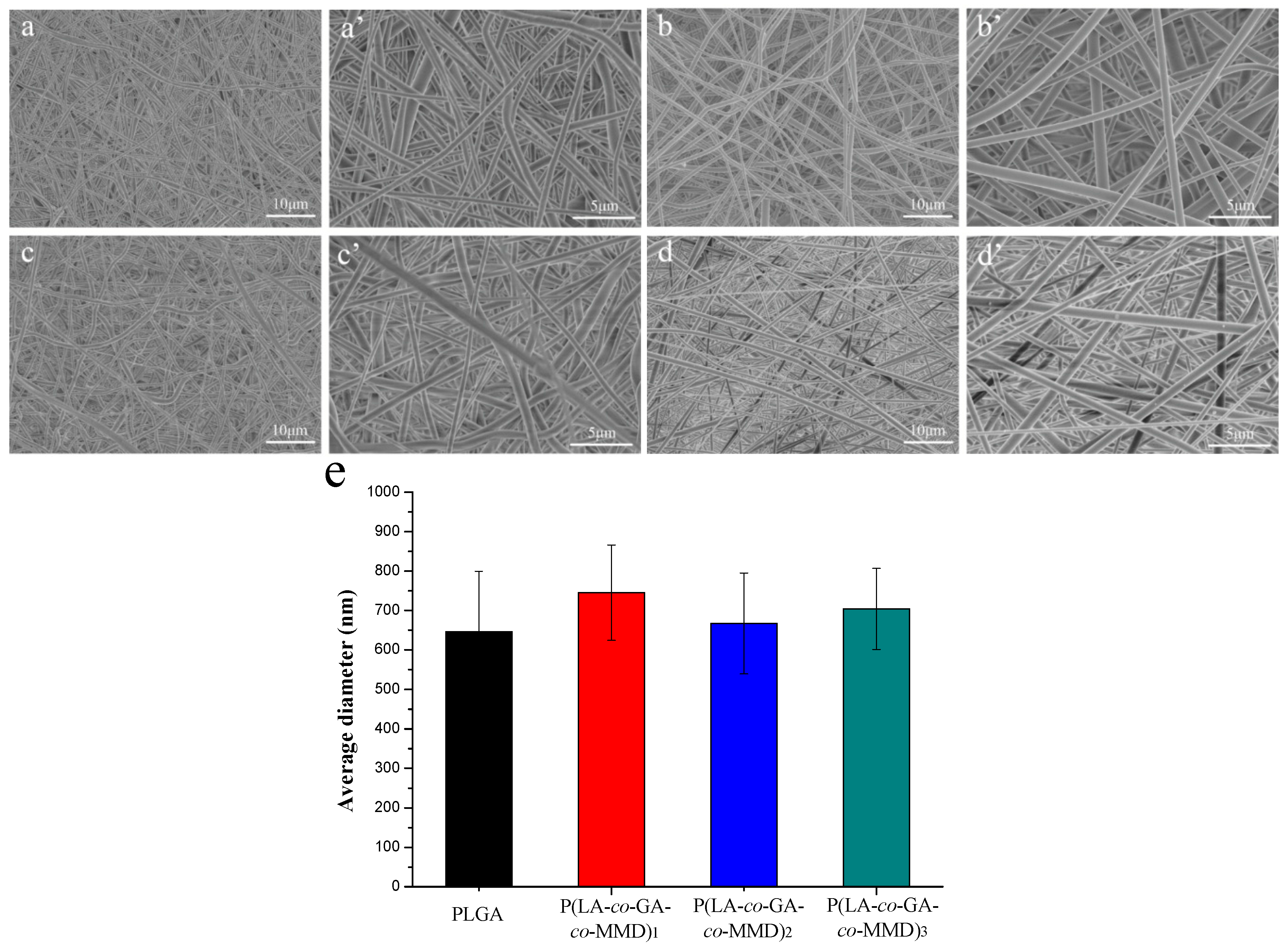
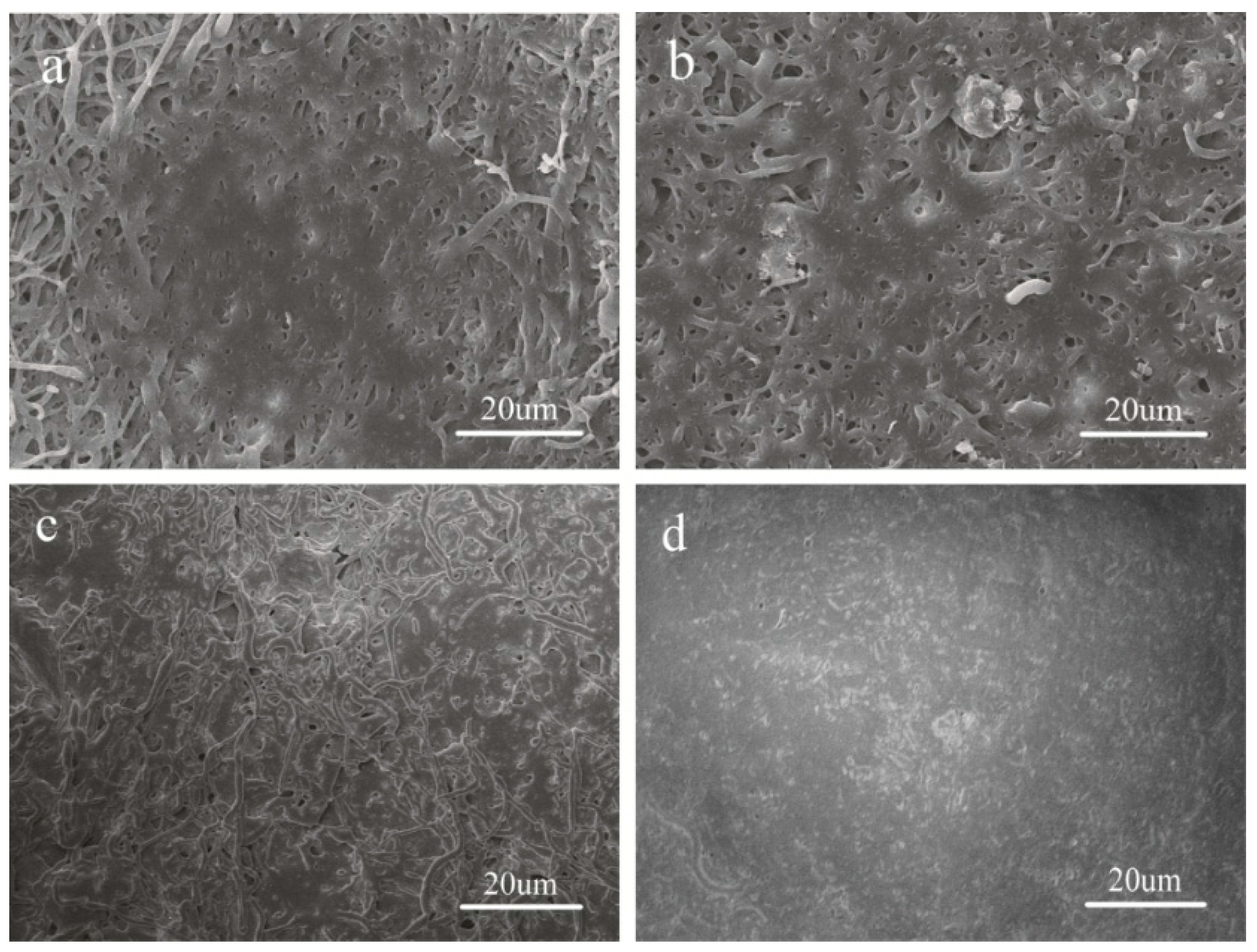
3.2. Morphology of PLGA and P(LA-co-GA-co-MMD) Scaffolds
3.3. Mechanical Properties of PLGA and P(LA-co-GA-co-MMD) Scaffolds
| Sample ID | Tensile Strength (MPa) | Elongation at Break (%) | ||
|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |
| PLGA | 2.89 ± 0.31 | 2.20 ± 0.12 | 84 ± 5 | 114 ± 20 |
| P(LA-co-GA-co-MMD)1 | 2.63 ± 0.20 | 2.46 ± 0.27 | 105 ± 28 | 242 ± 34 |
| P(LA-co-GA-co-MMD)2 | 2.66 ± 0.07 | 1.89 ± 0.46 | 109 ± 25 | 363 ± 32 |
| P(LA-co-GA-co-MMD)3 | 3.23 ± 0.61 | 2.20 ± 0.68 | 117 ± 9 | 559 ± 82 |
3.4. In Vitro Degradation of PLGA and P(LA-co-GA-co-MMD) Scaffolds
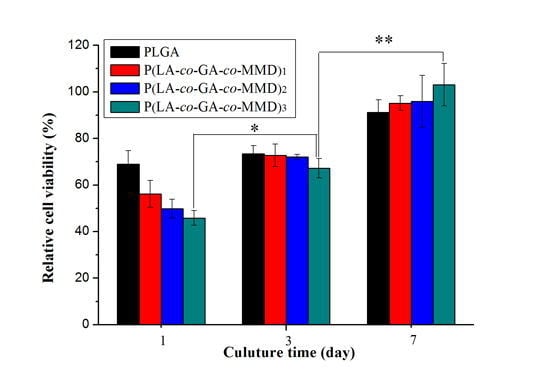
3.5. Proliferation of HUVECs on PLGA and P(LA-co-GA-co-MMD) Scaffolds

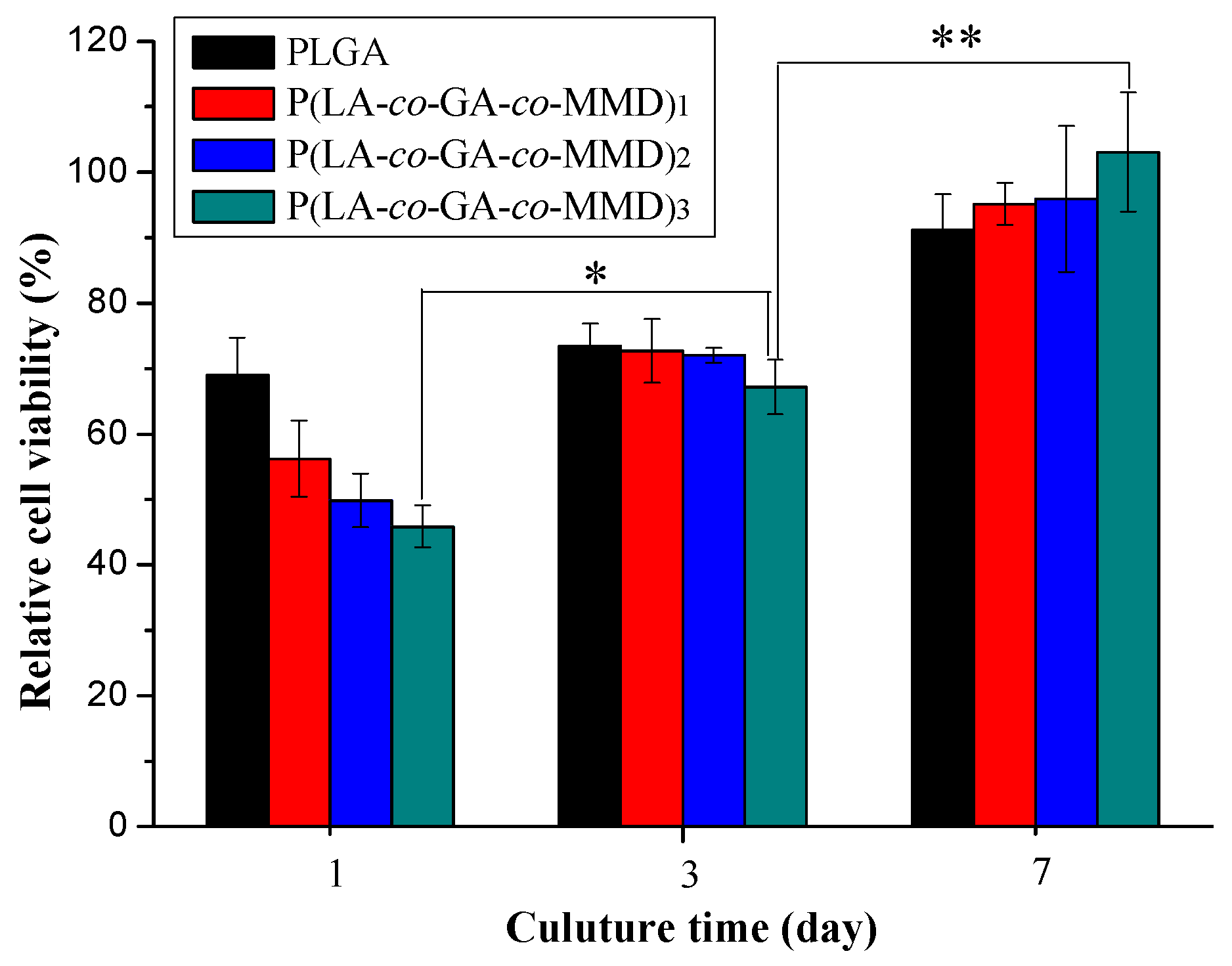
3.6. Tissue Response of Scaffold in Subcutaneous Implantation
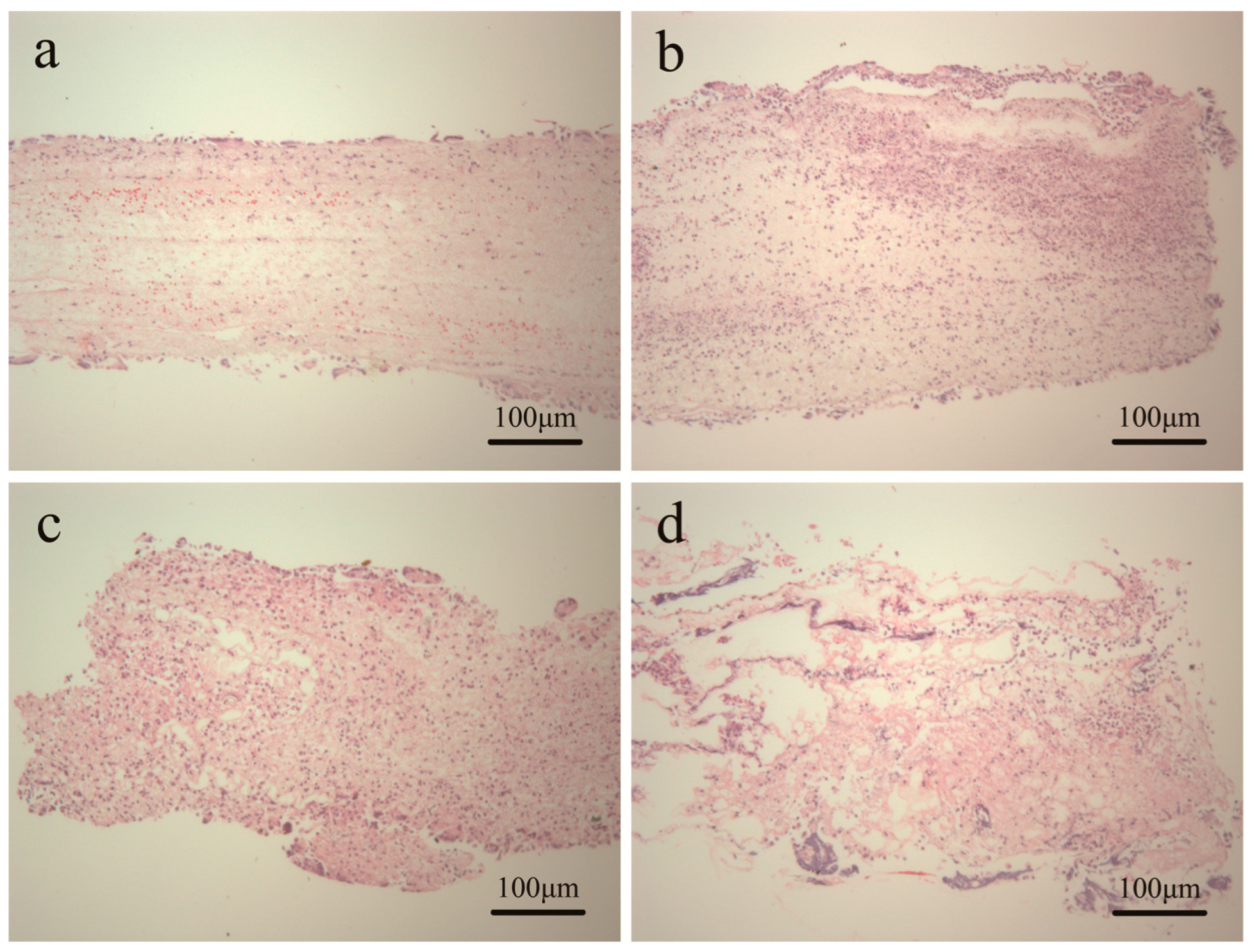
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Feng, Y.; Zhao, H.; Xiao, R.; Lu, J.; Zhang, L.; Guo, J. Electrospun hemocompatible PU/gelatin-heparin nanofibrous bilayer scaffolds as potential artificial blood vessels. Macromol. Res. 2012, 20, 347–350. [Google Scholar] [CrossRef]
- Lovett, M.; Cannizzaro, C.; Daheron, L.; Messmer, B.; Vunjak-Novakovic, G.; Kaplan, D.L. Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007, 28, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Degradation behaviors of electrospun resorbable polyester nanofibers. Tissue Eng. B Rev. 2009, 15, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano-and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Feng, Y.; Yang, J.; Fan, J.; Lv, J.; Zhang, L.; Guo, J.; Ren, X.; Zhang, W. Electrospun scaffolds of silk fibroin and poly(lactide-co-glycolide) for endothelial cell growth. J. Mater. Sci. Mater. Med. 2015, 26, 5386. