Temperature-, pH- and CO2-Sensitive Poly(N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization of PNIPAAm-co-PAA Copolymers in Aqueous Solutions and Films
3. Results and Discussion
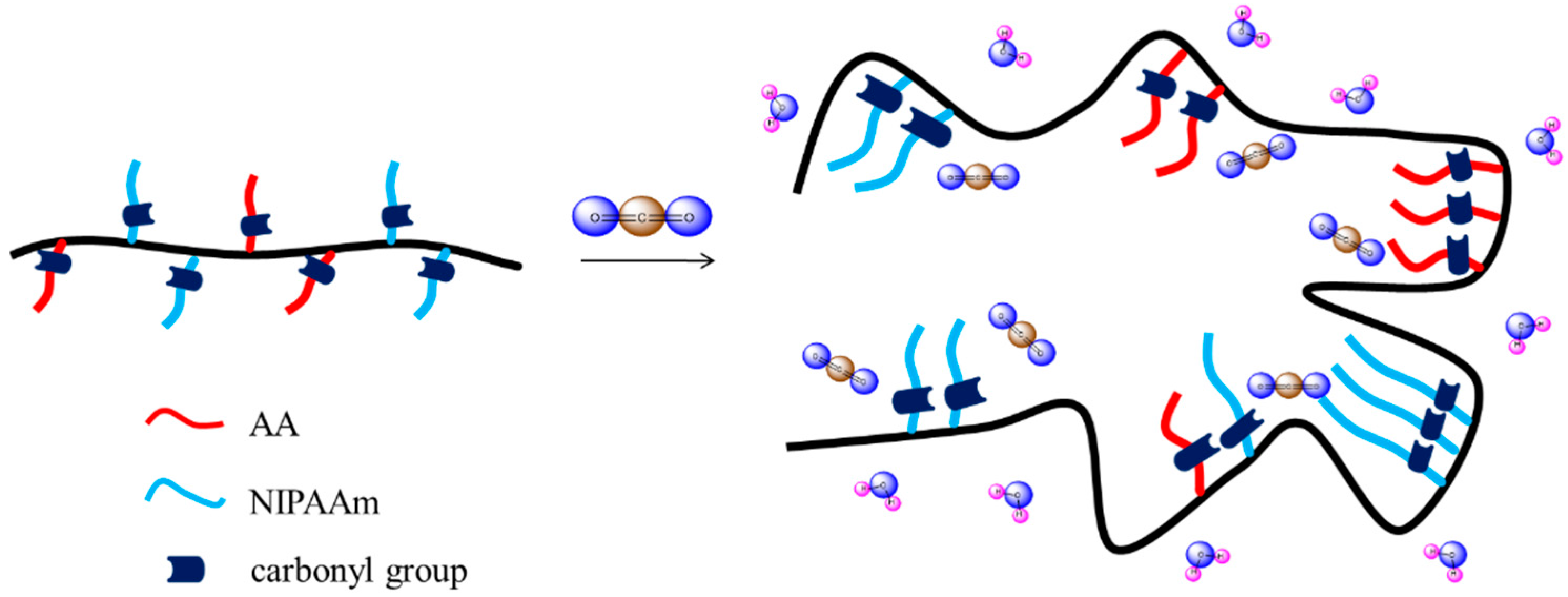
3.1. Synthesis of PNIPAAm-co-PAA Random Copolymers
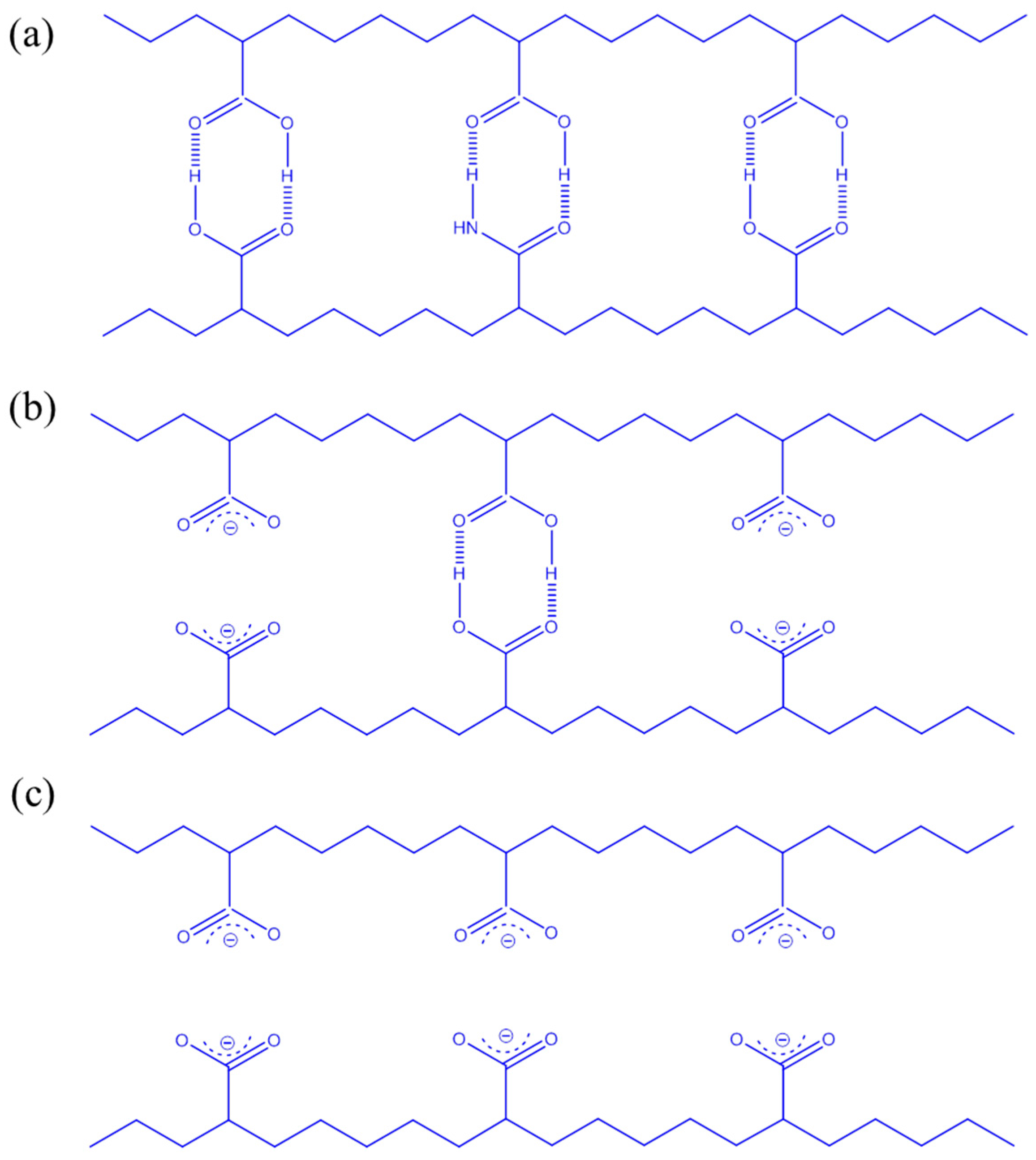
3.2. Thermal Properties of PNIPAAm-co-PAA Random Copolymers
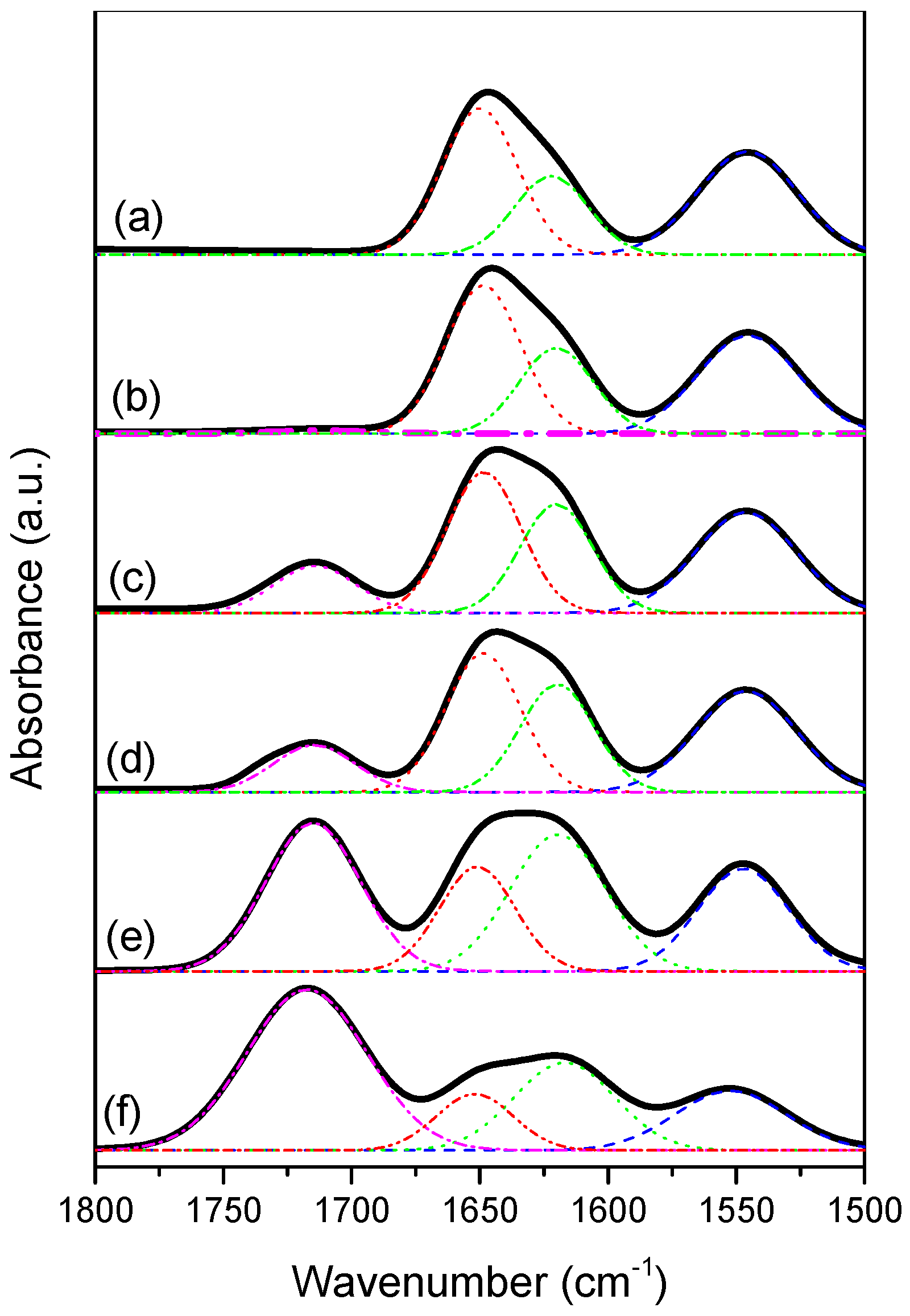
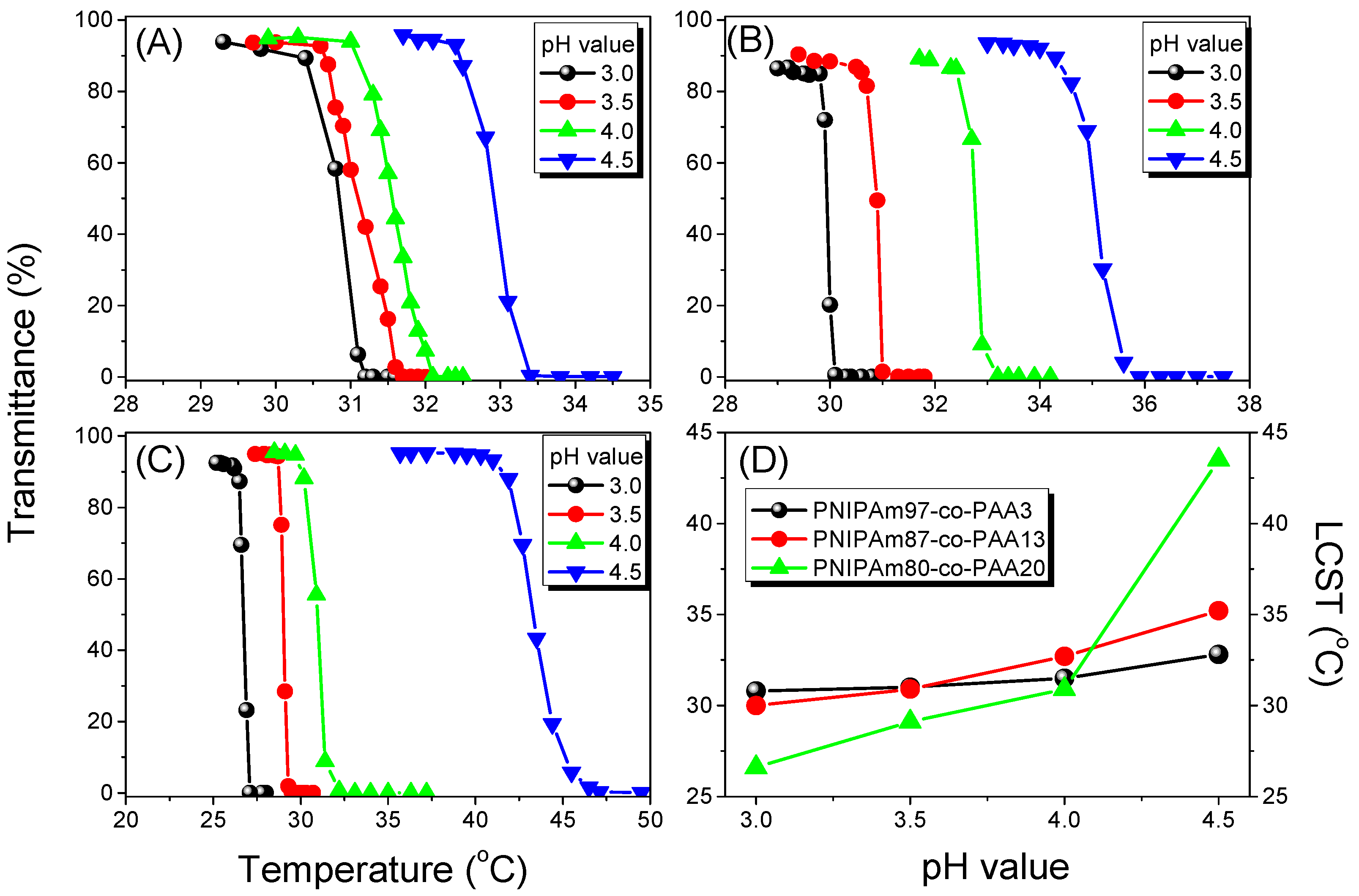
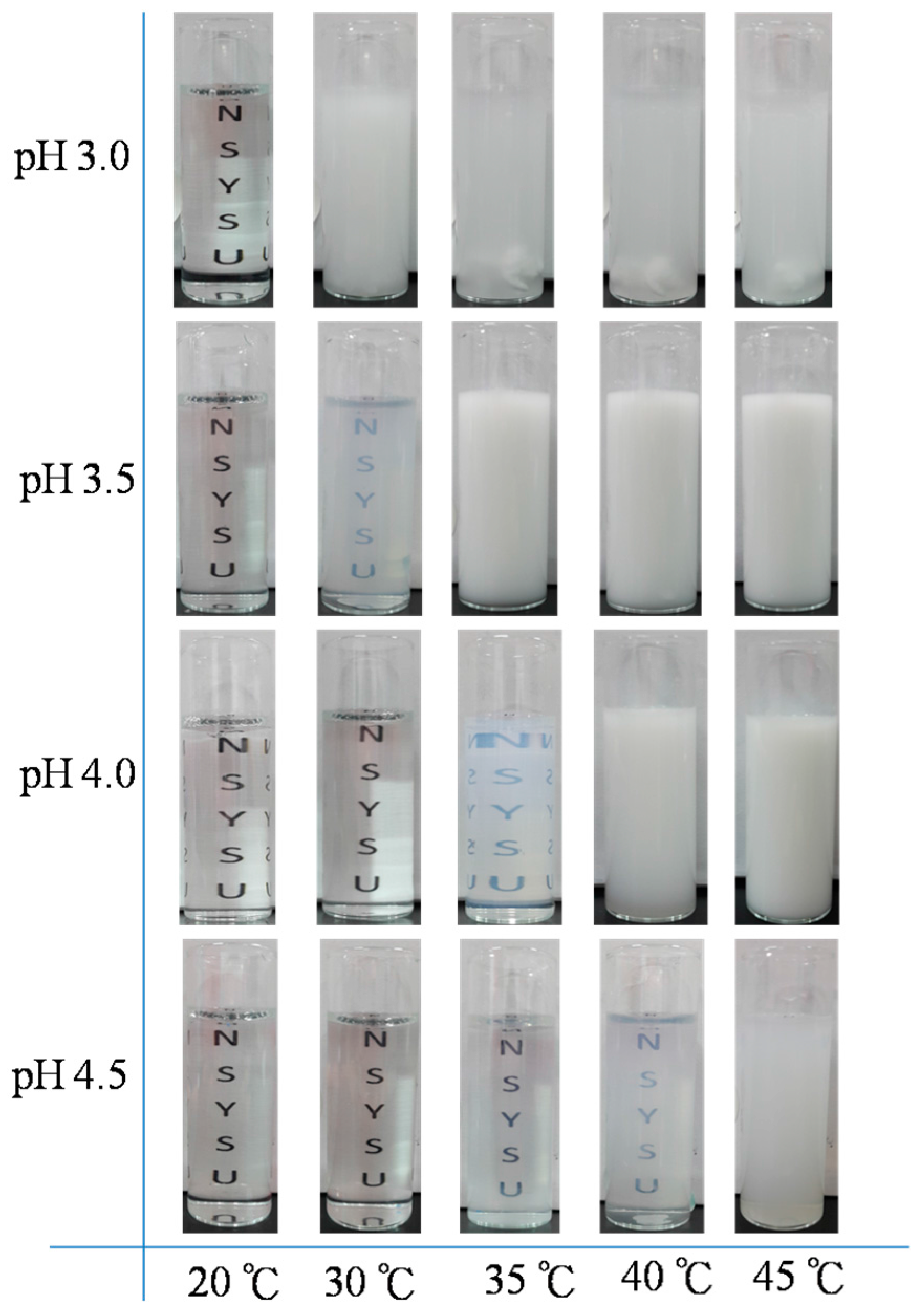
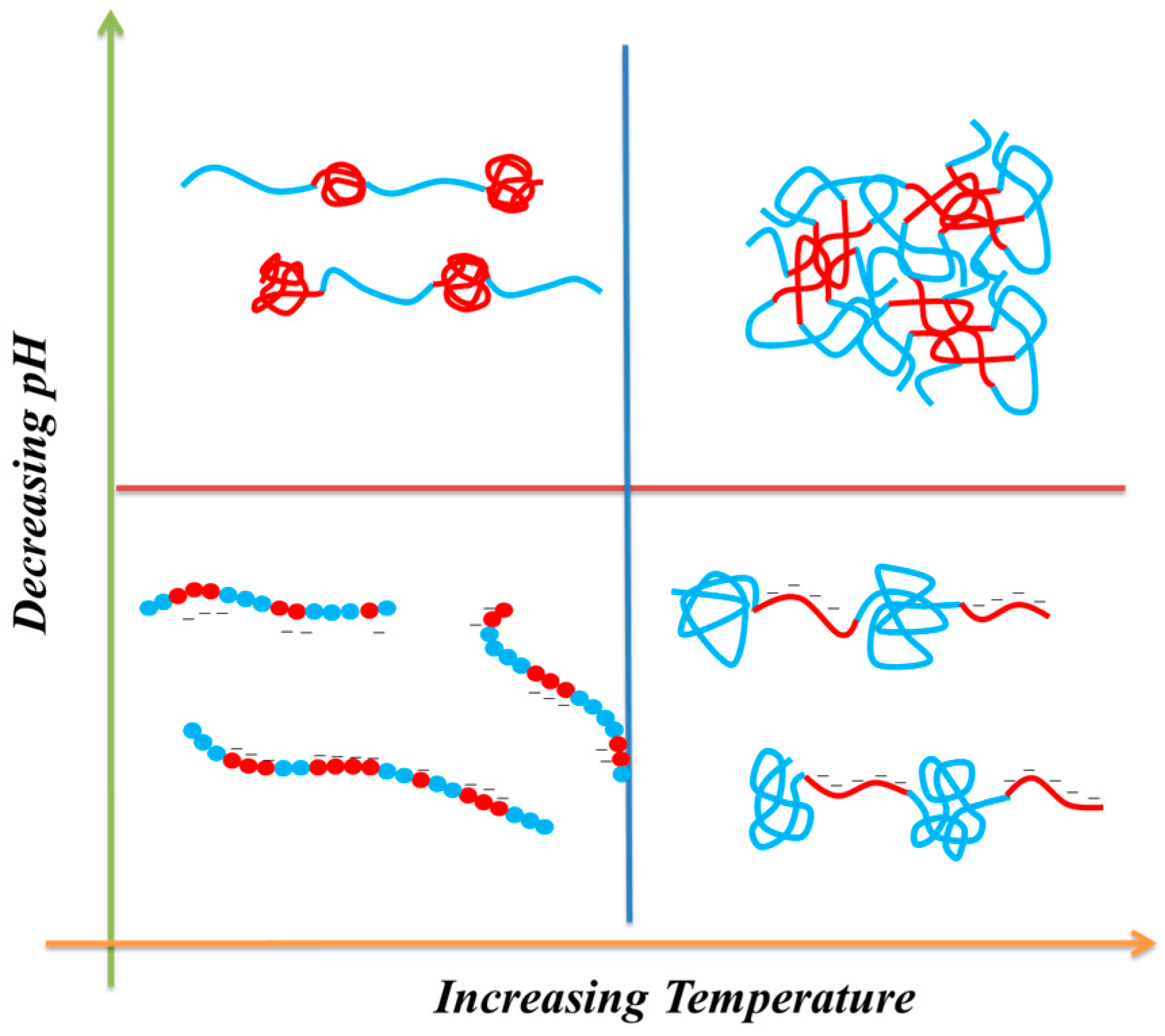
3.3. LCST Behaviors of PNIPAAm-co-PAA Random Copolymers
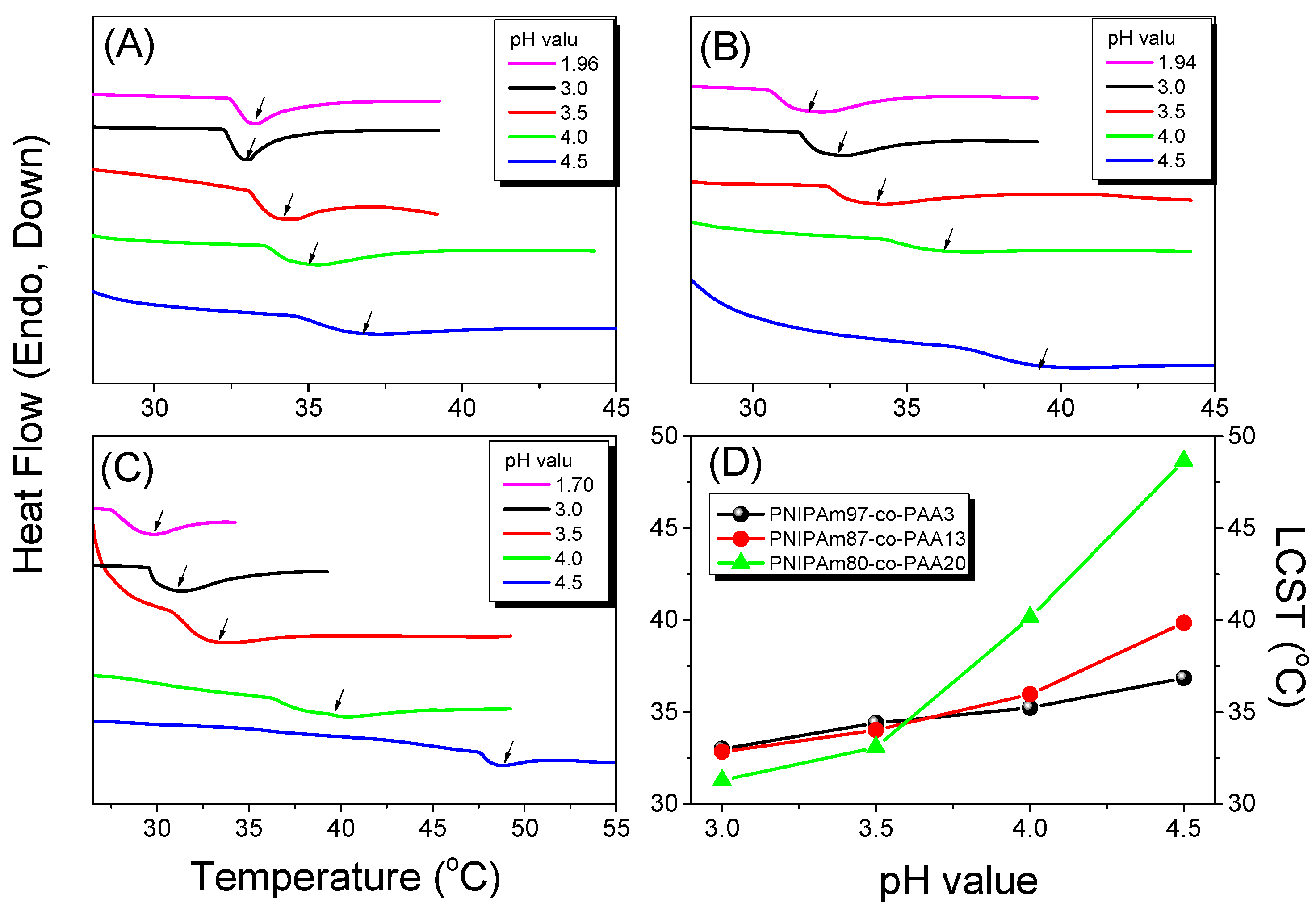
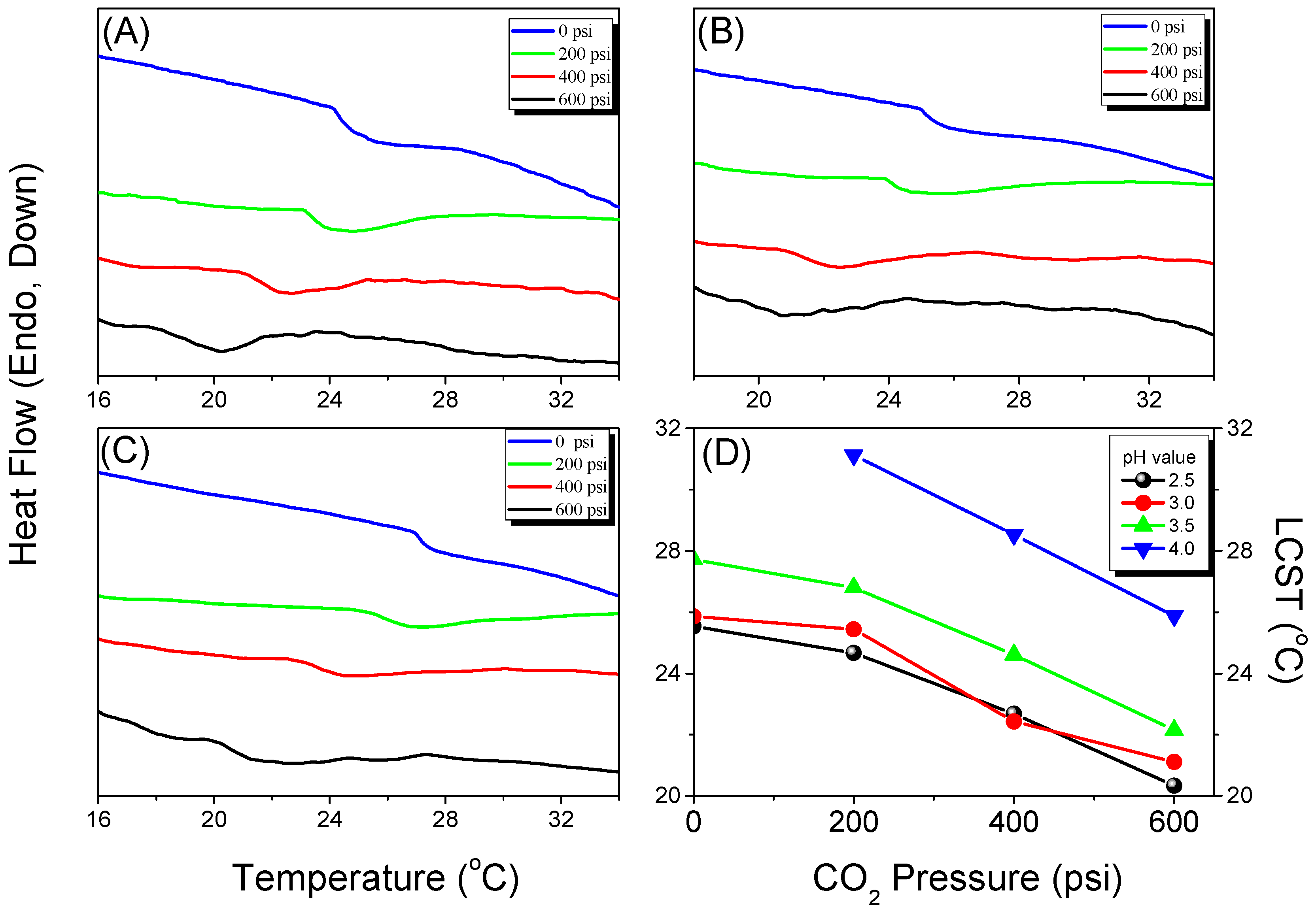
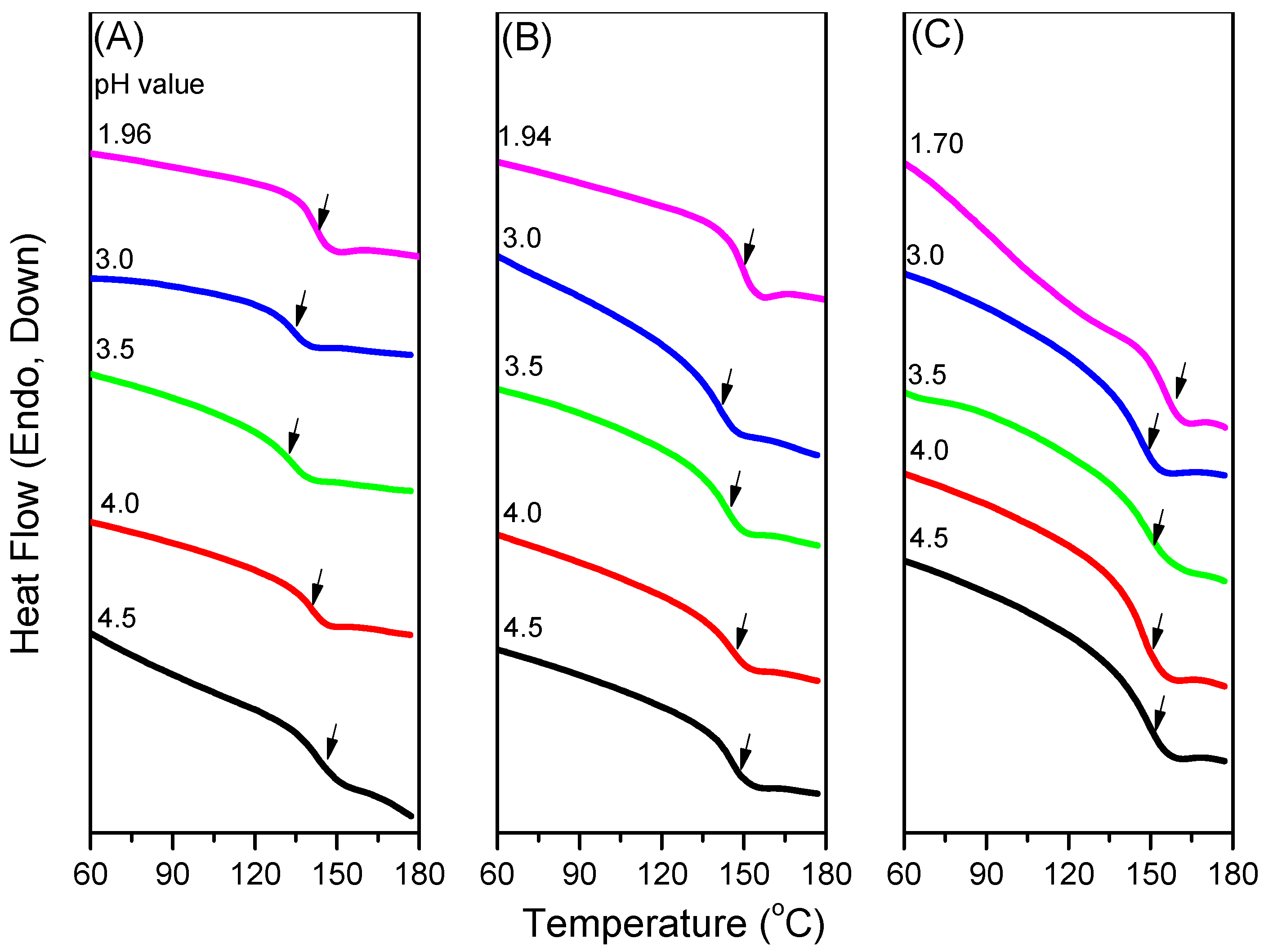
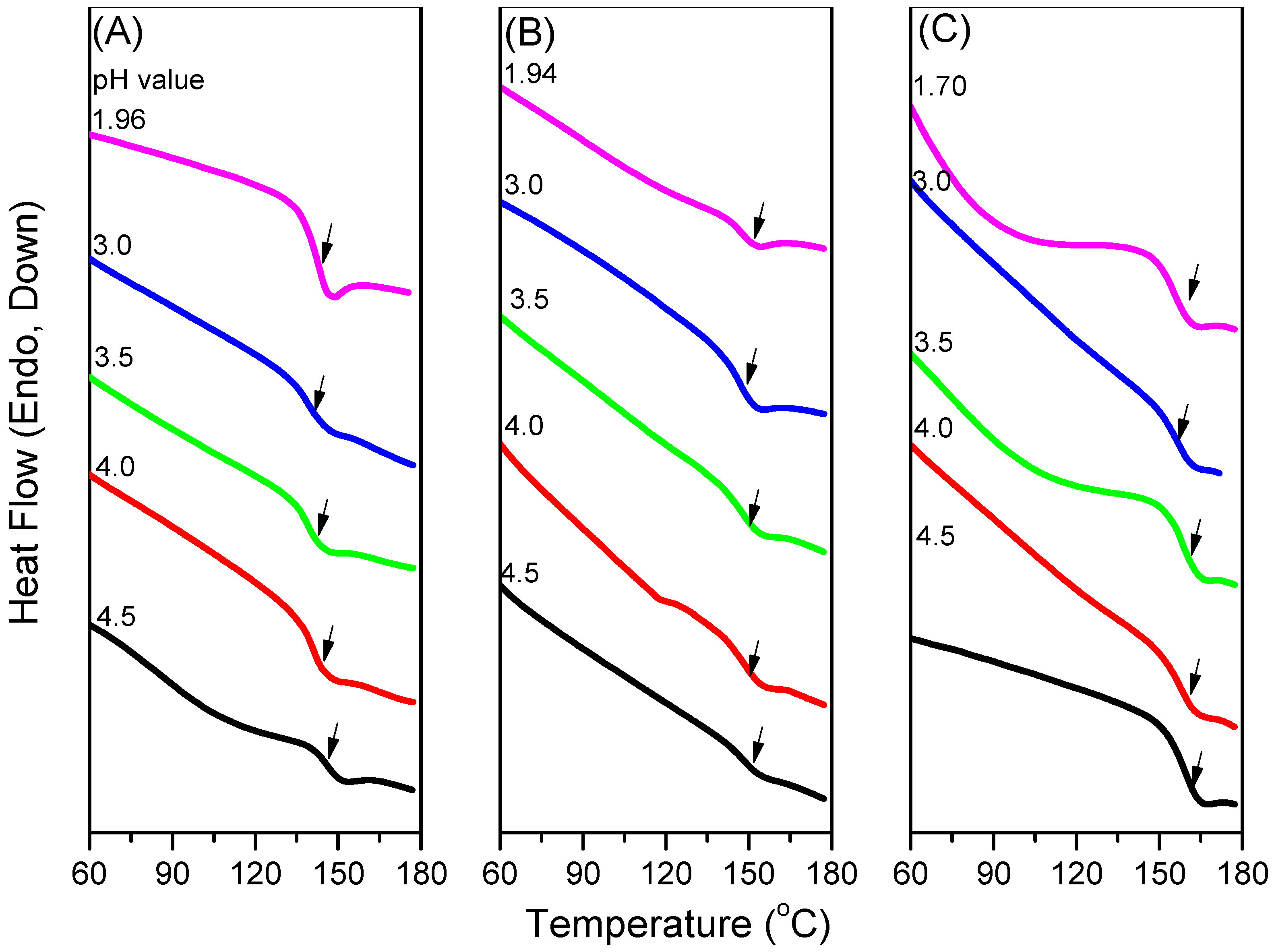
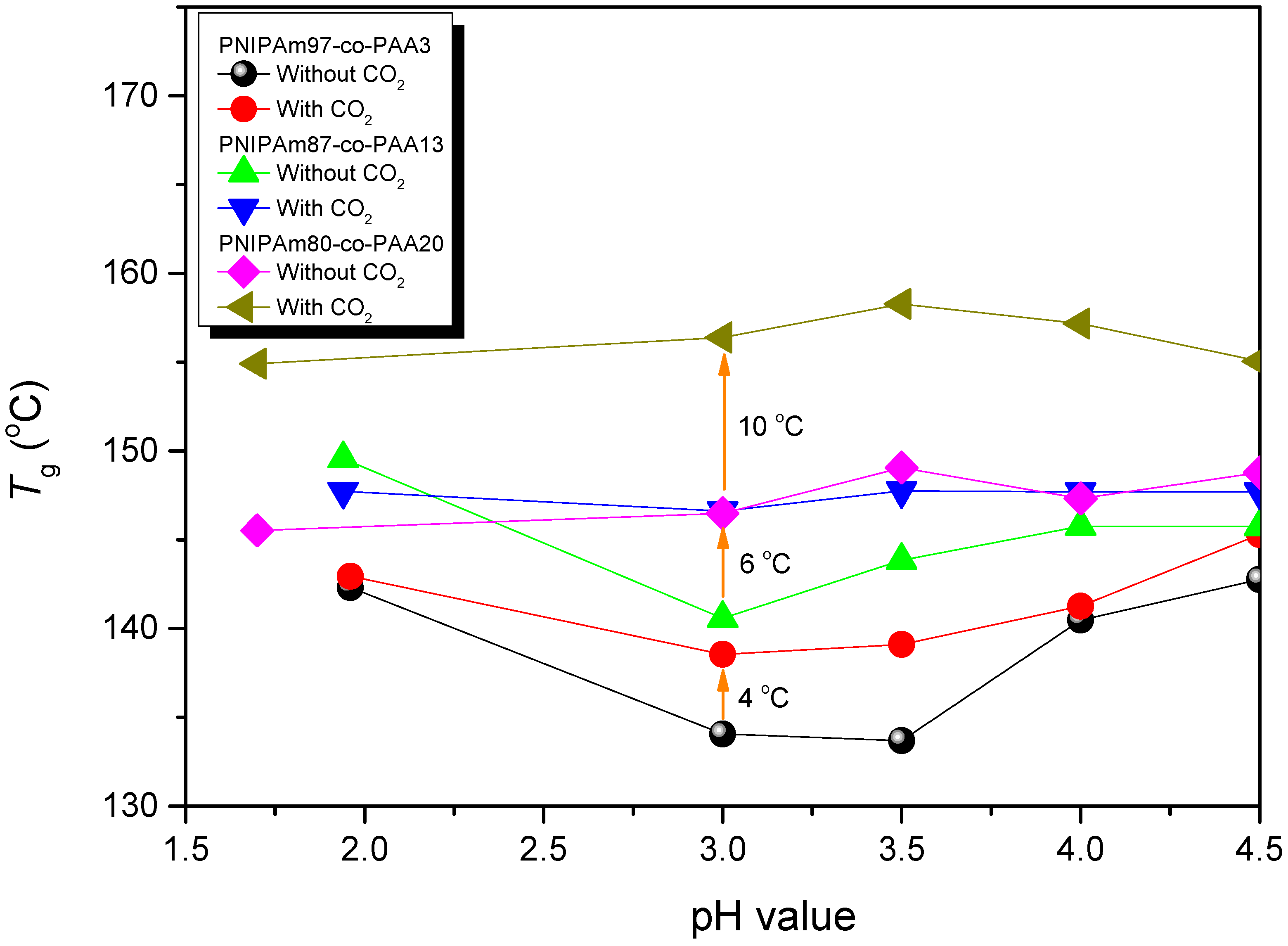
3.4. LCSTs and Values of Tg of PNIPAAm-co-PAA under CO2 and after scCO2 Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, Y.S.; Lin, H.T.; Wang, C.F. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Shi, Y.; Yoonessi, M.; Weiss, R.A. High temperature shape memory polymers. Macromolecules 2013, 46, 4160–4167. [Google Scholar] [CrossRef]
