Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
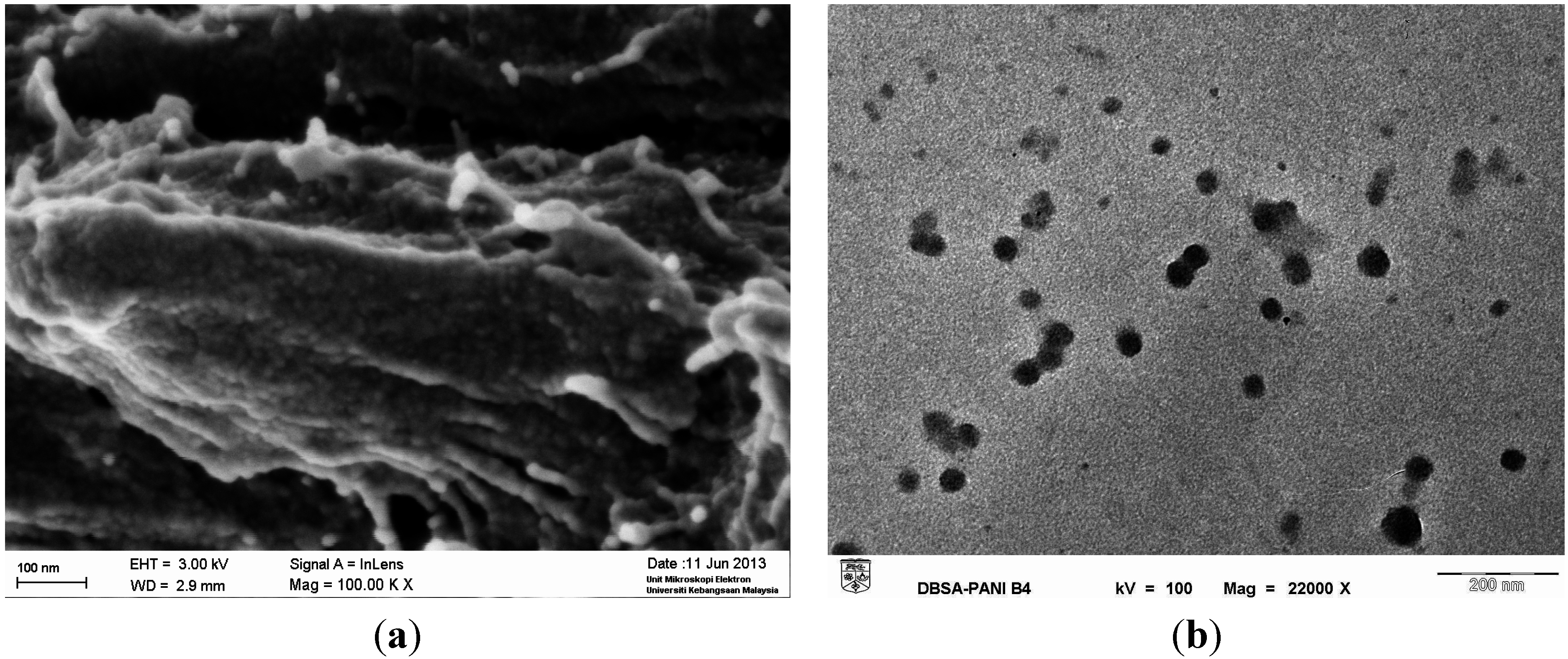
2.2. Synthesis of DBSA-Doped PANI Nanoparticles
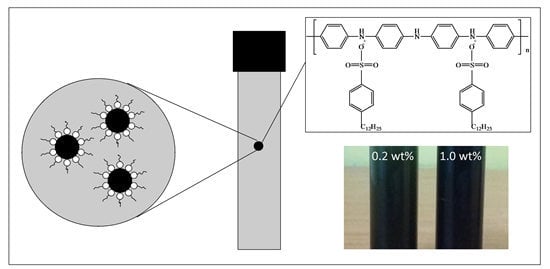
2.3. Preparation of Nanofluid of DBSA-Doped PANI Nanoparticles and Water
2.4. Characterization of DBSA-Doped PANI Nanoparticles