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Feng, Y.; Li, Q.; Hao, X.; Liu, W.; Zhou, W.; Shi, C.; Ren, X.; Zhang, W. PLGA/SF blend scaffolds modified with plasmid complexes for enhancing proliferation of endothelial cells. React. Funct. Polym. 2015, 91–92, 19–27. [Google Scholar] [CrossRef]
- Nain, A.S.; Wong, J.C.; Amon, C.; Sitti, M. Drawing suspended polymer micro-/nanofibers using glass micropipettes. Appl. Phys. Lett. 2006, 89, 183105. [Google Scholar] [CrossRef]
- Tao, S.L.; Desai, T.A. Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, L.; Wei, G.; Won, Y.; Ma, P.X. Surface engineering of nano-fibrous poly (l-lactic acid) scaffolds via self-assembly technique for bone tissue engineering. J. Biomed. Nanotechnol. 2005, 1, 54–60. [Google Scholar] [CrossRef]
- Paramonov, S.E.; Jun, H.W.; Hartgerink, J.D. Self-assembly of peptide-amphiphile nanofibers: The roles of hydrogen bonding and amphiphilic packing. J. Am. Chem. Soc. 2006, 128, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. I. Stability theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. II. Applications. Phys. Fluids 2001, 13, 2221–2236. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001, 89, 3018. [Google Scholar] [CrossRef]
- Khan, M.; Yang, J.; Shi, C.; Feng, Y.; Zhang, W.; Gibney, K.; Tew, G.N. Surface modification of polycarbonate urethane with zwitterionic polynorbornene via thiol-ene click-reactionto facilitate cell growth and proliferation. Macromol. Mater. Eng. 2015, 300, 802–809. [Google Scholar] [CrossRef]