- Boukis, A.C.; Llevot, A.; Meier, M.A.R. High glass transition temperature renewable polymers via biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Zhang, F.; Liu, L.; Liu, Y.; Zhang, S.; Hu, Y.; Leng, J. Shape-memory polymers with adjustable high glass transition temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Gibbs, J.H.; DiMarzio, E.A. Nature of the glass transition and the glassy state. J. Chem. Phys. 1948, 28, 373. [Google Scholar] [CrossRef]
- Dalnoki-Veress, K.; Forrest, J.A.; Murray, C.; Gigault, C.; Dutcher, J.R. Molecular weight dependence of reductions in the glass transition temperature of thin, freely standing polymer films. Phys. Rev. E 2001, 63, 031801. [Google Scholar] [CrossRef] [PubMed]
- Kwei, T.K. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
- Painter, P.C.; Graf, J.F.; Coleman, M.M. Effect of hydrogen bonding on the enthalpy of mixing and the composition dependence of the glass transition temperature in polymer blends. Macromolecules 1991, 24, 5630–5638. [Google Scholar] [CrossRef]
- Tsui, O.K.C.; Zhang, H.F. Effects of chain ends and chain entanglement on the glass transition temperature of polymer thin films. Macromolecules 2001, 34, 9139–9142. [Google Scholar] [CrossRef]
