Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials
Abstract
:1. Introduction
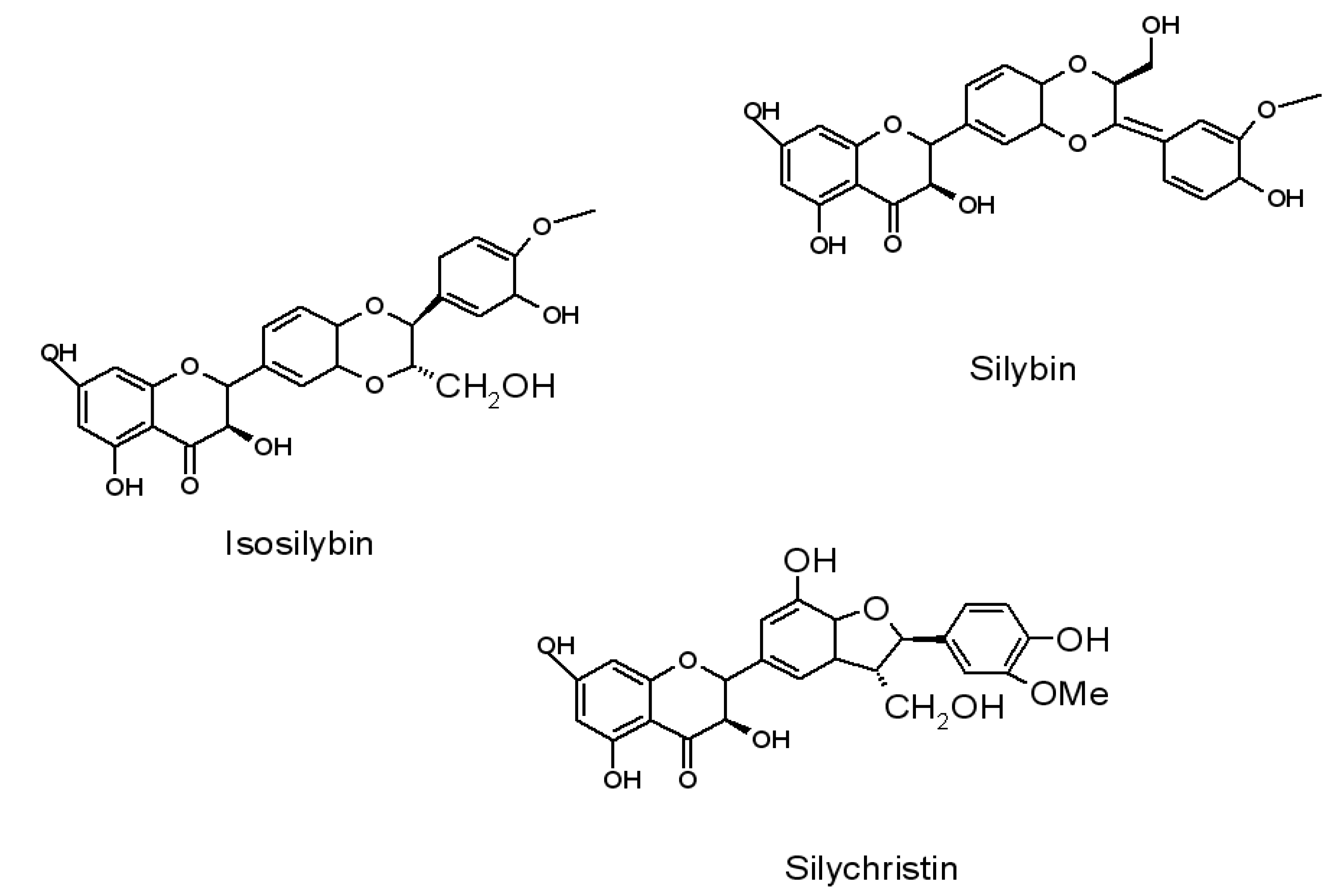
2. Application of Flavonoids to Polymer Stabilization and the Use of Natural Phytocompounds as Color Indicators of Polymer Ageing Time
3. Experimental Section
3.1. Chemicals
3.2. Measurement Methods
3.2.1. EPM Impregnation with Silymarin in Chloroform
3.2.2. Traditional Method of Processing EPM
3.2.3. Accelerated Ageing
3.2.4. The Dispersion Degree of Additives
3.2.5. Attenuated Total Reflectance Fourier Transforms Infrared (ATR-FTIR) Spectroscopy
3.2.6. Color Measurement
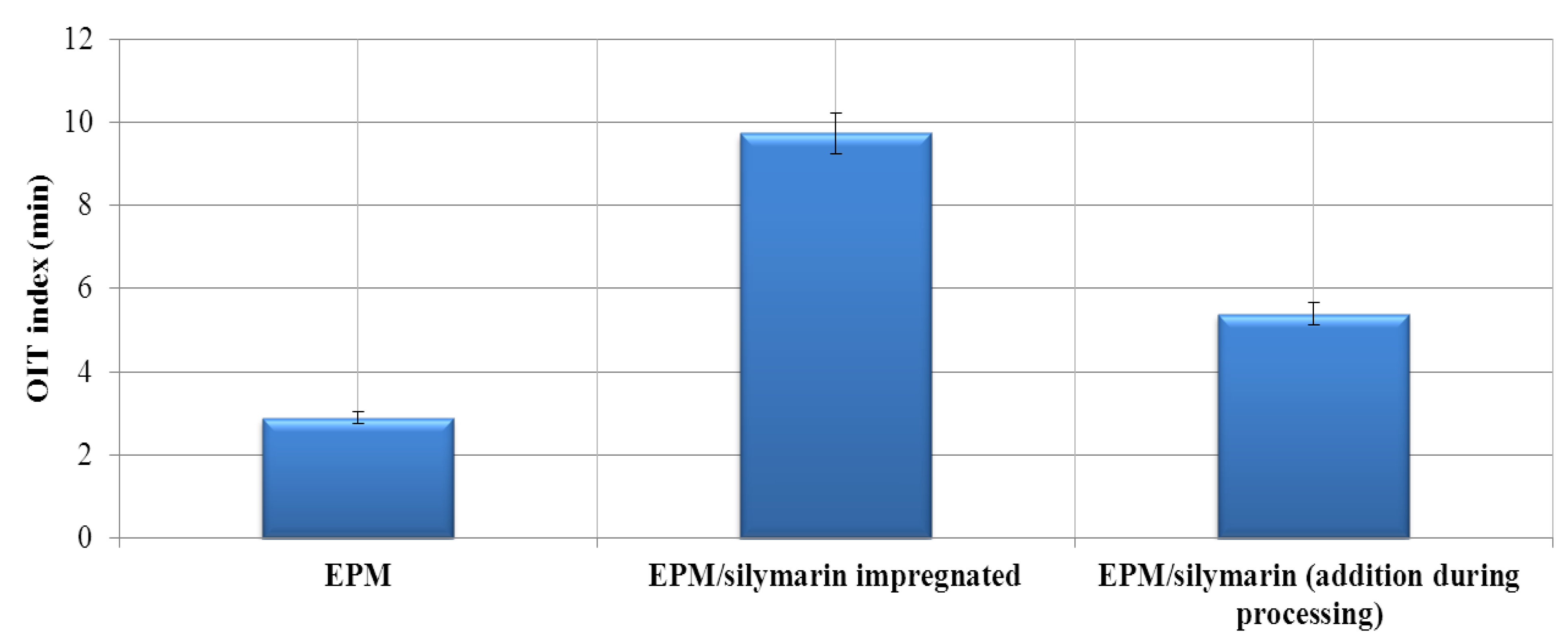
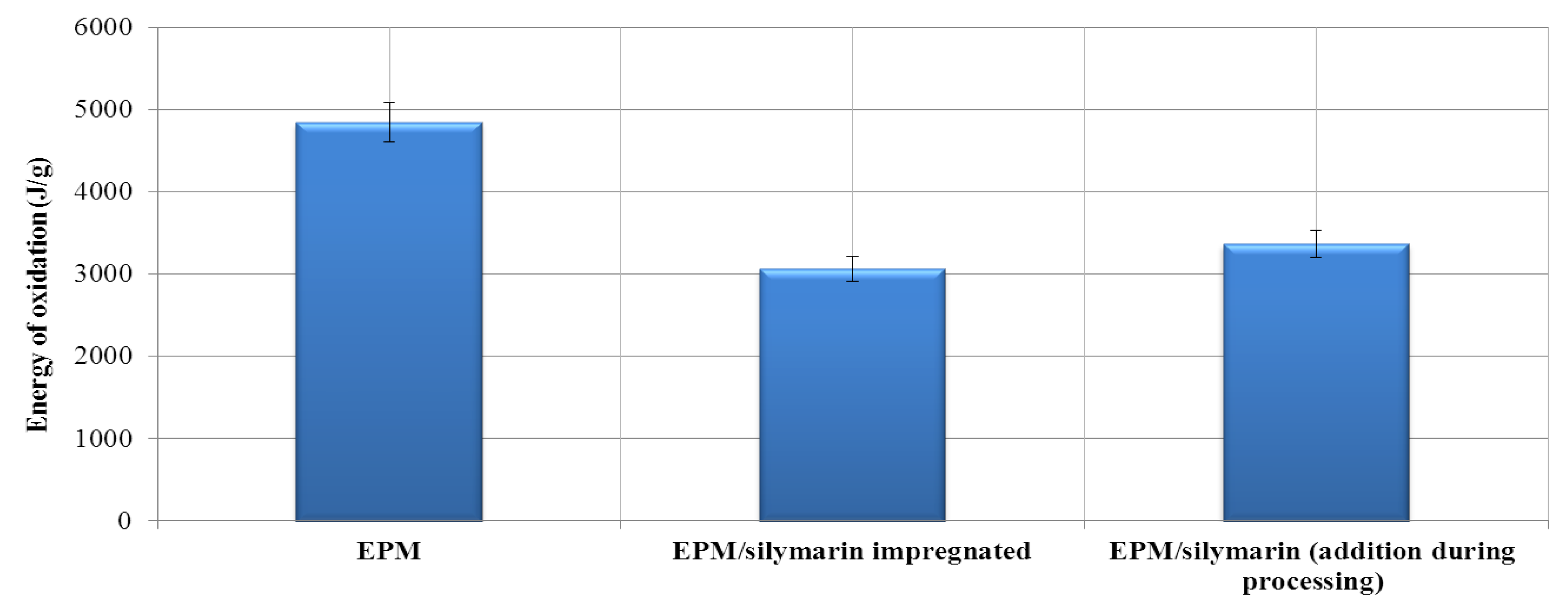
3.2.7. The Oxygen Induction time (OIT)
3.2.8. Thermogravimetric Analysis (TGA)
4. Results and Discussion (Application of Flavonoids for Polymer Stabilization and Use Natural Phytocompounds such as Color Indicator of Polymers Ageing Time)
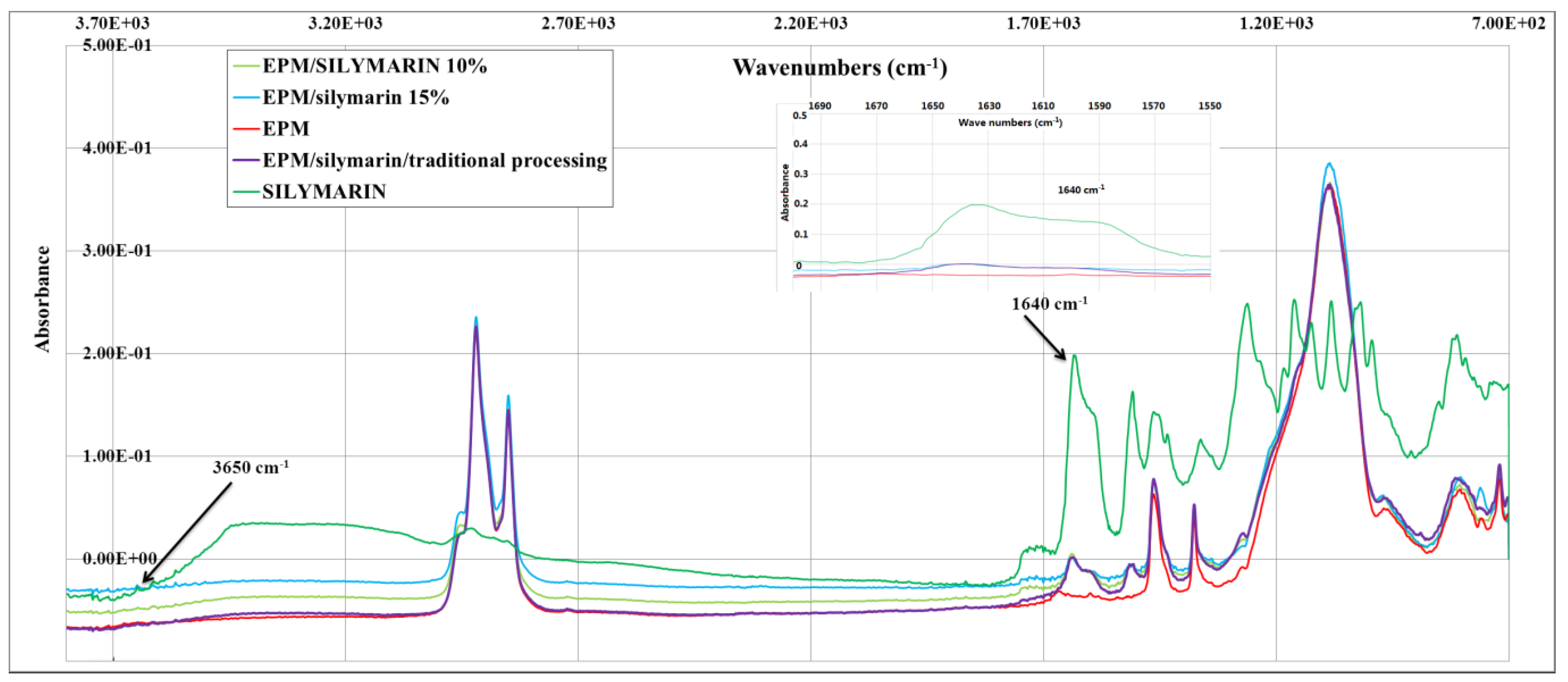
| Material | Atmosphere | Decomposition stage | ||
|---|---|---|---|---|
| First | Second | Third | ||
| Silymarin | Weight loss (%) | 0.63 | 0.89 | 38.43 |
| Temperature range (°C) | 58–99 | 108–150 | From 240 | |
| EPM | Weight loss (%) | 62.24 | 13.81 | – |
| Temperature range (°C) | 267–408 | From 471 | – | |
| EPM/silymarin | Weight loss (%) | 66.33 | 13.05 | – |
| Temperature range (°C) | 283–406 | From 474 | – | |

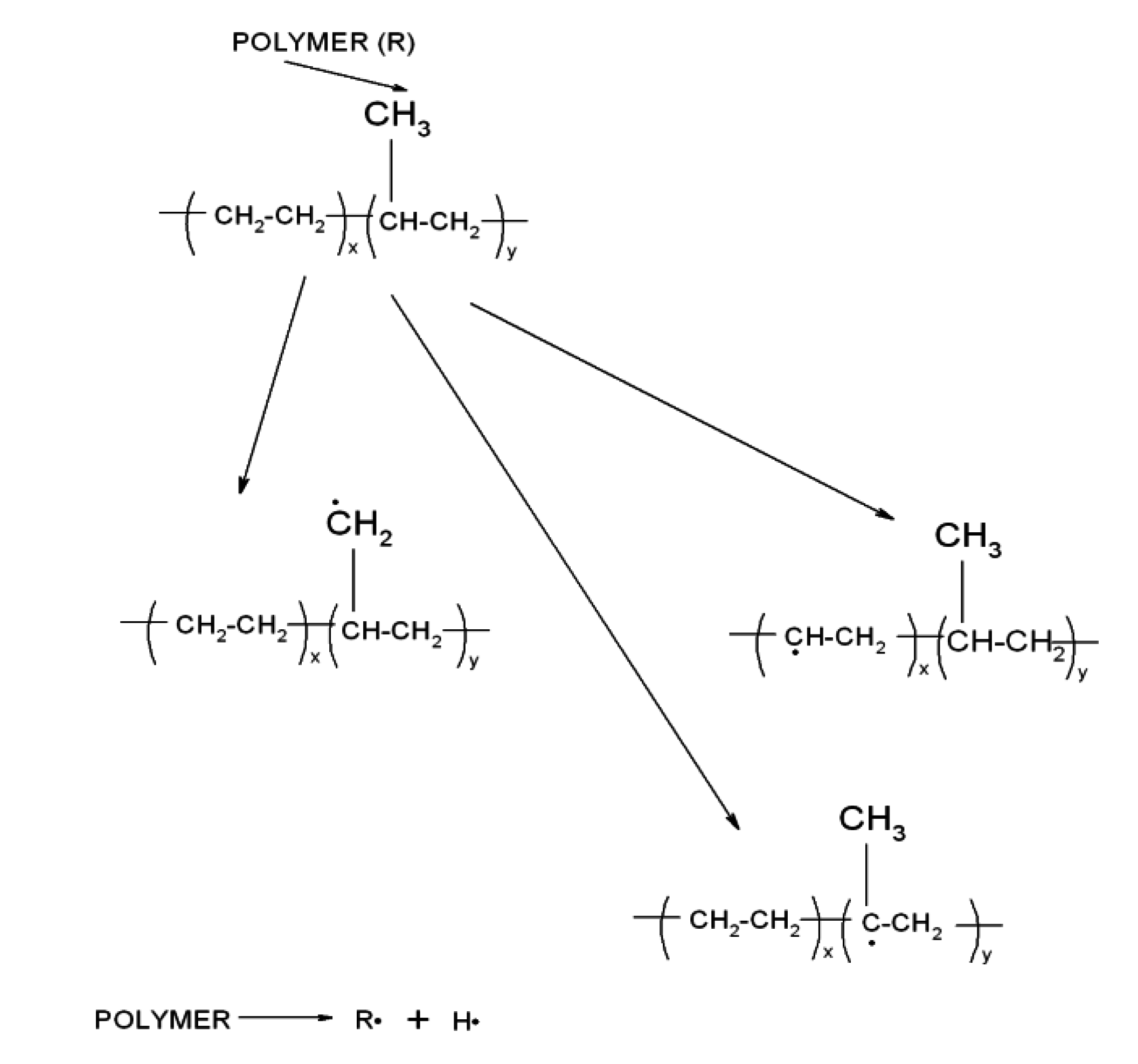
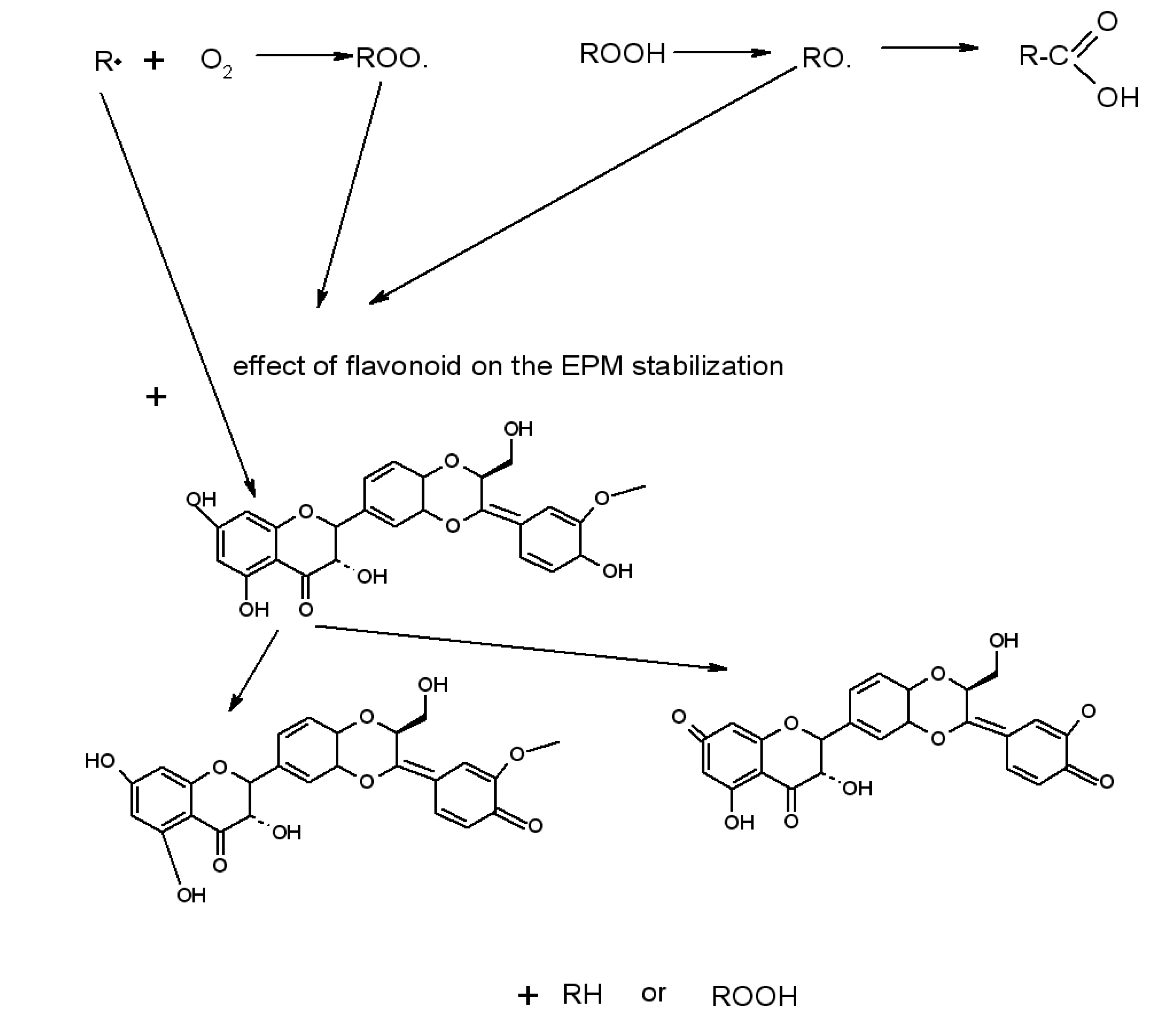
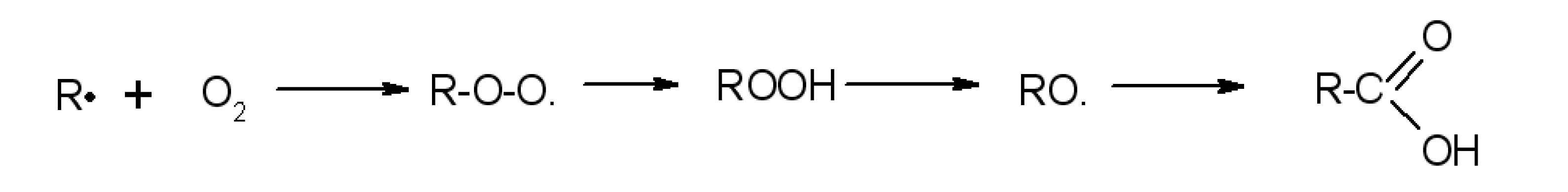
Flavonoid Influence on the Color Stability of Polymeric Materials
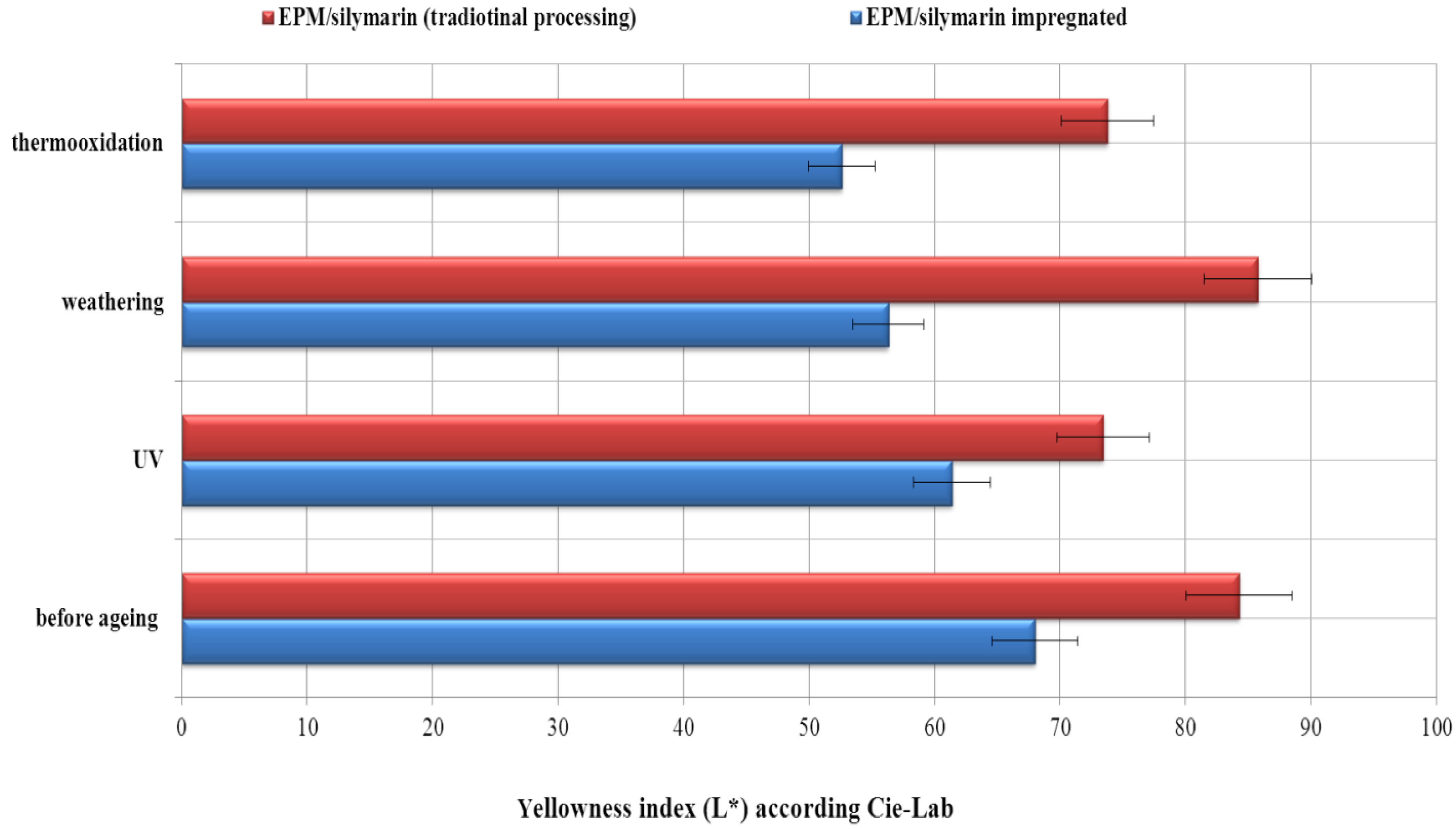
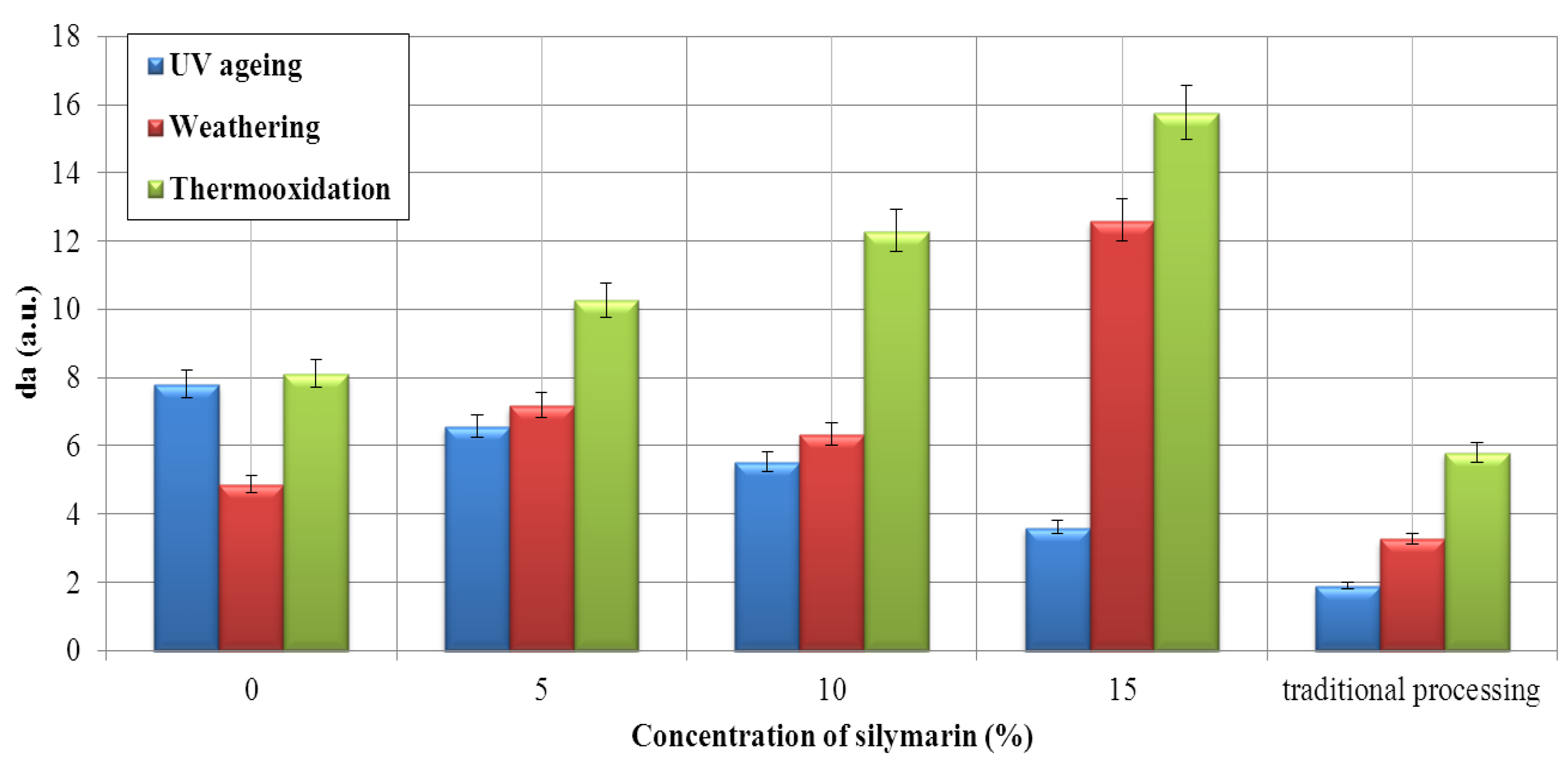

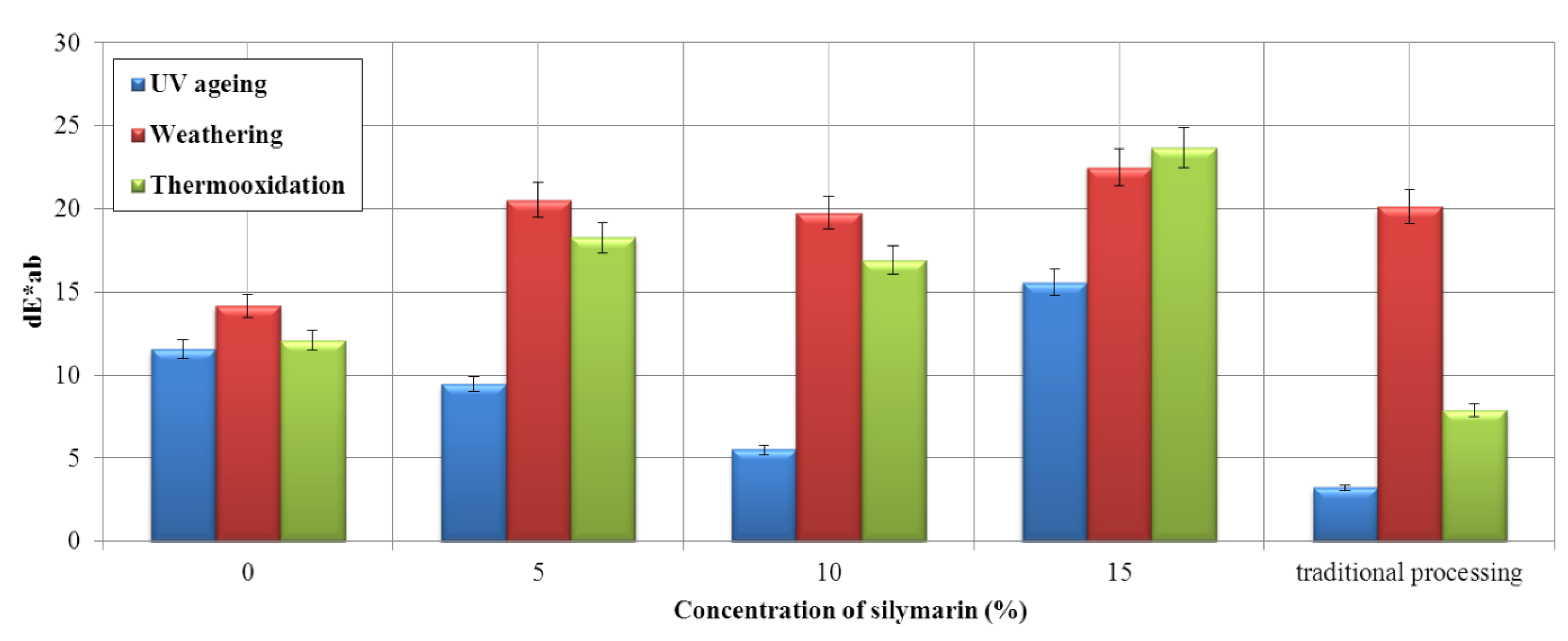
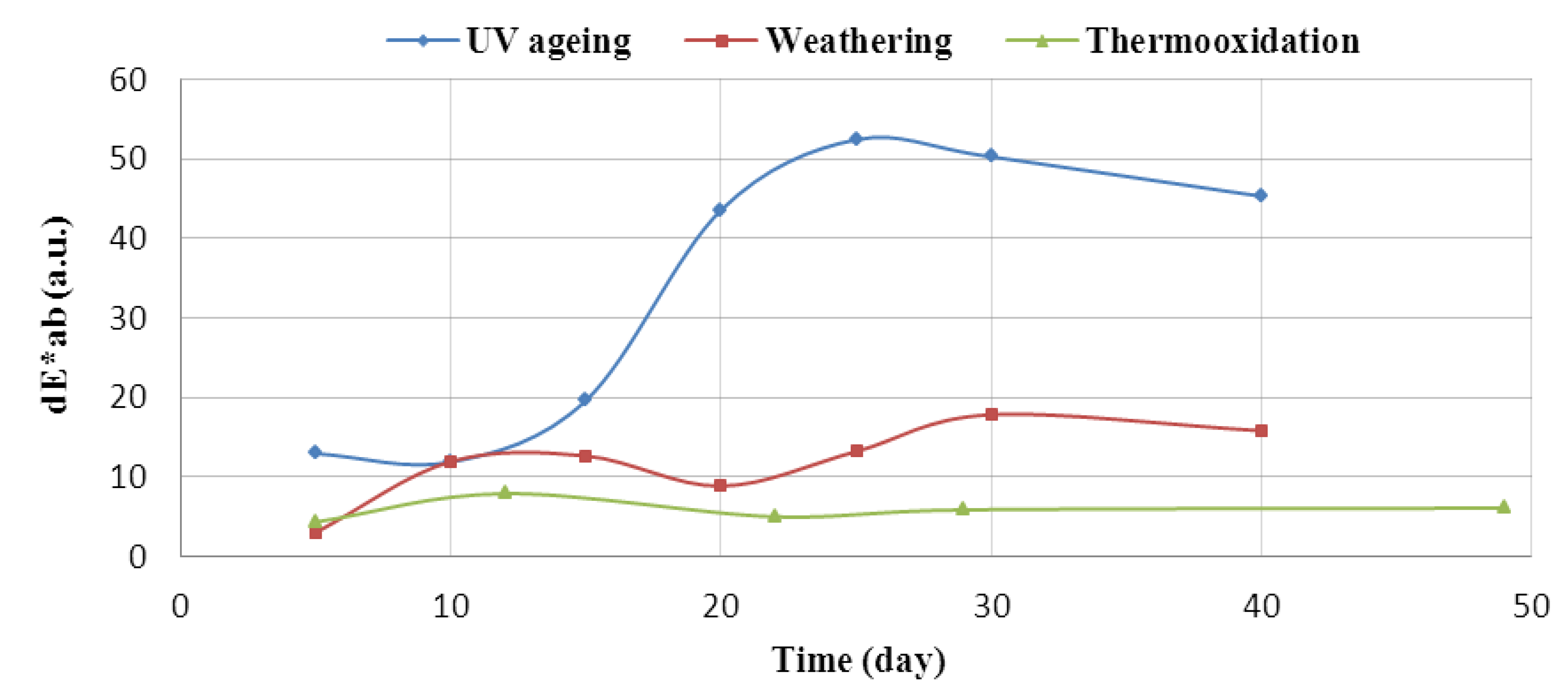
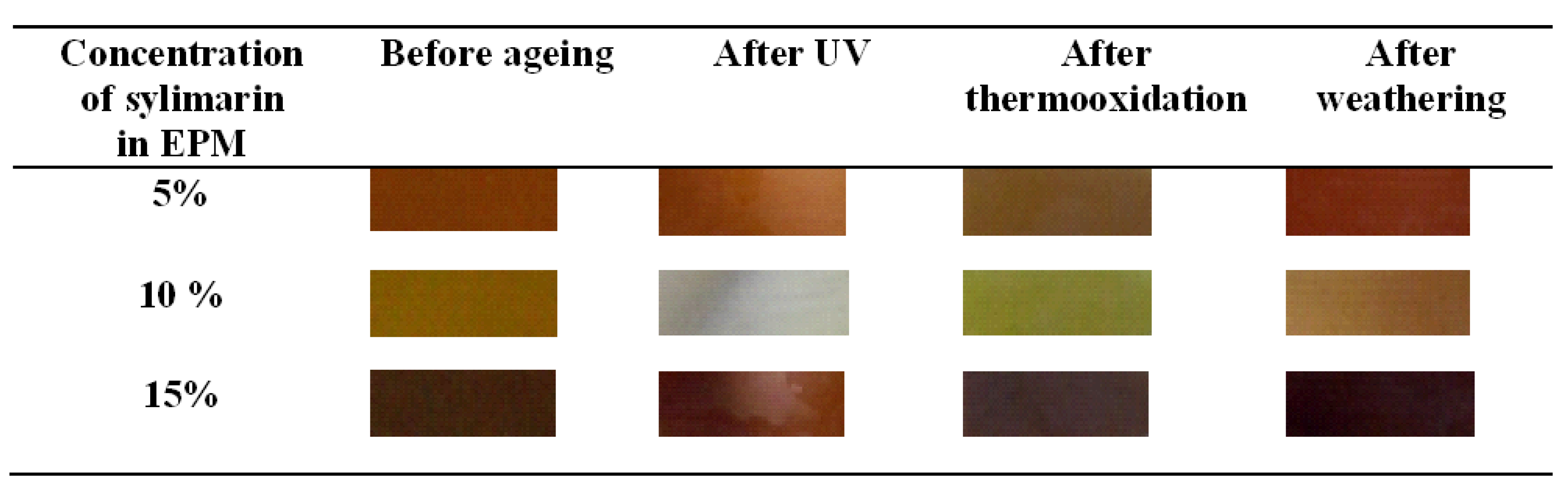
