Core/Shell Structure of Ni/NiO Encapsulated in Carbon Nanosphere Coated with Few- and Multi-Layered Graphene: Synthesis, Mechanism and Application
Abstract
:1. Introduction
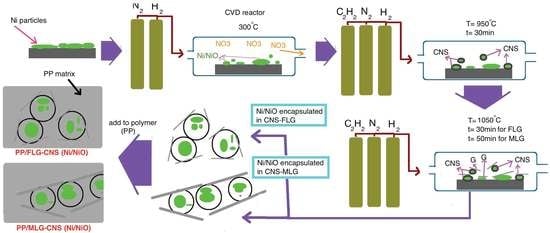
2. Materials and Methods
3. Results and Discussion
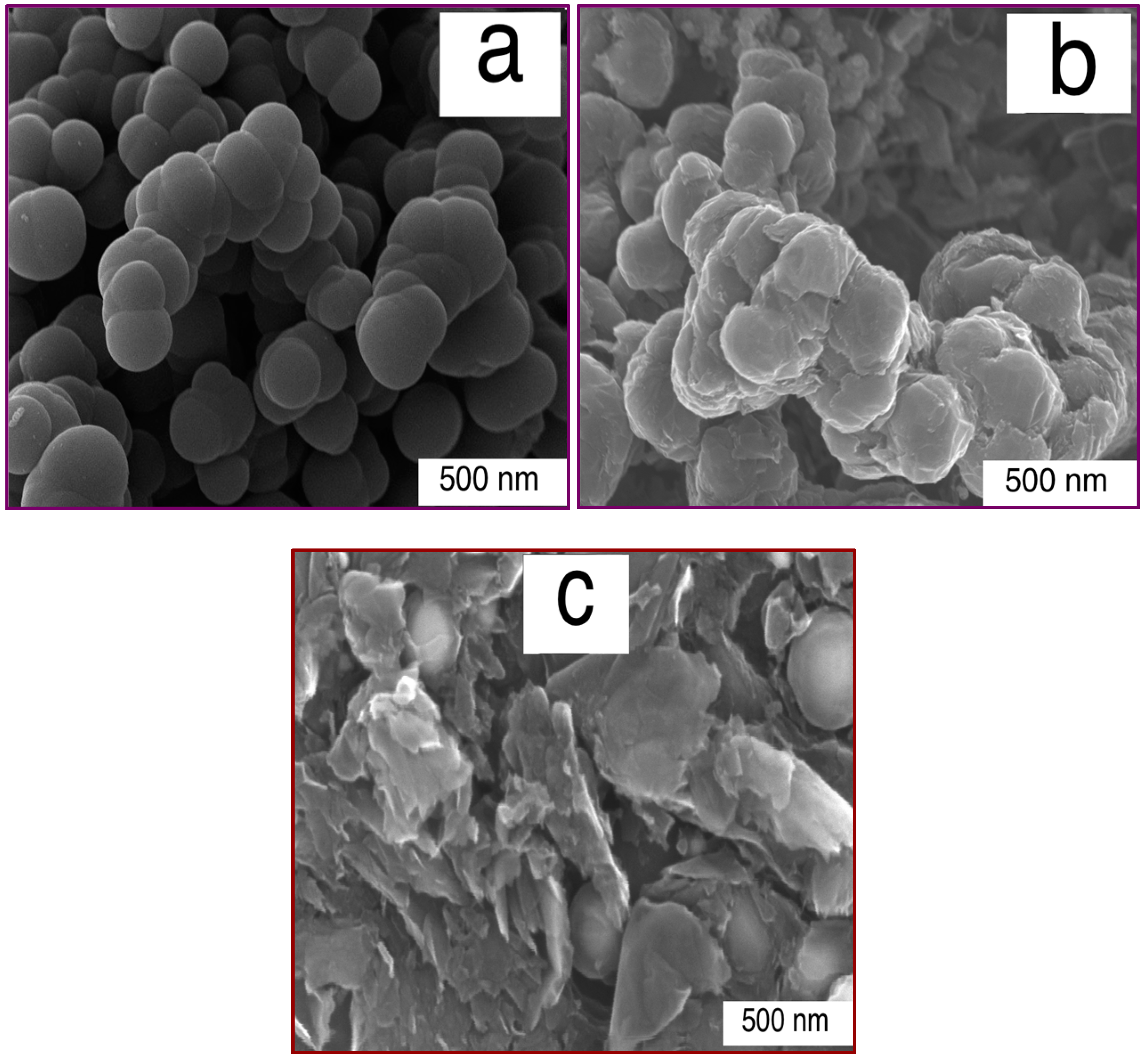
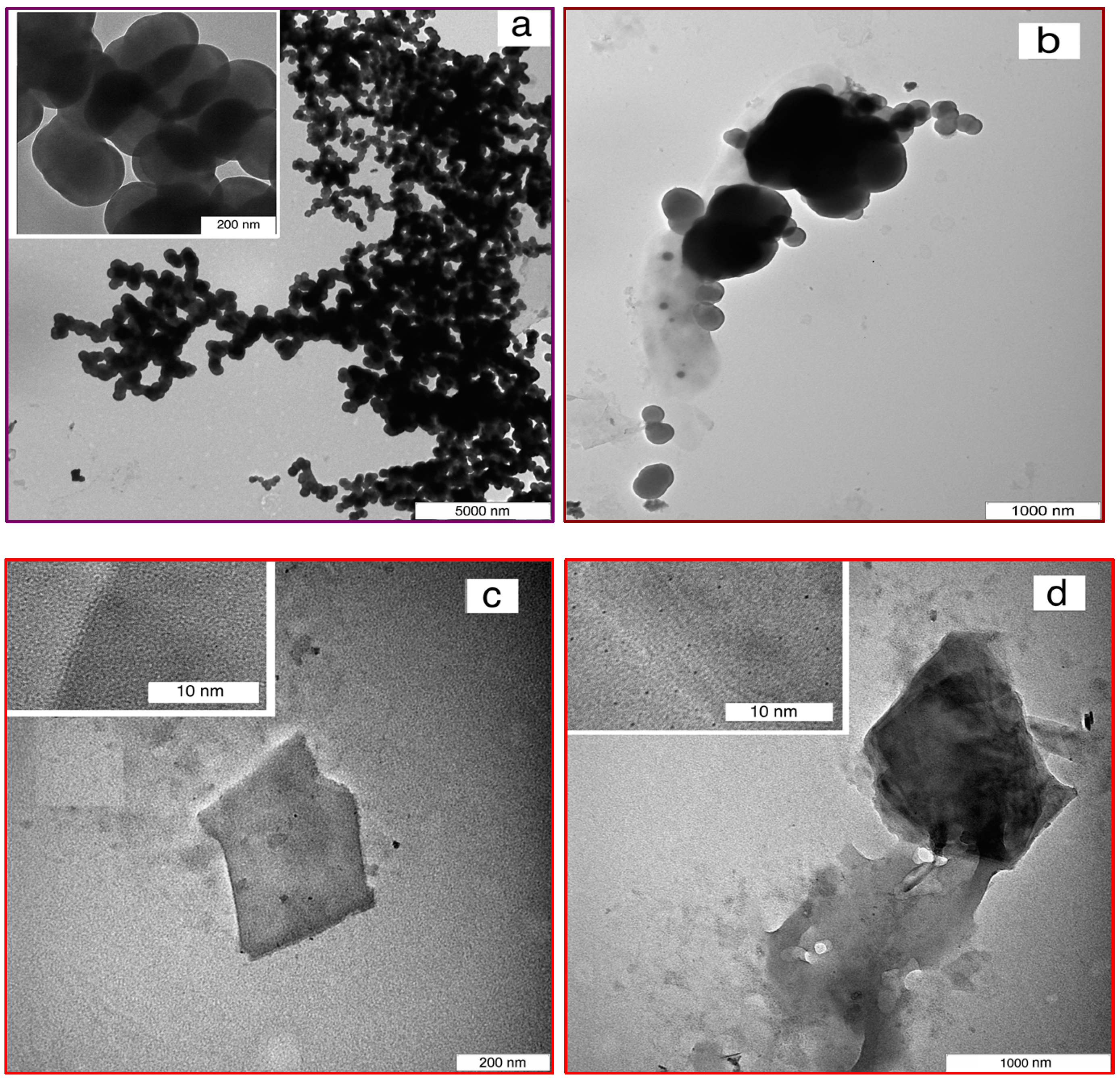
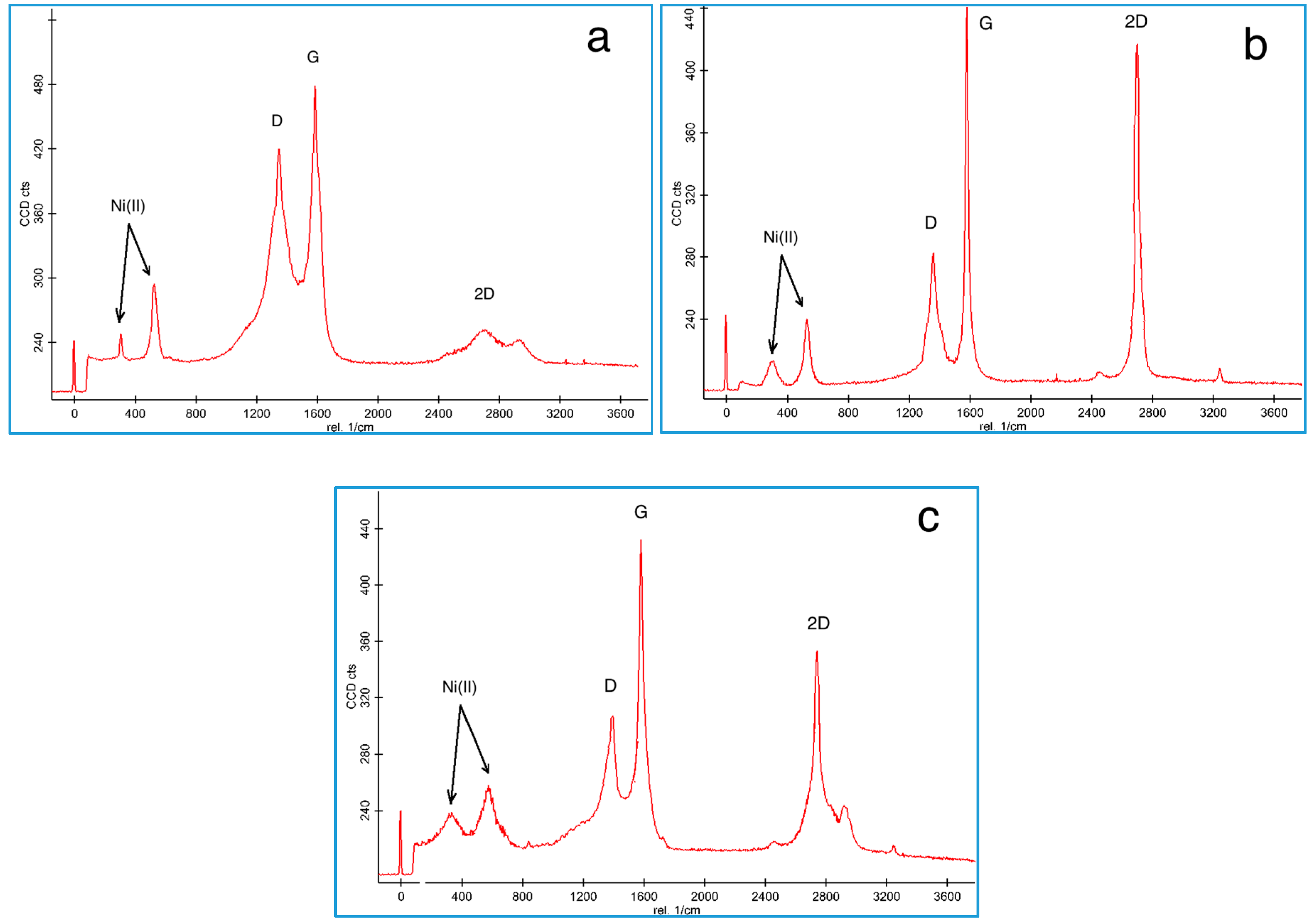