- Kuo, S.W.; Xu, H.; Huang, C.F.; Chang, F.C. Significant glass transition temperature increase in hydrogen bonded copolymers. J. Polym. Sci. B Polym. Phys. 2002, 40, 2313–2323. [Google Scholar] [CrossRef]
- Kuo, S.W.; Kao, H.C.; Chang, F.C. Thermal behavior and specific interaction in high glass transition temperature PMMA copolymer. Polymer 2003, 44, 6873–6882. [Google Scholar] [CrossRef]
- Chen, J.K.; Kuo, S.W.; Kao, H.C.; Chang, F.C. Thermal property, specific interactions and surface energy of PMMA terpolymers having high glass transition temperatures and low moisture absorptions. Polymer 2005, 46, 2354–2364. [Google Scholar] [CrossRef]
- Yeh, S.L.; Zhu, C.Y.; Kuo, S.W. Transparent heat-resistant PMMA copolymers for packing light emitting diode materials. Polymers 2015, 7, 1379–1388. [Google Scholar] [CrossRef]
- Coleman, M.M.; Xu, Y.; Painter, P.C. Compositional heterogeneities in hydrogen-bonded polymer blends: Infrared spectroscopic results. Macromolecules 1994, 27, 127–134. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Gil, E.R.; Spoelstra, A.B.; Bastiaansen, C.W.M.; Broer, D.J.; Schenning, A.P.H.J. Stimuli-responsive materials based on interpenetrating polymer liquid crystal hydrogels. Adv. Funct. Mater. 2015, 25, 3314–3320. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Q.; Ju, G.; Zhang, Y.; Jiang, L.; Shi, F. Bell-shaped superhydrophilic–superhydrophobic–superhydrophilic double transformation on a pH-responsive smart surface. Adv. Mater. 2014, 26, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Cheng, H.; Shen, L.; Wu, C. LLS and FTIR studies on the hysteresis in association and dissociation of poly(N-isopropylacrylamide) chains in water. Macromolecules 2006, 39, 2325–2329. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug. Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kwan, C.M.S.; Wu, C. Laser light scattering study of the formation and structure of poly(N-isopropylacrylamide-co-acrylic acid) nanoparticles. Macromolecules 1997, 30, 6090–6094. [Google Scholar] [CrossRef]
- Shibayama, M.; Fujikawa, Y.; Nomura, S. Dynamic light scattering study of poly(N-isopropylacrylamide-co-acrylic acid) gels. Macromolecules 1996, 29, 6535–6540. [Google Scholar] [CrossRef]