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kriston, I.; Orbán-Mester, Á.; Nagy, G.; Staniek, P.; Földes, E.; Pukánszky, B. Melt stabilisation of Phillips type polyethylene, Part II: Correlation between additive consumption and polymer properties. Polym. Degrad. Stab. 2009, 94, 1448–1456. [Google Scholar] [CrossRef]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin, Germany, 1998; p. 44. [Google Scholar]
- Holmström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. II. Structural changes occurring in low-density polyethylene at an oxygen content less than 0.0005%. J. Appl. Polym. Sci. 1974, 18, 761–778. [Google Scholar] [CrossRef]
- Holström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. III. Structural changes occurring in low-density polyethylene at oxygen contents below 1.2%. J. Appl. Polym. Sci. 1974, 18, 779–804. [Google Scholar] [CrossRef]
- Massey, S.; Adnot, A.; Rjeb, A.; Roy, D. Action of water in the degradation of low-density polyethylene studied by X-ray photoelectron spectroscopy. Express. Polym. Lett. 2007, 8, 506–515. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Relationship between chemical l structure and performance of primary antioxidants in PBD. Polym. Degrad. Stab. 1999, 66, 31–39. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Study of the effects of additive interaction in polymer stabilization. Polym. Prepr. 2001, 42, 365–366. [Google Scholar]
- Anandhan, S.; Viknesh, C.J.; Othman, N.; Sasidharan, S. A new processing additive for natural rubber from agricultural waste. Kaut. Schuk Gummi Kunst. 2011, 64, 44–51. [Google Scholar]