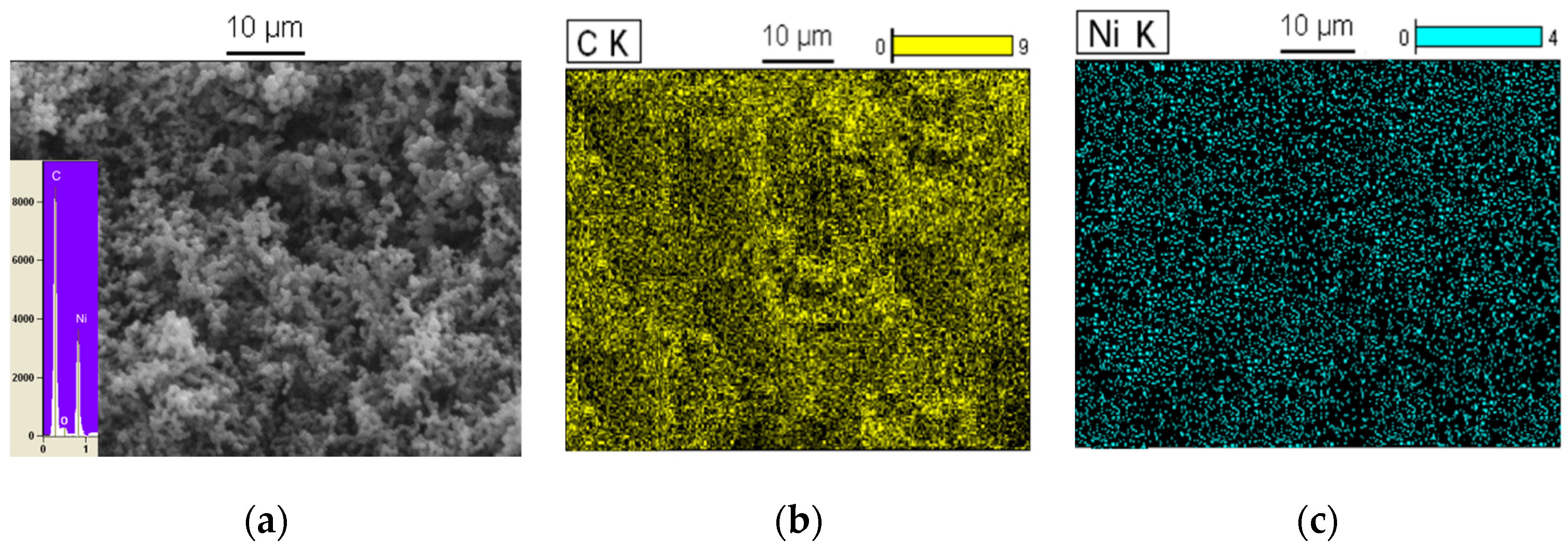
3.1. Nanomaterial Characterization
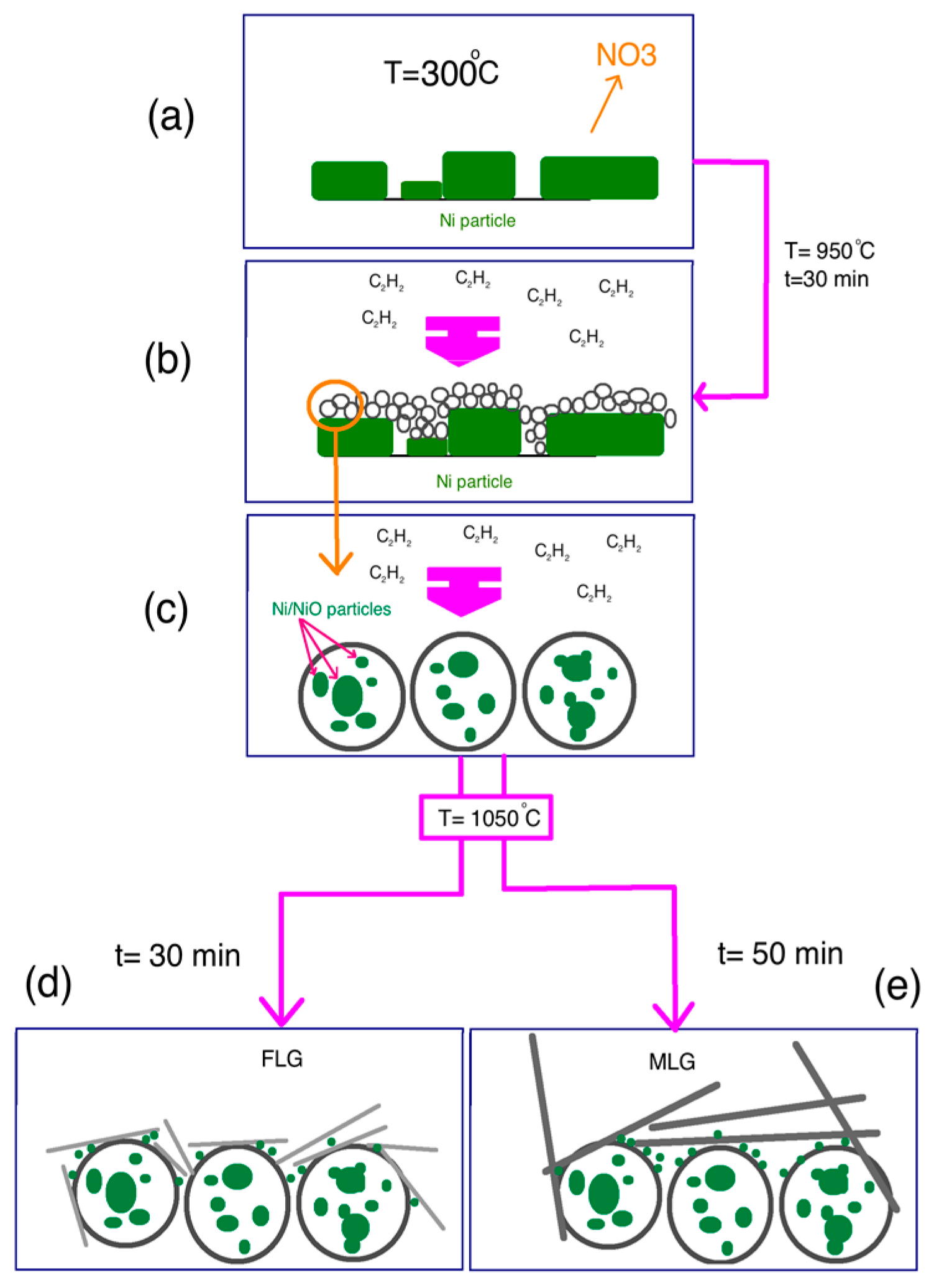
3.2. Mechanism