- Khan, M.; Yang, J.; Shi, C.; Lv, J.; Feng, Y.; Zhang, W. Surface tailoring for selective endothelialization and platelet inhibition via a combination of SI-ATRP and click chemistry using Cys-Al-Gly-peptide. Acta Biomater. 2015, 20, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lv, J.; Gao, B.; Zhang, L.; Yang, D.; Shi, C.; Guo, J.; Li, W.; Feng, Y. Modification of polycarbonateurethane surface with poly(ethylene glycol) monoacrylate and phosphorylcholine glyceraldehyde for anti-platelet adhesion. Front. Chem. Sci. Eng. 2014, 8, 188–196. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, W.; Khan, M.; Li, Q.; Feng, Y.; Yao, F.; Zhang, W. Hydrophilic PCU scaffolds prepared by grafting PEGMA and immobilizing gelatin to enhance cell adhesion and proliferation. Mater. Sci. Eng. C 2015, 50, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.O.; Aid-Launais, R.; Chew, S.Y.; Le Visage, C. Polysaccharide electrospun fibers with sulfated poly(fucose) promote endothelial cell migration and VEGF-mediated angiogenesis. Biomater. Sci. 2014, 2, 843–852. [Google Scholar] [CrossRef]
- Vatankhah, E.; Semnani, D.; Prabhakaran, M.P.; Tadayon, M.; Razavi, S.; Ramakrishna, S. Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta Biomater. 2014, 10, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Kai, D.; Ramakrishna, S. Biocompatibility evaluation of protein-incorporated electrospun polyurethane-based scaffolds with smooth muscle cells for vascular tissue engineering. J. Mater. Sci. 2013, 48, 5113–5124. [Google Scholar] [CrossRef]