- Fenollar, O.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Lopez, J.; Balart, R. Effect of the epoxidized linseed oil concentration as natural plasticizer in vinyl plastisols. J. Mater. Sci. 2010, 45, 4406–4413. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosanfilms. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Garrigós, M.C.; Jiménez, A. Characterization and thermal stability of poly(vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym. Degrad. Stab. 2010, 95, 2207–2221. [Google Scholar] [CrossRef]
- Yurttas, H.C.; Shafer, H.W.; Warthesen, J.J. Antioxidant activity of nontocopherol hazelnut (Corylus spp.) phenolics. J. Food Sci. 2000, 65, 276–280. [Google Scholar] [CrossRef]
- Zalacain, A.; Carmona, M.; Lorenzo, C.; Blazquez, I.; Alonso, G.L. Antiradical efficiency of different vegetable tannin extracts. J. Am. Leather Chem. Assoc. 2002, 97, 137–142. [Google Scholar]
- Schwarzenbach, K.; Gilg, B.; Muller, D.; Knobloch, G.; Pauquet, J.R.; Rota-Graziosi, P. Antioxidants. In Plastic Additives Handbook; Zweifel, H., Ed.; Hanser: Munich, Germany, 2001; pp. 29–31. [Google Scholar]
- Torikai, A.; Takeuchi, A.; Nagaya, S.; Fueki, K. Photodegradation of polyethylene: Effect of crosslinking on the oxygenated products and mechanical properties. Polym. Photochem. 1986, 7, 199–211. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wile, D.M. The photodegradation of polypropylene films. II. Photolysis of ketonic oxidation products. Macromolecules 1969, 2, 587–597. [Google Scholar] [CrossRef]
- Philippart, J.L.; Sinturel, C.; Arnaud, R.; Gardette, J.L. In fluence of the exposure parameters on the mechanism of photooxidation of polypropylene. Polym. Degrad. Stab. 1999, 64, 213–225. [Google Scholar] [CrossRef]
- Mita, I.; Jellinek, H.H. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978; p. 24. [Google Scholar]