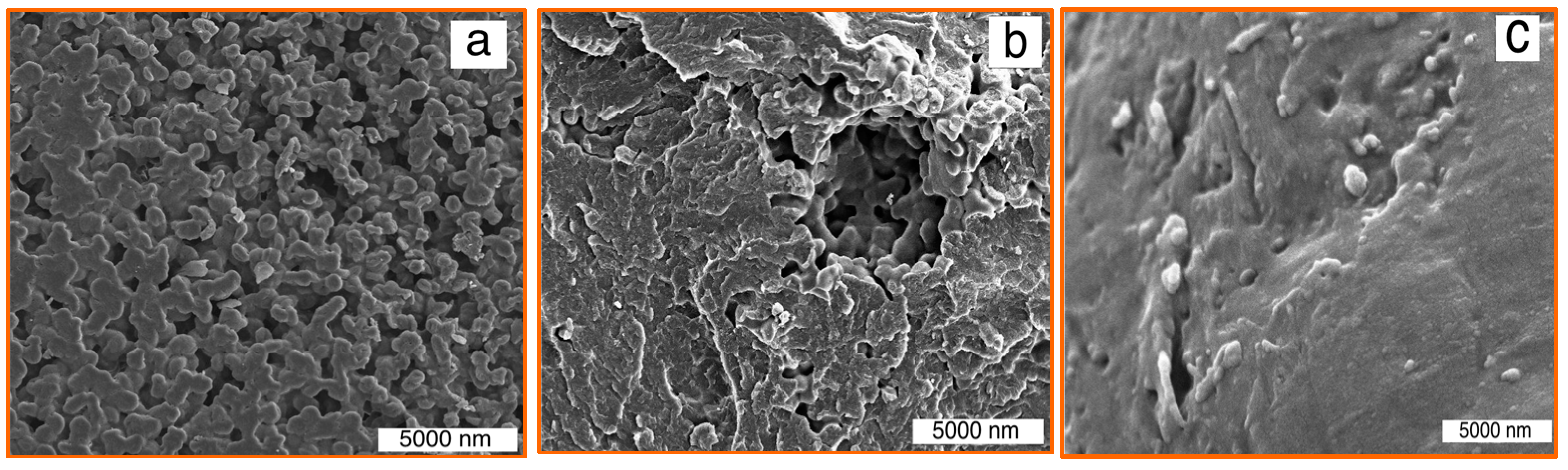
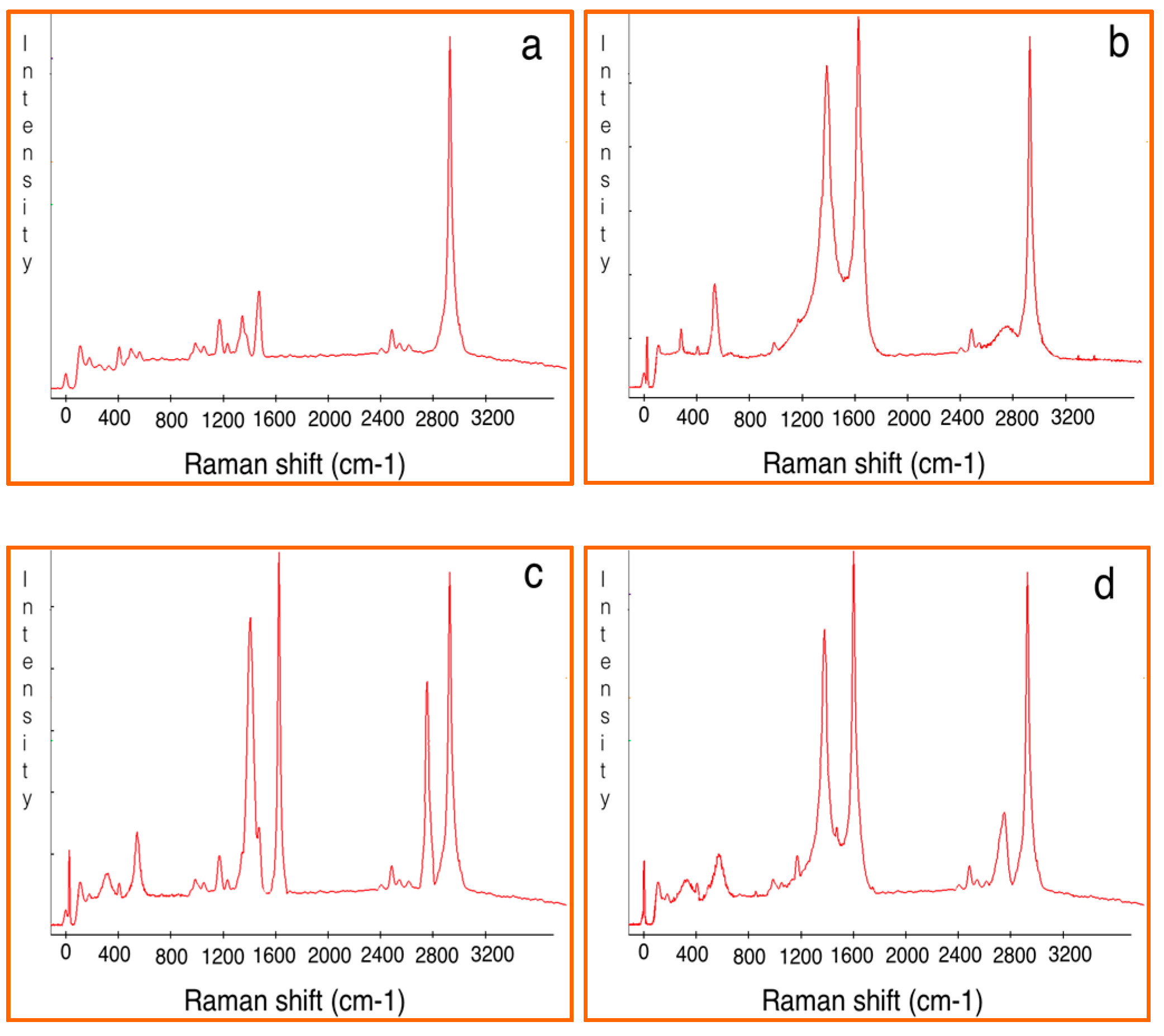
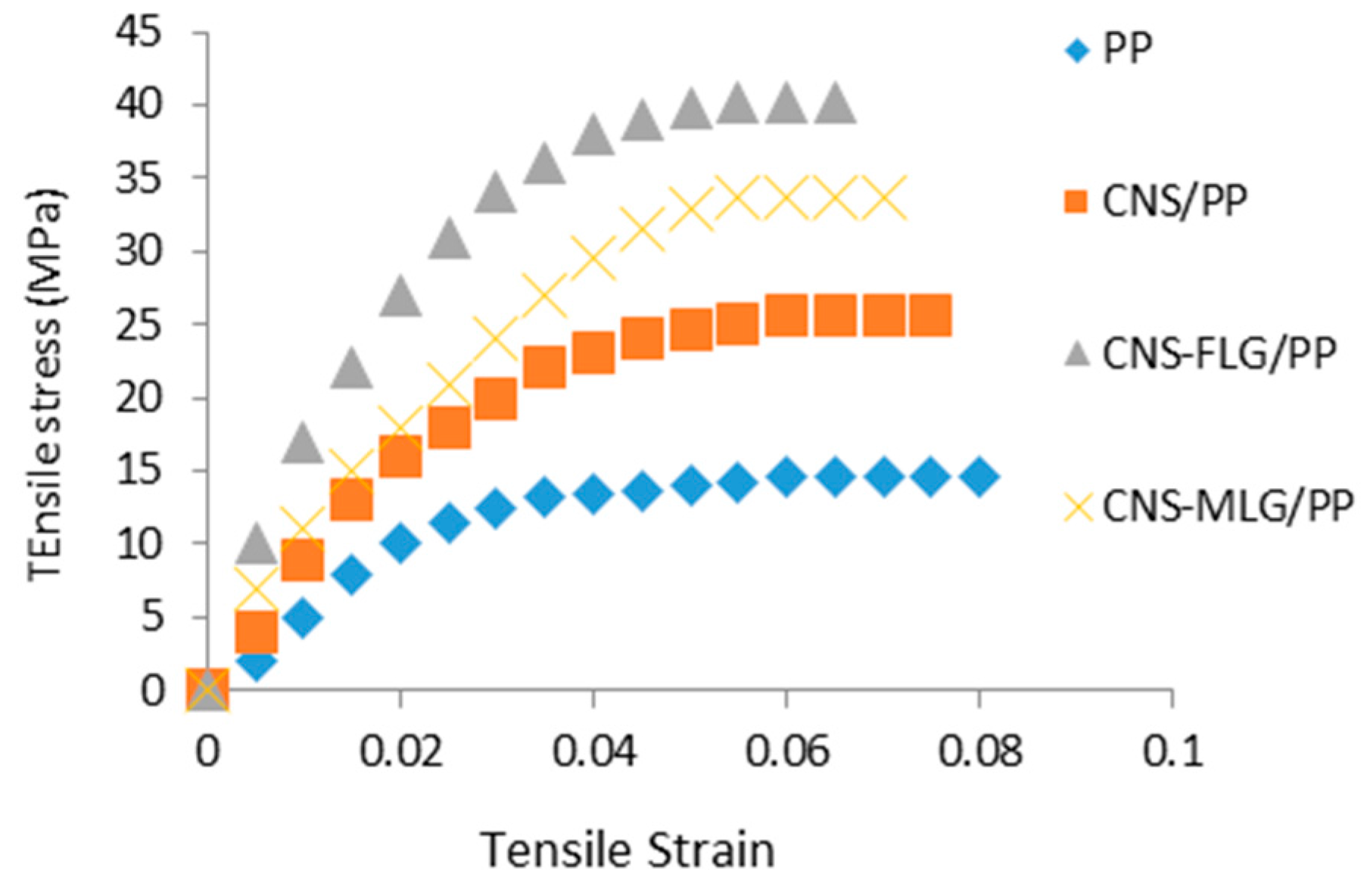
3.3. Composite Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Presser, V. (Eds.) Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2013.