- Wang, G.; Hu, X.; Lin, W.; Dong, C.; Wu, H. Electrospun PLGA-silk fibroin-collagen nanofibrous scaffolds for nerve tissue engineering. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Y.; Yang, J.; Guo, J.; Zhang, W. Targeting REDV peptide functionalized polycationic gene carrier for enhancing the transfection and migration capability of human endothelial cells. J. Mater. Chem. B 2015, 3, 3379–3391. [Google Scholar] [CrossRef]
- Aghdam, R.M.; Najarian, S.; Shakhesi, S.; Khanlari, S.; Shaabani, K.; Sharifi, S. Investigating the effect of PGA on physical and mechanical properties of electrospun PCL/PGA blend nanofibers. J. Appl. Polym. Sci. 2012, 124, 123–131. [Google Scholar] [CrossRef]
- Hao, X.; Li, Q.; Lv, J.; Yu, L.; Ren, X.; Zhang, L.; Feng, Y.; Zhang, W. CREDVW-linked polymeric micelles as a targeting gene transfer vector for selective transfection and proliferation of endothelial cells. ACS Appl. Mater. Interfaces 2015, 7, 12128–12140. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W.E.; Anderson, J.M.; van den Beucken, J.; Jansen, J.A. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials 2012, 33, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Li, H.F.; Sun, Z.Z.; Zheng, W.; Zheng, Y.F. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2011, 12, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, P.; Stride, E.; Edirisinghe, M. Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm. Res. 2013, 30, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lu, J.; Behl, M.; Lendlein, A. Progress in depsipeptide-based biomaterials. Macromol. Biosci. 2010, 10, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Behl, M.; Kelch, S.; Lendlein, A. Biodegradable multiblock copolymers based on oligodepsipeptides with shape-memory properties. Macromol. Biosci. 