- Kellen, T. Polymer Degradation; Van Nostrand Reinhold: New York, NY, USA, 1983; p. 7. [Google Scholar]
- Ivan, B.; Kellen, T.; Tudos, F.; Jellinek, H.H.; Kachi, H. Degradation and Stabilisation of Polymers; Elsevier: Amsterdam, The Netherlands, 1989; volume 2, p. 570. [Google Scholar]
- Cooray, B.; Scott, G.; Scott, G. Developments in Polymer Stabilisation–2; Applied Science Publishers: London, UK, 1980; p. 419. [Google Scholar]
- Al-Malaika, S.; Ashley, H.; Issenhuth, S. The antioxidant role of α-tocopherol in polymers. I. The nature of transformation products of α-tocopherol formed during melt processing of LDPE. J. Polym. Sci. A 1994, 32, 3099–3113. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Goodwin, C.; Issenhuth, S.; Burdick, D. The antioxidant role of alpha-tocopherol in polymers II. Melt stabilising effect inpolypropylene. Polym. Degrad. Stab. 1999, 64, 145–156. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Issenhuth, S. The antioxidant role of alpha-tocopherol in polymers III. Nature of transformation products during polyolefins extrusion. Polym. Degrad. Stab. 1999, 65, 143–151. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Kosmalska, A. Derivatives of flavonoides as anti-aging substances in elastomers. Compt. Rendus Chim. 2011, 14, 483–488. [Google Scholar] [CrossRef]
- Peng, Z.; Liang, X.; Zhang, Y.; Zhang, Y. Reinforcement of EPDM by in situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2002, 84, 1339–1345. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Basfar, A.A. Evaluation of some antioxidants in radiation vulcanized ethylene-propylene diene (EPDM) rubber. Nucl. Instrum. Meth. B 2001, 185, 346–350. [Google Scholar] [CrossRef]