2.5. Stability of Nanofluids
2.6. Thermal Conductivity Measurement of Nanofluid
2.7. Specific Heat Capacity Measurement of Nanofluid
3. Results and Discussion
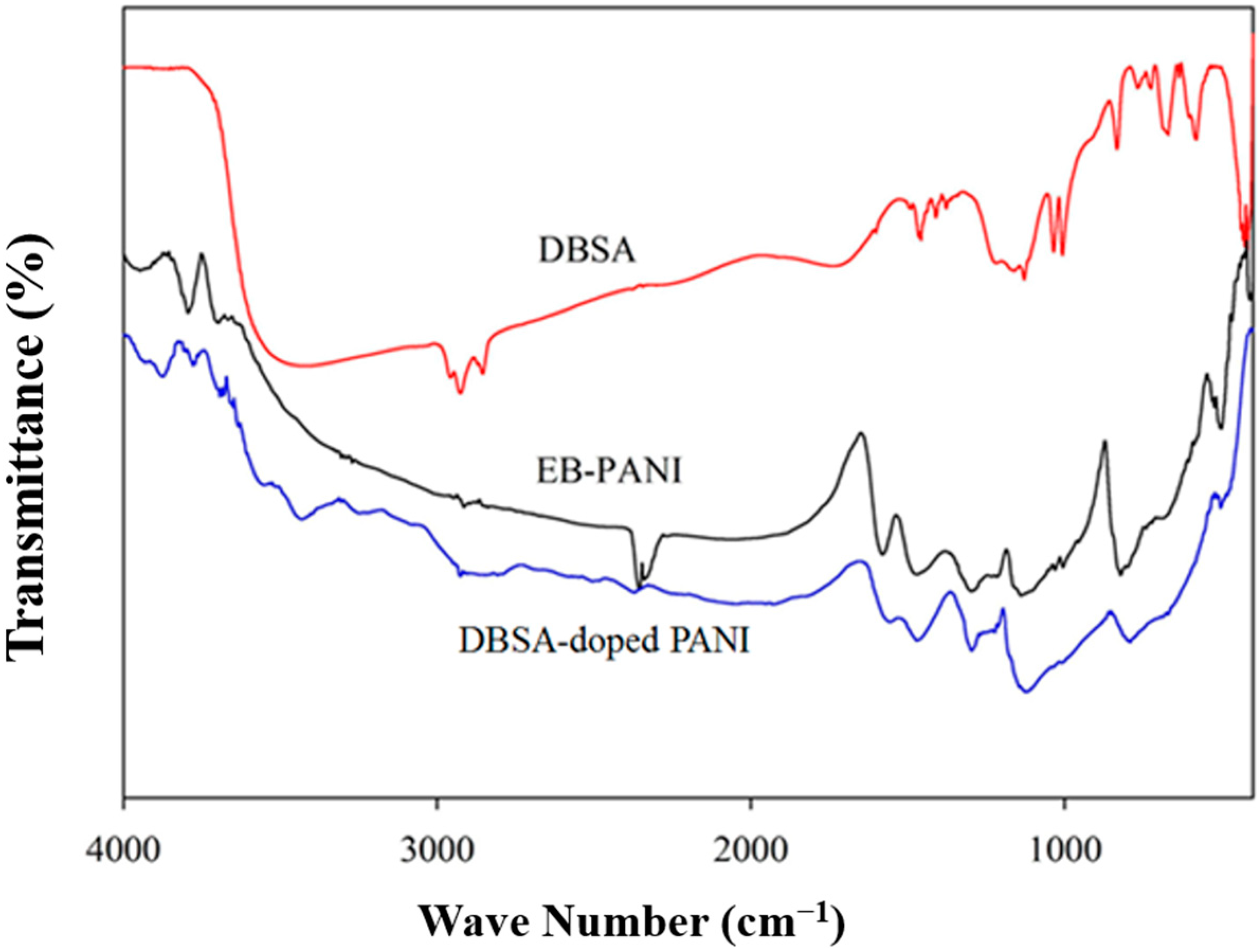
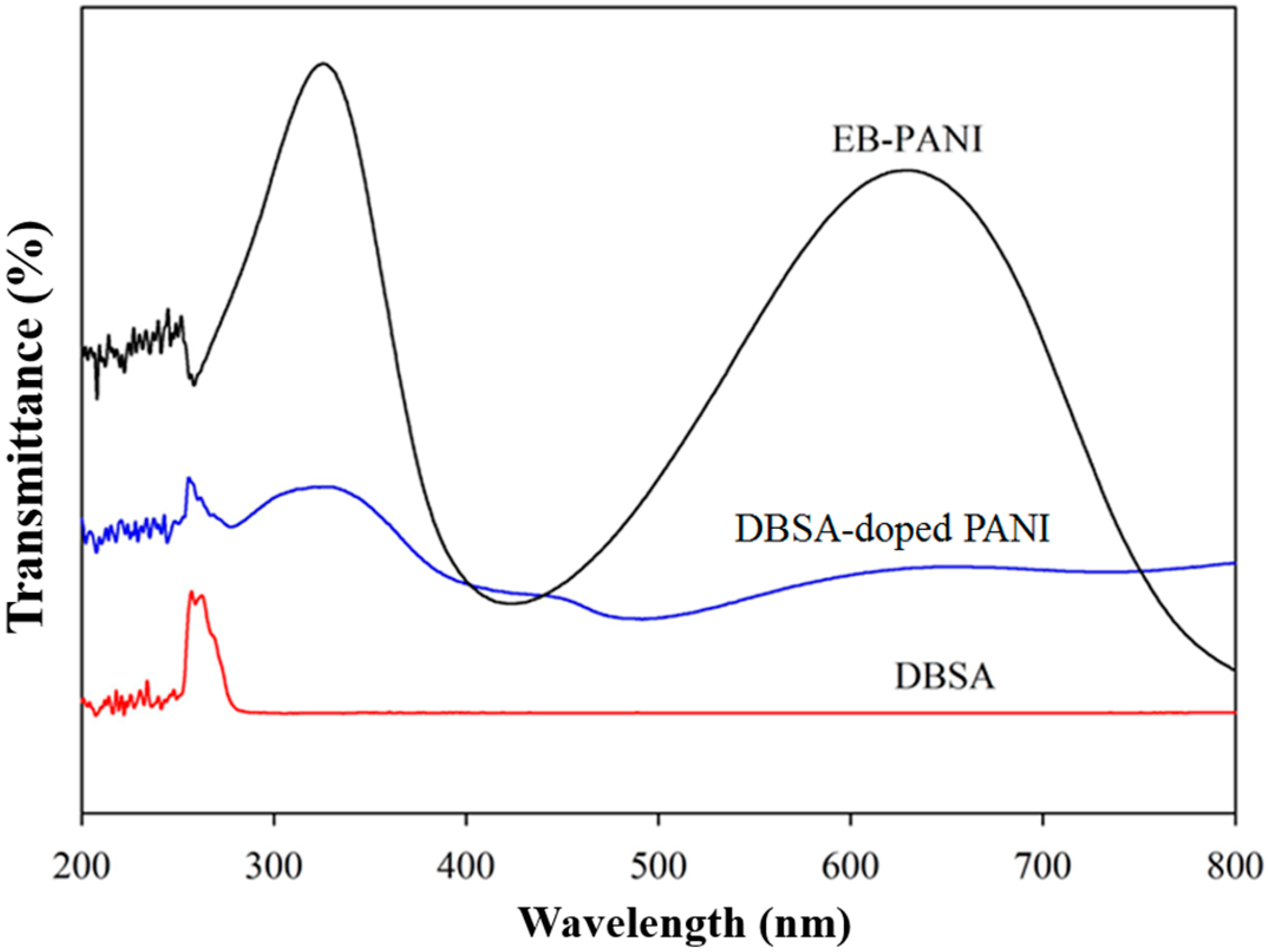
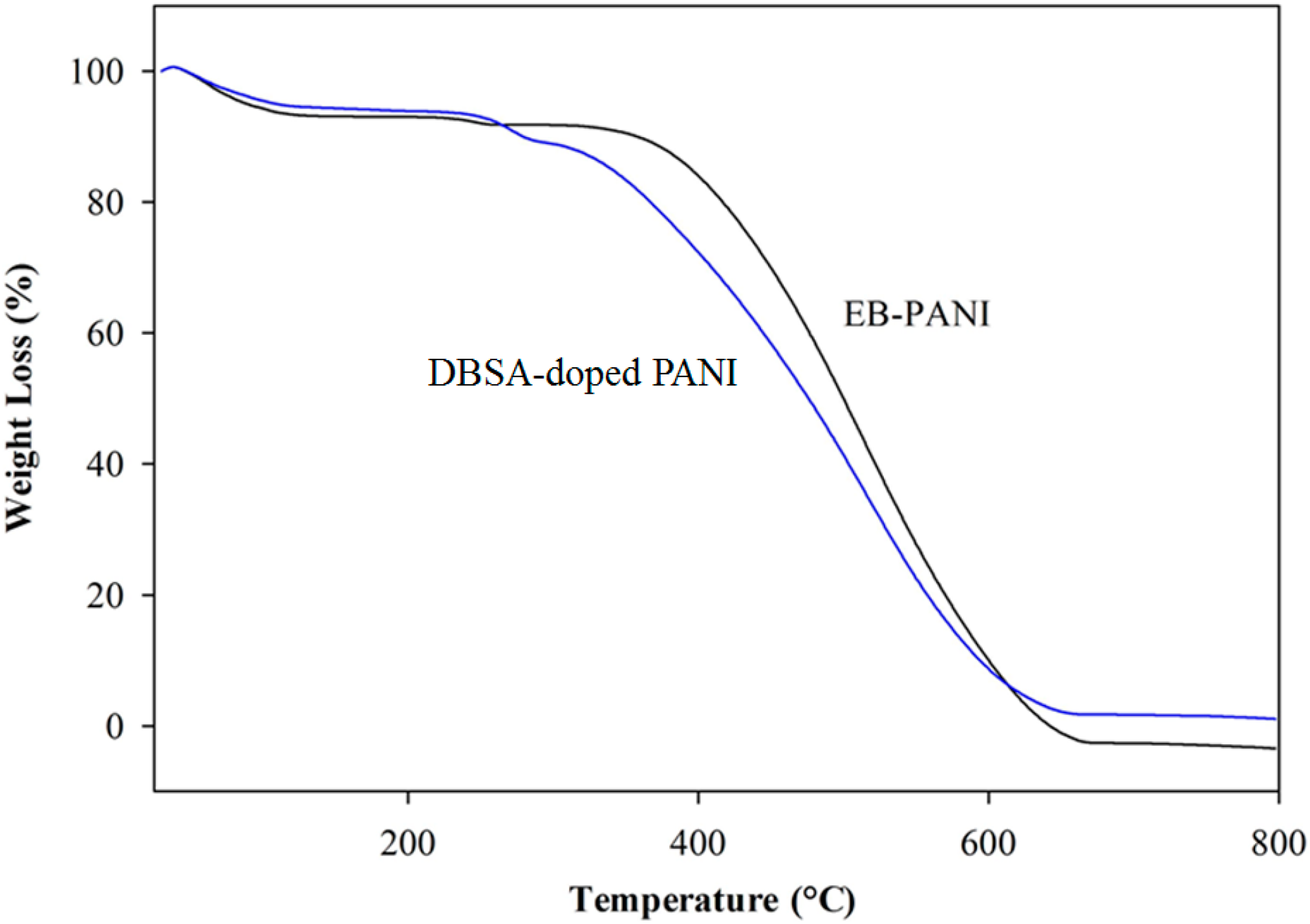