2009, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Y.; Tian, H.; Shi, C.; Zhao, M.; Guo, J. Controlled release of doxorubicin from amphiphilic depsipeptide-PDO-PEG-based copolymer nanosized microspheres. React. Funct. Polym. 2013, 73, 1281–1289. [Google Scholar] [CrossRef]
- Helder, J.; Kohn, F.E.; Sato, S.; van den Berg, J.W.; Feijen, J. Synthesis of poly[oxyethylidenecarbonylimino (2-oxoethylene)][poly(glycine-d,l-lactic acid)] by ring opening polymerization. Die Makromol. Chem. Rapid Commun. 1985, 6, 9–14. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, J. Biodegradable polydepsipeptides. Int. J. Mol. Sci. 2009, 10, 589–615. [Google Scholar] [CrossRef] [PubMed]
- Abayasinghe, N.K.; Perera, K.P.U.; Thomas, C.; Daly, A.; Suresh, S.; Burg, K.; Harrison, G.M.; Smith, D.W. Amido-modified polylactide for potential tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Detrembleur, C.; Jérôme, R. Novel aliphatic polyesters based on functional cyclic (di)esters. Macromol. Rapid Commun. 2003, 24, 161–172. [Google Scholar] [CrossRef]
- Feng, Y.; Klee, D.; Keul, H.; Höcker, H. Lipase-catalyzed ring-opening polymerization of morpholine-2,5-dione derivatives: A novel route to the synthesis of poly(ester amide)s. Macromol. Chem. Phys. 2000, 201, 2670–2675. [Google Scholar] [CrossRef]
- Feng, Y.; Klee, D.; Höcker, H. Lipase-catalyzed ring-opening polymerization of 6(S)-methyl-morpholine-2,5-dione. J. Polym. Sci. A Polym. Chem. 2005, 43, 3030–3039. [Google Scholar] [CrossRef]