- Noronha, F.; Angelini, J.; Góis, N.; Mei, L. Performance development requirementsfor elastomers of electric power network insulators. J. Mater. Process. Technol. 2005, 162, 102–108. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Zhang, J.; Shi, D. Influence of different antioxidants on cure kinetics and aging behaviours of ethylene propylene diene rubber/low density polyethylene blends. Plast. Rubber Compos. 2009, 38, 187–194. [Google Scholar] [CrossRef]
- Tsepalov, V.F.; Kharitonova, A.A.; Gladyshev, G.P.; Emanuel, N.M. Determination of the rate constants and inhibition coefficients of phenol antioxidants with the aid of model chain reactions. Kinet. Catal. 1977, 18, 1034–1041. [Google Scholar]
- Gugumus, F. New trends in the stabilization of polyolefin fibers. Polym. Degrad. Stab. 1994, 44, 273–297. [Google Scholar] [CrossRef]
- Thompson, C.R.; Moore, J.C.; Saal, S.F.; Swan, H.S. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, F.; Albertsson, A.C. Rapid test methods for analysing degradable polyolefins with a pro-oxidant system. J. Appl. Polym. Sci. 2001, 79, 2309–2316. [Google Scholar] [CrossRef]
- Kelen, T. Polymer Degradation; Van Nostrand Reinhold: New York, NY, USA, 1983; p. 421. [Google Scholar]
- Dixon, R.K. Thermal ageing predictions from an Arrhenius plot with only one data point. IEEE T. Dielect. El. In. 1980, E1–15, 331–340. [Google Scholar]
- Gugumus, F. The Use of Accelerated Tests in the Evaluation of Antioxidantsand Light Stabilizers. In Developments in Polymer Stabilization; Scott, G., Ed.; Applied Science Publishers: Barking, UK, 1987; pp. 239–289. [Google Scholar]
- Scoponi, M.; Pradella, F.; Carassiti, V. Photodegradable polyolefins. Photo-oxidation mechanisms of innovative polyolefin copolymers containing double bonds. Coordin. Chem. Rev. 1993, 125, 219–230. [Google Scholar] [CrossRef]