- Zhang, Z.; Zhang, M.; Wang, Y.; Tan, Q.; Lv, X.; Zhong, Z.; Su, F. Amorphous silicon–carbon nanospheres synthesized by chemical vapor deposition using cheap methyltrichlorosilane as improved anode materials for Li-ion batteries. Nanoscale 2013, 5, 5384–5389. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.S.; Sbravati, N.D.; Janorkar, A.V. Mechanical & cell culture properties of elastin-like polypeptide, collagen, bioglass, and carbon nanosphere composites. Ann. Biomed. Eng. 2013, 41, 2042–2055. [Google Scholar] [PubMed]
- Fang, Y.; Zheng, G.; Yang, J.; Tang, H.; Zhang, Y.; Kong, B.; Zhang, F. Dual-pore mesoporous carbon@ silica composite core–shell nanospheres for multidrug delivery. Angew. Chem. 2014, 126, 5470–5474. [Google Scholar] [CrossRef]
- Choi, B.G.; Chang, S.J.; Kang, H.W.; Park, C.P.; Kim, H.J.; Hong, W.H.; Huh, Y.S. High performance of a solid-state flexible asymmetric supercapacitor based on graphene films. Nanoscale 2012, 4, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, F.; Ahmadian, A.; Yunus, R.; Salleh, M.A.M.; Senu, N. Effect of growing graphene flakes on branched carbon nanofibers based on carbon fiber on mechanical and thermal properties of polypropylene. RSC Adv. 2015, 5, 9925–9932. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Huang, B.C.; Coville, N.J. The Fe (CO)5 catalyzed pyrolysis of pentane: Carbon nanotube and carbon nanoball formation. Carbon 2002, 40, 2791–2799. [Google Scholar] [CrossRef]
- Yadhukulakrishnan, G.B.; Karumuri, S.; Rahman, A.; Singh, R.P.; Kalkan, A.K.; Harimkar, S.P. Spark plasma sintering of graphene reinforced zirconium diboride ultra-high temperature ceramic composites. Ceram. Int. 2013, 39, 6637–6646. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z.; Li, F.; Che, R.; Zhao, D. A Low-Concentration Hydrothermal Synthesis of Biocompatible Ordered Mesoporous Carbon Nanospheres with Tunable and Uniform Size. Angew. Chem. Int. Ed. Engl. 2010, 49, 7987–7991. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Suh, W.H.; Suslick, K.S. Magnetic and porous nanospheres from ultrasonic spray pyrolysis. J. Am. Chem. Soc. 2005, 127, 12007–12010. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kang, Y.C. Fe3O4-decorated hollow graphene balls prepared by spray pyrolysis process for ultrafast and long cycle-life lithium ion batteries. Carbon 2014, 79, 58–66. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Q.; Zheng, W. Synthesis and characterization of amorphous hollow carbon spheres. J. Mater. Sci. 2012, 47, 2072–2077. [Google Scholar] [CrossRef]
- Koprinarov, N.; Konstantinova, M. Preparation of carbon spheres by low-temperature pyrolysis of cyclic hydrocarbons. J. Mater. Sci. 2011, 46, 1494–1501. [Google Scholar] [CrossRef]
- Ghaemi, F.; Yunus, R.; Mohd Salleh, M.A.; Lim, H.N.; Rashid, S.A. Bulk production of high-purity carbon nanosphere by combination of chemical vapor deposition methods. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 669–675. [Google Scholar] [CrossRef]
- Ghaemi, F.; Yunus, R.; Ahmadian, A.; Ismail, F.; Salleh, M.A.M.; Rashid, S.A. Few-and multi-layer graphene on carbon fibers: Synthesis and application. RSC Adv. 2015, 5, 81266–81274. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Cabrero-Vilatela, A.; Weatherup, R.S.; Braeuninger-Weimer, P.; Caneva, S.; Hofmann, S. Towards a general growth model for graphene CVD on transition metal catalysts. Nanoscale 2016, 8, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, M.; Buqa, H.; Krumeich, F.; Novak, P.; Petrat, F.M.; Veit, C. Chemical Vapor Deposited Silico/Graphite Compound Material as Negative Electrode for Lithium-Ion Batteries. Electrochem. Solid State Lett. 2005, 8, A516–A520. [Google Scholar] [CrossRef]
- Wilson, A.M.; Dahn, J.R. Lithium insertion in carbons containing nanodispersed silicon. J. Electrochem. Soc. 1995, 142, 326–332. [Google Scholar] [CrossRef]