- Shi, C.; Yao, F.; Huang, J.; Han, G.; Li, Q.; Khan, M.; Feng, Y.; Zhang, W. Proliferation and migration of human vascular endothelial cells mediated by ZNF580 gene complexed with mPEG-b-P(MMD-co-GA)-g-PEI microparticles. J. Mater. Chem. B 2014, 2, 1825–1837. [Google Scholar] [CrossRef]
- Hägglund, B.; Sandberg, G. Effect of l-alanine and some other amino acids on thymocyte proliferation in vivo. Immunobiology 1993, 188, 62–69. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S.; Sabzalian, M.R. Studies on synthesis and in vitro biodegradability of novel optically active nanostructure poly(ester-imide)s containing l-phenylalanine and l-isoleucine linkages. Colloid Polym. Sci. 2011, 289, 93–100. [Google Scholar] [CrossRef]
- Deshmukh, M.; Singh, Y.; Gunaseelan, S.; Gao, D.; Stein, S.; Sinko, P.J. Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010, 31, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, C.; Zhang, W.; Behl, M.; Lendlein, A.; Feng, Y. Nanoparticles complexed with gene vectors to promote proliferation of human vascular endothelial cells. Adv. Healthc. Mater. 2015, 4, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Y.; Tian, H.; Zhao, M.; Khan, M.; Guo, J. Amphiphilic depsipeptide-based block copolymers as nanocarriers for controlled release of ibuprofen with doxorubicin. J. Polym. Sci. A Polym. Chem. 2013, 51, 3213–3226. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Khan, M.; Ren, X.; Guo, J.; Feng, Y. Biodegradable depsipeptide-PDO-PEG-based block copolymer micelles as nanocarriers for controlled release of doxorubicin. React. Funct. Polym. 2014, 82, 89–97. [Google Scholar] [CrossRef]