3.1. FTIR Characterizations of DBSA-PANI Nanoparticles
| Composition | EB-PANI (%) | DBSA-Doped PANI (%) |
|---|---|---|
| Carbon | 71.3 | 65.5 |
| Hydrogen | 8.6 | 8.7 |
| Nitrogen | 16.2 | 6.4 |
| Sulfur | – | 5.4 |
| Oxygen | 3.9 | 14.0 |
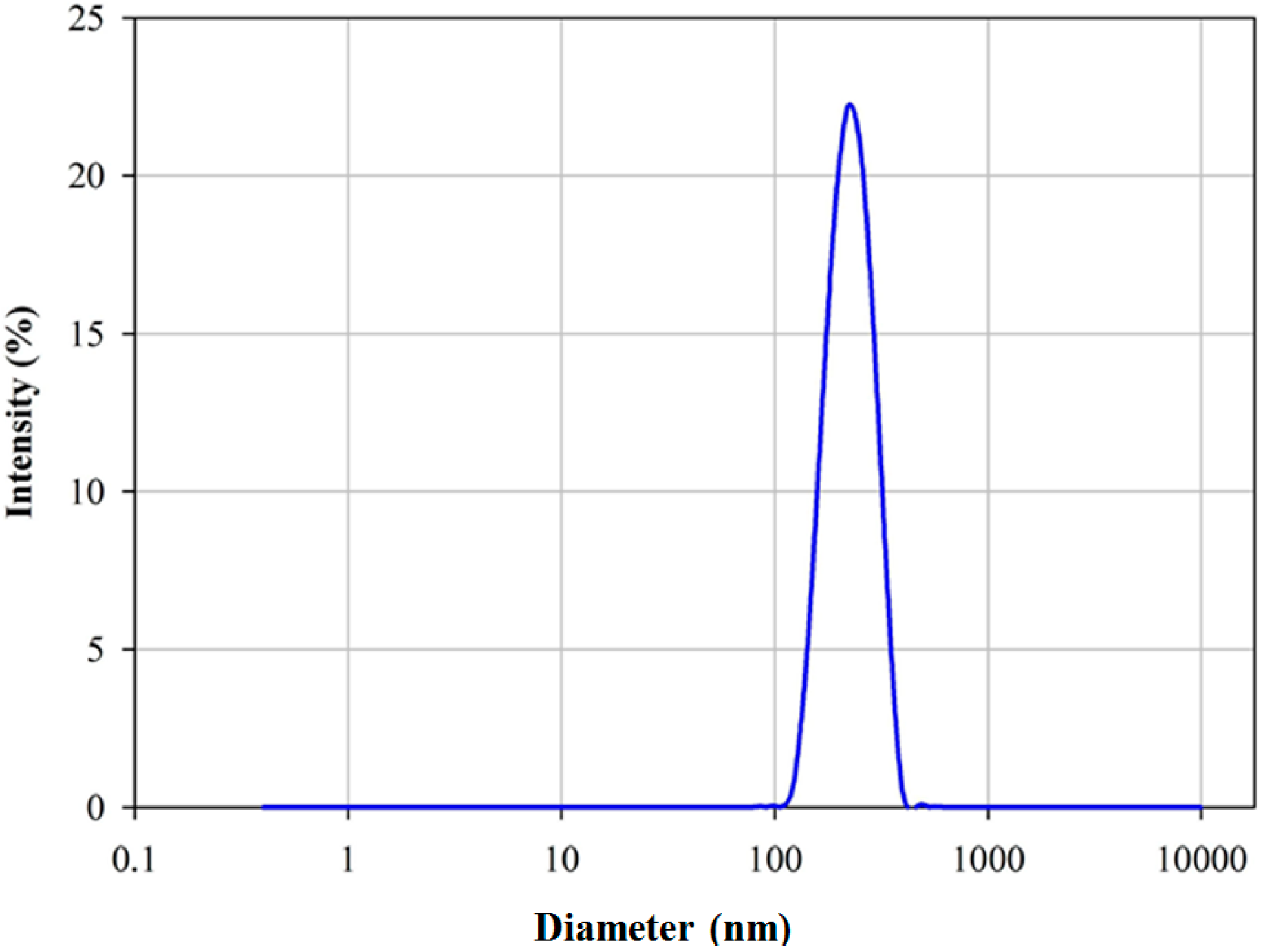