- Davis, A.; Sims, D. Weathering of Polymers; Applied Science Publishers: London, UK, 1983; pp. 55–57. [Google Scholar]
- Gijsman, P.; Hennekens, J.; Janssen, K. Polymer Durability, Degradation, Stabilization and Lifetime Prediction; Clough, R.L., Billingham, N.C., Gillen, K.T., Eds.; Advances in Chemistry Series 249; American Chemical Society: Washington, DC, USA, 1996; pp. 622–648. [Google Scholar]
- Thorat, H.B.; Prabhu, C.S.; Sureshkumar, K.; Pandya, M.V. Stabilization of ethylene-propylene copolymer against g-ray induced degradation. Radiat. Phys. Chem. 1998, 51, 215. [Google Scholar]
- Zaharescu, T.; Giurginca, M.; Jipa, S.; Podina, C. Radiochemical oxidation of ethylene–propylene elastomers in the presence of some phenolic antioxidants. Polym. Degrad. Stab. 1999, 63, 245–251. [Google Scholar] [CrossRef]
- Mitra, S.; Ghanbari-Siahkali, A.; Kingshott, P.; Hvilsted, S.; Almdal, K. An investigation on changes in chemical properties of pure ethylene-propylene-diene rubber in aqueous acidic environments. Mater. Chem. Phys. 2006, 98, 248–255. [Google Scholar] [CrossRef]
- Rivaton, A.; Cambon, S.; Gardette, J.L. Radiochemical ageing of ethylene–propylene–diene monomer elastomers. 1. Mechanism of degradation under inert atmosphere. J. Polym. Sci. Polym. Chem. 2004, 42, 1239–1248. [Google Scholar] [CrossRef]
- Gillen, K.T.; Clough, R.L.; Wise, J. Prediction of Elastomer Lifetimes from Accelerated Thermal-Ageing Experiments, ASC 249. In Polymer Durability; American Chemical Society Publications: Washington, DC, USA, 1993; pp. 557–575. [Google Scholar]