- Shi, C.; Yao, F.; Li, Q.; Khan, M.; Ren, X.; Feng, Y.; Huang, J.; Zhang, W. Regulation of the endothelialization by human vascular endothelial cells by ZNF580 gene complexed with biodegradable microparticles. Biomaterials 2014, 35, 7133–7145. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, C.; Zhang, L.; Tian, H.; Yuan, W. Synthesis and characterization of novel copolymers based on 3(S)-methyl-morpholine-2,5-dione. Trans. Tianjin Univ. 2012, 18, 315–319. [Google Scholar] [CrossRef]
- Yao, J.; Pantano, M.F.; Pugno, N.M.; Bastiaansen, C.W.; Peijs, T. High-performance electrospun co-polyimide nanofibers. Polymer 2015, 76, 105–112. [Google Scholar] [CrossRef]
- He, W.; Yong, T.; Teo, W.E.; Ma, Z.; Ramakrishna, S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: Potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005, 11, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on PLLA/PBLG/collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Lu, W.; Ren, X.; Liu, W.; Guo, M.; Ullah, I.; Zhang, W. Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering. Polymers 2016, 8, 13. https://doi.org/10.3390/polym8020013
Feng Y, Lu W, Ren X, Liu W, Guo M, Ullah I, Zhang W. Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering. Polymers. 2016; 8(2):13. https://doi.org/10.3390/polym8020013
Chicago/Turabian StyleFeng, Yakai, Wei Lu, Xiangkui Ren, Wen Liu, Mengyang Guo, Ihsan Ullah, and Wencheng Zhang. 2016. "Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering" Polymers 8, no. 2: 13. https://doi.org/10.3390/polym8020013
APA StyleFeng, Y., Lu, W., Ren, X., Liu, W., Guo, M., Ullah, I., & Zhang, W. (2016). Electrospun Poly(lactide-co-glycolide-co-3(S)-methyl-morpholine-2,5-dione) Nanofibrous Scaffolds for Tissue Engineering. Polymers, 8(2), 13. https://doi.org/10.3390/polym8020013