3.2. Stability of Nanofluids
3.3. Thermal Conductivity of Nanofluids
| Mass Fraction of DBSA-PANI Nanoparticles (%) | Thermal Conductivity (W/m·K) | Enhancement in Thermal Conductivity (%) |
|---|---|---|
| 0 | 0.596 ± 0.018 | – |
| 0.2 | 0.602 ± 0.018 | 1.0 |
| 0.4 | 0.604 ± 0.024 | 1.4 |
| 0.6 | 0.607 ± 0.004 | 1.9 |
| 0.8 | 0.616 ± 0.019 | 3.4 |
| 1.0 | 0.628 ± 0.012 | 5.4 |
3.4. Specific Heat Capacity of Nanofluids
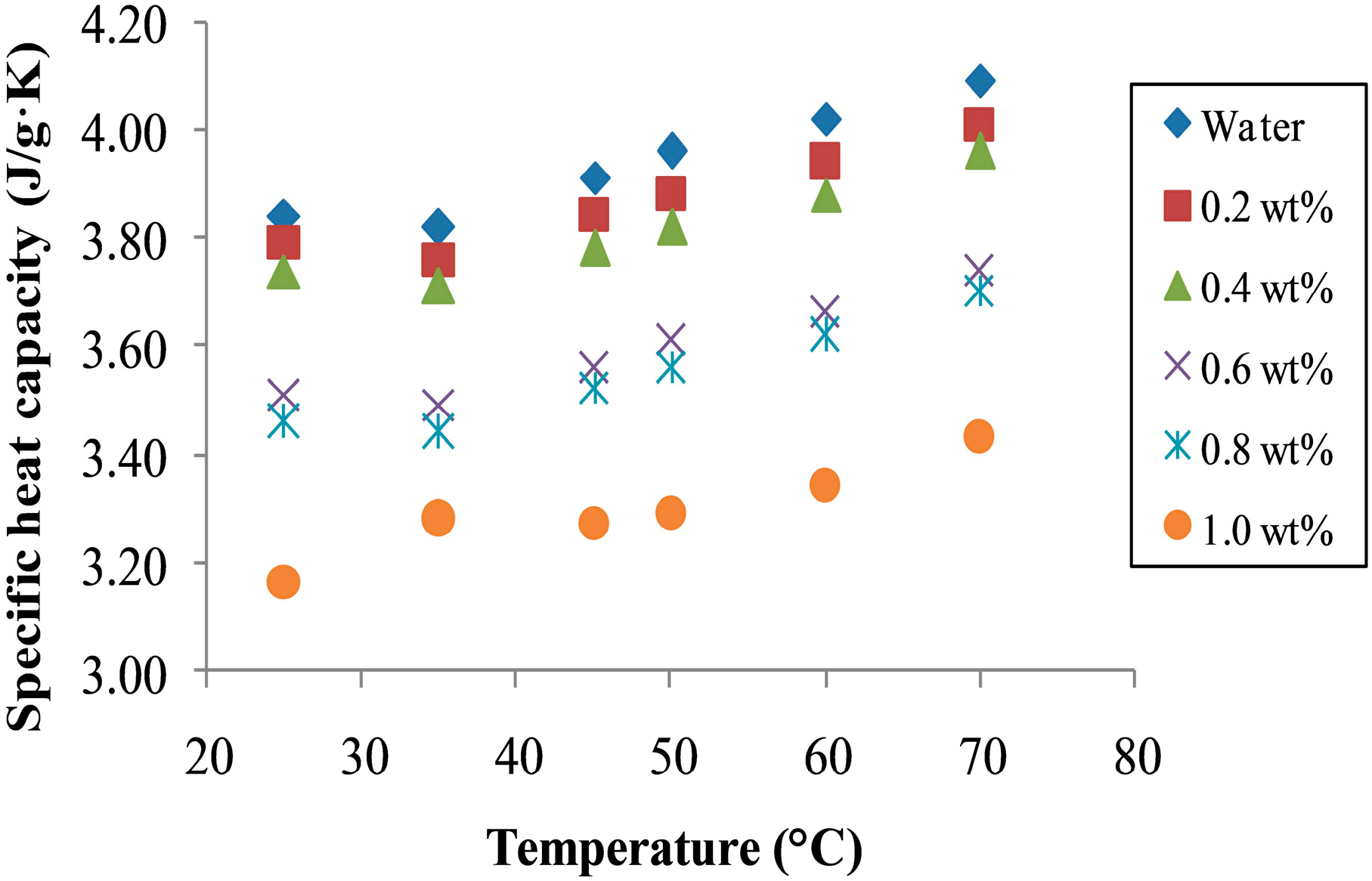
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Thermophysical and electrokinetic properties of nanofluids—A critical review. Appl. Therm. Eng. 2008, 28, 2109–2125. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammad, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57, 582–594. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress & Exposition, San Francisco, CA, USA, November 1995; pp. 99–105.