- Ghaemi, F.; Yunus, R.; Salleh, M.A.M.; Rashid, S.A.; Ahmadian, A.; Lim, H.N. Effects of the surface modification of carbon fiber by growing different types of carbon nanomaterials on the mechanical and thermal properties of polypropylene. RSC Adv. 2015, 5, 28822–28831. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene−carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Cheng, K.; Peng, T.; Pan, M.; Mu, S. Graphene/carbon nanospheres sandwich supported PEM fuel cell metal nanocatalysts with remarkably high activity and stability. J. Mater. Chem. A 2013, 1, 2126–2132. [Google Scholar] [CrossRef]
- Guo, C.X.; Li, C.M. A self-assembled hierarchical nanostructure comprising carbon spheres and graphene nanosheets for enhanced supercapacitor performance. Energy Environ. Sci. 2011, 4, 4504–4507. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in grapheme. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Gao, L.; Liu, B.; Jiang, C.; Cheng, H.M. Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, K.; Gong, X.L.; Zhang, Z. Creep and recovery of polypropylene/carbon nanotube composites. Int. J. Plast. 2011, 27, 1239–1251. [Google Scholar] [CrossRef]
- Rezaei, F.; Yunus, R.; Ibrahim, N.A. Effect of fiber length on thermomechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2009, 30, 260–263. [Google Scholar] [CrossRef]
- ASTM Standard, D638: Standard Test Method for Tensile Properties of Plastics; ASTM Internationa: West Conshohocken, PA, USA, 2010.
- Fu, S.Y.; Lauke, B.; Mäder, E.; Yue, C.Y.; Hu, X. Tensile properties of short-glass-fiber-and short-carbon-fiber-reinforced polypropylene composites. Compos. A Appl. Sci. Manuf. 2000, 31, 1117–1125. [Google Scholar] [CrossRef]
- Vishkaee, M.S.; Salleh, M.A.M.; Yunus, R.; Biak, D.R.A.; Danafar, F.; Mirjalili, F. Effect of short carbon fiber surface treatment on composite properties. J. Compos. Mater. 2010, 45, 1885–1891. [Google Scholar] [CrossRef]
- Patange, M.; Biswas, S.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D. Morphology-controlled synthesis of monodispersed graphitic carbon coated core/shell structured Ni/NiO nanoparticles with enhanced magnetoresistance. Phys. Chem. Chem. Phys. 2015, 17, 32398–32412. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Jorio, A.; Ferreira, E.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 2008, 93, 113103. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni (NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]


| Sample | Tensile stress (MPa) | Young’s modulus (MPa) |
|---|---|---|
| PP | 15.1 ± 0.3 | 480.2 ± 11.7 |
| CNS/PP | 25.6 ± 0.4 | 830.2 ± 31.2 |
| CNS-FLG/PP | 73.8 ± 0.6 | 1,243.6 ± 57.1 |
| CNS-MLG/PP | 40.1 ± 0.7 | 1,097.5 ± 48.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaemi, F.; Abdullah, L.C.; Tahir, P. Core/Shell Structure of Ni/NiO Encapsulated in Carbon Nanosphere Coated with Few- and Multi-Layered Graphene: Synthesis, Mechanism and Application. Polymers 2016, 8, 381. https://doi.org/10.3390/polym8110381
Ghaemi F, Abdullah LC, Tahir P. Core/Shell Structure of Ni/NiO Encapsulated in Carbon Nanosphere Coated with Few- and Multi-Layered Graphene: Synthesis, Mechanism and Application. Polymers. 2016; 8(11):381. https://doi.org/10.3390/polym8110381
Chicago/Turabian StyleGhaemi, Ferial, Luqman Chuah Abdullah, and Paridah Tahir. 2016. "Core/Shell Structure of Ni/NiO Encapsulated in Carbon Nanosphere Coated with Few- and Multi-Layered Graphene: Synthesis, Mechanism and Application" Polymers 8, no. 11: 381. https://doi.org/10.3390/polym8110381
APA StyleGhaemi, F., Abdullah, L. C., & Tahir, P. (2016). Core/Shell Structure of Ni/NiO Encapsulated in Carbon Nanosphere Coated with Few- and Multi-Layered Graphene: Synthesis, Mechanism and Application. Polymers, 8(11), 381. https://doi.org/10.3390/polym8110381