- Zhuang, J.; Gordon, M.R.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-stimuli responsive macromolecules and their assemblies. Chem. Soc. Rev. 2013, 42, 7421–7435. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L. Specific intermolecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Shih, Y.T.; Liu, K.H. The effect of carbonyl group on sorption of CO2 in glassy polymers. J. Supercrit. Fluids 2003, 25, 261–268. [Google Scholar] [CrossRef]
- Xia, F.; Feng, L.; Wang, S.; Sun, T.; Song, W.; Jiang, W.; Jiang, L. Dual-responsive surfaces that switch between superhydrophilicity and superhydrophobicity. Adv. Mater. 2006, 18, 432–436. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.Y.; Li, Y.K.; Lee, Y.M. Dual thermo- and pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Polymer 2007, 48, 1718–1728. [Google Scholar] [CrossRef]
- Clarke, K.C.; Dunham, S.N.; Lyon, L.A. Core/shell microgels decouple the pH and temperature responsivities of microgel films. Chem. Mater. 2015, 27, 1391–1396. [Google Scholar] [CrossRef]
- Leonforte, F.; Muller, M. Functional poly(N-isopropylacrylamide)/poly(acrylic acid) mixed brushes for controlled manipulation of nanoparticles. Macromolecules 2016, 49, 5256–5265. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Zhou, T.Y.; Kuo, S.W. Carbon dioxide affects the phase transition of poly(N-isopropylacrylamide). RSC Adv. 2016, 6, 75032–75037. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. Effect of copolymer composition on the miscibility of poly(styrene-co-acetoxystyrene) with phenolic resin. Polymer 2001, 42, 9843–9848. [Google Scholar] [CrossRef]
- Lin, C.L.; Chen, W.C.; Liao, C.S.; Su, Y.C.; Huang, C.F.; Kuo, S.W.; Chang, F.C. Sequence distribution and polydispersity index affect the hydrogen-bonding strength of poly(vinylphenol-co-methyl methacrylate) copolymers. Macromolecules 2005, 38, 6435–6444. [Google Scholar] [CrossRef]
- Wu, Y.C.; Kuo, S.W. Self-assembly supramolecular structure through complementary multiple hydrogen bonding of heteronucleobase-multifunctionalized polyhedral oligomeric silsesquioxane (POSS) complexes. J. Mater. Chem. 2012, 22, 2982–2991. [Google Scholar] [CrossRef]
- Boutris, C.; Chatzi, E.G.; Kiparissides, C. Characterization of the LCST behaviour of aqueous poly(N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer 1997, 38, 2567–2570. [Google Scholar] [CrossRef]