- Xu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2011, 2012. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.C.-C.; Tsai, C.Y.; Wang, C.-C. Enhancement of thermal conductivity with Cu for nanofluids using chemical reduction method. Int. J. Heat Mass Transf. 2006, 49, 3028–3033. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Lin, Y.-S.; Yin, Y.-S. A novel one-step chemical method for preparation of copper nanofluids. J. Colloid Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surfaces B 2010, 75, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Hou, B.; Lu, Z.; Wang, Z.; Chen, S. Thermal conductivity of nanofluids containing high aspect ratio fillers. Int. J. Heat Mass Transf. 2013, 64, 108–114. [Google Scholar] [CrossRef]
- Chung, S.J.; Leonard, J.P.; Nettleship, I.; Lee, J.K.; Soong, Y.; Martello, D.V.; Chyu, M.K. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Tajik, B.; Abbassi, A.; Saffar-Avval, M.; Najafabadi, M.A. Ultrasonic properties of suspensions of TiO2 and Al2O3 nanoparticles in water. Powder Technol. 2012, 217, 171–176. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Meibodi, M.E.; Vafaie-Sefti, M.; Rashidi, A.M.; Amrollahi, A.; Tabasi, M.; Kalal, H.S. The role of different parameters on the stability and thermal conductivity of carbon nanotube/water nanofluids. Int. Commun. Heat Mass Transf. 2010, 37, 319–323. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 2012, 55, 7969–7980. [Google Scholar] [CrossRef]
- Iranidokht, V.; Hamian, S.; Mohammadi, N.; Shafii, M.B. Thermal conductivity of mixed nanofluids under controlled pH conditions. Int. J. Therm. Sci. 2013, 74, 63–71. [Google Scholar] [CrossRef]
- Halelfadl, S.; Maré, T.; Estellé, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Estellé, P.; Halelfadl, S.; Maré, T. Thermal conductivity of CNT water based nanofluids: Experimental trends and models overview. J. Therm. Eng. 2015, 1, 381–390. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Khedkar, R.S.; Sonawane, S.S.; Wasewar, K.L. Influence of Cuo nanoparticles in enhancing the thermal conductivity of water and monoethylene glycol based nanofluids. Int. Commun. Heat Mass Transf. 2012, 39, 665–669. [Google Scholar] [CrossRef]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, Y.; Chen, L. Developing a novel form of thermal conductivity of nanofluids with brownian motion effect by means of fractal geometry. Powder Technol. 2013, 239, 409–414. [Google Scholar] [CrossRef]
- Xue, L.; Keblinski, P.; Phillpot, S.; Choi, S.-S.; Eastman, J. Effect of liquid layering at the liquid–solid interface on thermal transport. Int. J. Mass Transf. 2004, 47, 4277–4284. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Role of interfacial layer and clustering on the effective thermal conductivity of CuO-Gear oil nanofluids. Exp. Therm. Fluid Sci. 2011, 35, 1490–1495. [Google Scholar] [CrossRef]
- Han, M.G.; Cho, S.K.; Oh, S.G.; Im, S.S. Preparation and characterization of polyaniline nanoparticles synthesized from DBSA micellar solution. Synth. Metals 2002, 126, 53–60. [Google Scholar] [CrossRef]
- Han, D.; Chu, Y.; Yang, L.; Liu, Y.; Lv, Z. Reversed micelle polymerization: A new route for the synthesis of DBSA–polyaniline nanoparticles. Colloids Surfaces A 2005, 259, 179–187. [Google Scholar] [CrossRef]
- Han, Y.-G.; Kusunose, T.; Sekino, T. One-step reverse micelle polymerization of organic dispersible polyaniline nanoparticles. Synth. Metals 2009, 159, 123–131. [Google Scholar] [CrossRef]
- MacDiarmid, A.; Epstein, A.J. The concept of secondary doping as applied to polyaniline. Synth. Metals 1994, 65, 103–116. [Google Scholar] [CrossRef]
- Wan, M.; Yadav, R.; Yadav, K.; Yadaw, S. Synthesis and experimental investigation on thermal conductivity of nanofluids containing functionalized polyaniline nanofibers. Exp. Therm. Fluid Sci. 2012, 41, 158–164. [Google Scholar] [CrossRef]
- Haddad, Z.; Abu-Nada, E.; Oztop, H.F.; Mataoui, A. Natural convection in nanofluids: Are the thermophoresis and Brownian motion effects significant in nanofluid heat transfer enhancement? Int. J. Therm. Sci. 2012, 57, 152–162. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.C.; Wang, C. Enhancements of thermal conductivities with Cu, CuO, and carbon nanotube nanofluids and application of MWNT/water nanofluid on a water chiller system. Nanoscale Res. Lett. 2011, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Sahoo, S.; Pabi, S.; Ghosh, S. On the phononic and electronic contribution to the enhanced thermal conductivity of water-based silver nanofluids. Int. J. Therm. Sci. 2013, 64, 53–61. [Google Scholar] [CrossRef]
- Łużny, W.; Piwowarczyk, K. Hydrogen bonds in camphorsulfonic acid doped polyaniline. Polimery 2011, 56, 652–656. [Google Scholar]
- Shacklette, L. Dipole and hydrogen-bonding interactions in polyaniline: A mechanism for conductivity enhancement. Synth. Metals 1994, 65, 123–130. [Google Scholar] [CrossRef]
- Zhang, L.; Long, Y.; Chen, Z.; Wan, M. The effect of hydrogen bonding on self-assembled polyaniline nanostructures. Adv. Funct. Mater. 2004, 14, 693–698. [Google Scholar] [CrossRef]
- Jung, S.; Jo, B.; Shin, D.; Banerjee, D. Experimental validation of a simple analytical model for specific heat capacity of aqueous nanofluids. Development 2010, 1, 2087. [Google Scholar]
- O’Hanley, H.; Buongiorno, J.; McKrell, T.; Hu, L.-W. Measurement and model validation of nanofluid specific heat capacity with differential scanning calorimetry. Adv. Mech. Eng. 2012, 4, 181079. [Google Scholar] [CrossRef]
- Zhou, S.-Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 1–3. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2010, 1–4. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, T.S.; Daik, R.; Hamid, M.A.A. Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid. Polymers 2015, 7, 1221-1231. https://doi.org/10.3390/polym7071221
Chew TS, Daik R, Hamid MAA. Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid. Polymers. 2015; 7(7):1221-1231. https://doi.org/10.3390/polym7071221
Chicago/Turabian StyleChew, Tze Siong, Rusli Daik, and Muhammad Azmi Abdul Hamid. 2015. "Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid" Polymers 7, no. 7: 1221-1231. https://doi.org/10.3390/polym7071221
APA StyleChew, T. S., Daik, R., & Hamid, M. A. A. (2015). Thermal Conductivity and Specific Heat Capacity of Dodecylbenzenesulfonic Acid-Doped Polyaniline Particles—Water Based Nanofluid. Polymers, 7(7), 1221-1231. https://doi.org/10.3390/polym7071221