- Zhang, J.N.; Peppas, A. Synthesis and Characterization of pH-and Temperature-sensitive poly (methacrylic acid)/poly(N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 2000, 33, 102–107. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.V.; Kramer, E.J. Effects of High-pressure CO2 on the glass transition temperature and mechanical properties of polystyrene. J. Polym. Sci.: Polym. Phys. Ed. 1982, 20, 1371–1384. [Google Scholar] [CrossRef]
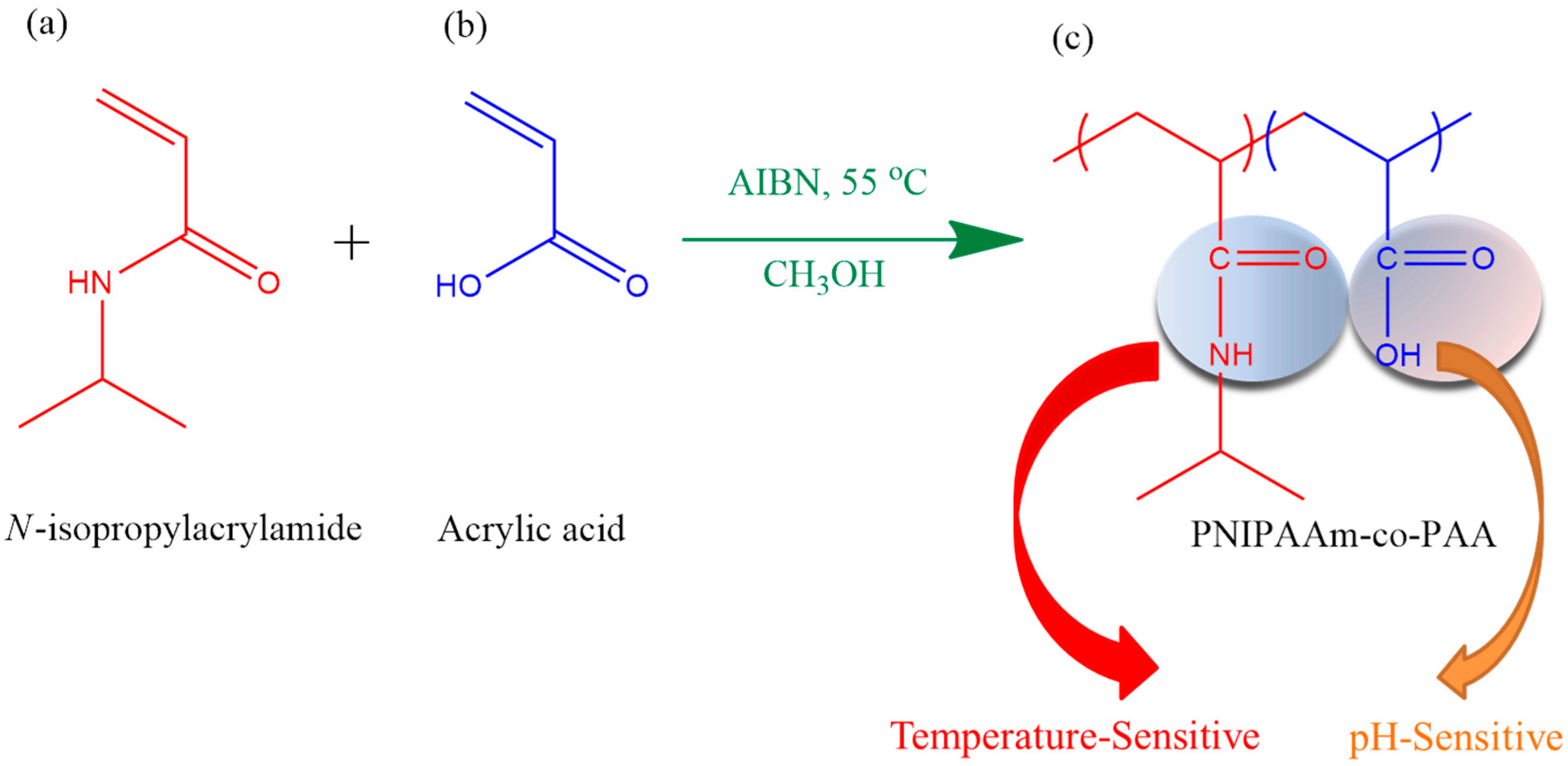
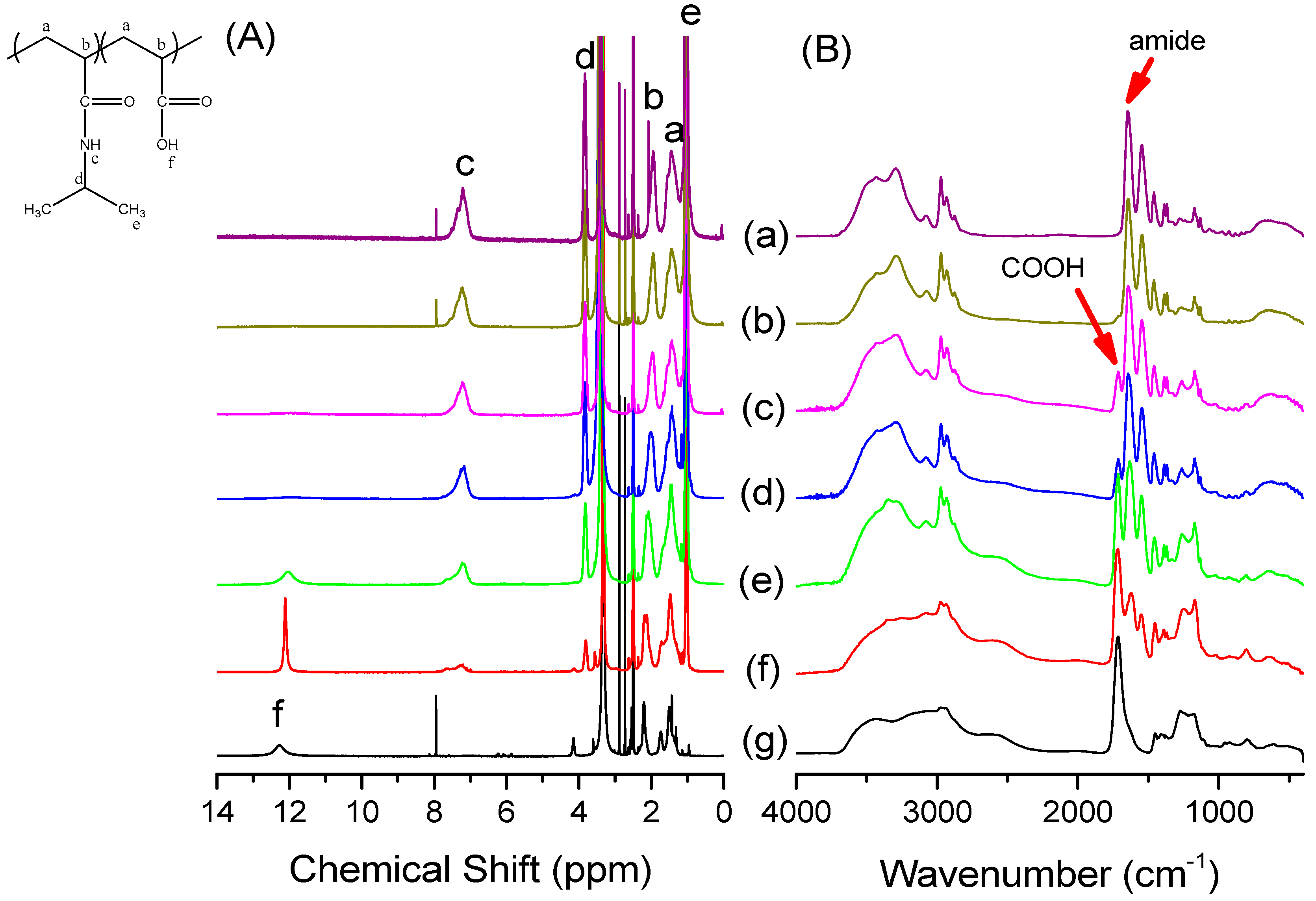

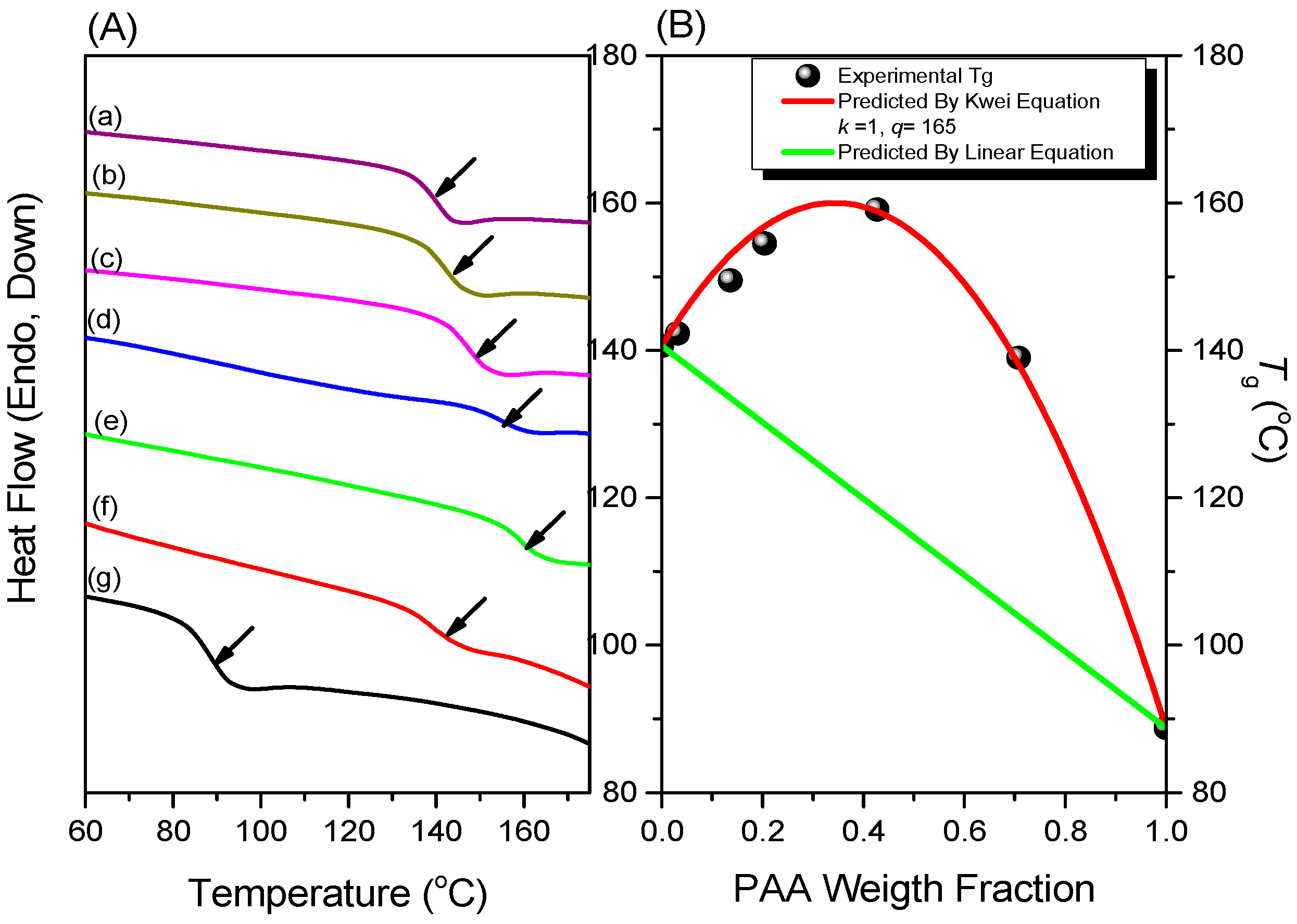
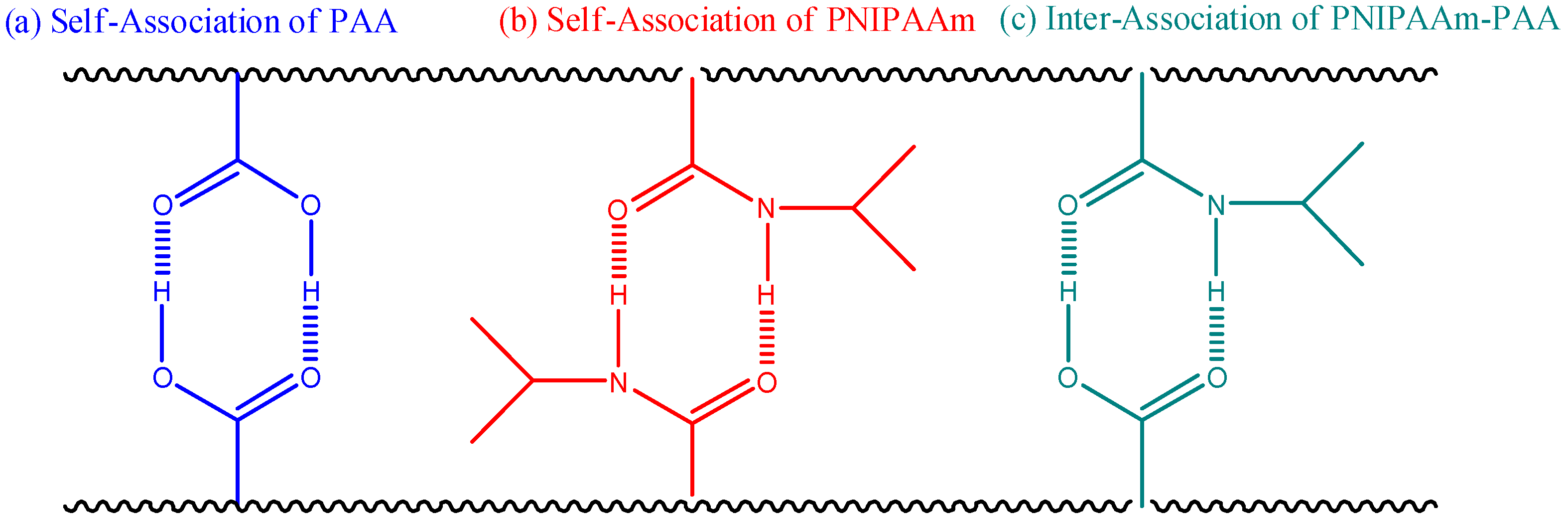

| Abbreviation | Monomer feed (mol %) | Polymer composition (mol %) | Tg (°C) | Mn (g/mol) | PDI | ||
|---|---|---|---|---|---|---|---|
| NIPAAm | AA | NIPAAm | AA | ||||
| PNIPAAm | 100 | 0 | 100 | 0 | 140.6 | 3.1 × 105 | 1.64 |
| PNIPAAm97-co-PAA3 | 95 | 5 | 96.9 | 3.1 | 142.3 | 4.2 × 106 | 1.70 |
| PNIPAAm87-co-PAA13 | 85 | 15 | 86.4 | 13.6 | 149.5 | 2.1 × 106 | 2.24 |
| PNIPAAm80-co-PAA20 | 75 | 25 | 79.6 | 20.4 | 154.5 | 6.8 × 106 | 1.31 |
| PNIPAAm57-co-PAA43 | 50 | 50 | 57.3 | 42.7 | 159.1 | 5.0 × 106 | 1.56 |
| PNIPAAm30-co-PAA70 | 25 | 75 | 29.2 | 70.8 | 140.0 | 8.9 × 106 | 1.59 |
| PAA | 0 | 100 | 0 | 100 | 88.8 | 3.1 × 105 | 1.64 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shieh, Y.-T.; Lin, P.-Y.; Chen, T.; Kuo, S.-W. Temperature-, pH- and CO2-Sensitive Poly(N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers 2016, 8, 434. https://doi.org/10.3390/polym8120434
Shieh Y-T, Lin P-Y, Chen T, Kuo S-W. Temperature-, pH- and CO2-Sensitive Poly(N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers. 2016; 8(12):434. https://doi.org/10.3390/polym8120434
Chicago/Turabian StyleShieh, Yeong-Tarng, Pei-Yi Lin, Tao Chen, and Shiao-Wei Kuo. 2016. "Temperature-, pH- and CO2-Sensitive Poly(N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures" Polymers 8, no. 12: 434. https://doi.org/10.3390/polym8120434
APA StyleShieh, Y.-T., Lin, P.-Y., Chen, T., & Kuo, S.-W. (2016). Temperature-, pH- and CO2-Sensitive Poly(N-isopropylacryl amide-co-acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers, 8(12), 434. https://doi.org/10.3390/polym8120434