- Arakawa, K.; Seguchi, T.; Hayakawa, N.; Machi, S. Radiation-induced oxidation of polymers. Effect of antioxidant and antirad agent on oxygen consumption and gas evolution. J. Polym. Sci. 1983, 21, 1173–1181. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Characteristics of curcumin using cyclic voltammetry, UV–VIS, fluorescence and thermogravimetric analysis. Electrochem. Acta 2013, 107, 441–447. [Google Scholar] [CrossRef]
- Heller, H.J. Photochemistry of dyed and pigmented polymers. Eur. Polym. J. Suppl. 1969, 99–105. [Google Scholar]
- Rabek, J.F. Polymer photodegradation-mechanisms and experimental methods. Chapman Hall: Cambridge, UK, 1995; pp. 300–600. [Google Scholar]
- Rabek, J.F. Polymer Photodegradation of Polymers: Physical Characteristic and Applications; Springer-Verlag: Berlin, Germany, 1996. [Google Scholar]
- Ranby, B.G.; Rabek, J.F. Photodegradation, Photooxidation and Photostabilization of Polymers; John Wiley & Sons: New York, NY, USA, 1975; pp. 101–102. [Google Scholar]
- Masek, A.; Chrzescijanska, E.; Zaborski, M.; Piotrowska, M. Dodecyl gallate as a pro-ecological antioxidant for food packing materials. Compt. Rendus Chim. 2014, 17, 1116–1127. [Google Scholar] [CrossRef]
- Wypych, J. Handbook of Polymers; Chem. Tec. Publishing: Toronto, Ontario, Canada, 2012; pp. 200–450. [Google Scholar]
- Cirillo, G.; Iemma, F. Antioxidant Polymers: Synthesis, Properties, and Applications; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Ryun Oh, D.; Hyun-Kyu, K.; Lee, N.; Ho Chae, K.; Kaang, S.; Sung Lee, M.; Hyeon Kim, T. Synthesis of new polymeric antioxidants. Bull. Korean Chem. Soc. 2001, 22, 629–632. [Google Scholar]
- Masek, A.; Zaborski, M.; Chrześciajańska, E. Morin hydrate as pro-ecological antioxidant and pigment for polyolefin polymers. Compt. Rendus Chim. 2013, 16, 990–996. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M. Polymer biocomposites ENR/PCL from renewable resources. Compt. Rendus Chim. 2014, 17, 944–951. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Piotrowska, M. Controlled degradation of biocomposites ENR/PCL containing natural antioxidants. Compt. Rendus Chim. 2014, 17, 1128–1135. [Google Scholar] [CrossRef]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Antioxidative Properties of silymarin, 7-aminoflavone, neohesperidin dihydrochalcone and trihydroxyethylenorutin studied by the electrochemical methods. Int. J. Electrochem. Sci. 2014, 9, 7875–7889. [Google Scholar]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Electrochemical properties of catechin in non-aqueous media. Int. J. Electrochem. Sci. 2015, 10, 2504–2514. [Google Scholar]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Voltammetric and FTIR spectroscopic studies of the oxidation of retinyl propionate at Pt electrode in non-aqueous media. Int. J. Electrochem. Sci. 2014, 6, 6809–6820. [Google Scholar]
- Garcia, J.P.D.; Hsieh, M.F.; Doma, B.T., Jr.; Peruelo, D.C.; Chen, I.-H.; Lee, H.-M. Synthesis of Gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from Camellia sinensis. Polymers 2014, 6, 39–58. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of liquid natural rubber via oxidative degradation of natural rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Pretsch, T. Review on the functional determinants and durability of shape memory polymers. Polymers 2010, 2, 120–158. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Protective effects of silymarin, a milk thistle (silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J. Biochem. Biophys. 2006, 43, 306–311. [Google Scholar] [PubMed]
- Galhardi, F.; Mesquita, K.; Monserrat, J.M.; Barros, D.M. Effect of silymarin on biochemical parameters of oxidative stress in aged and young rat brain. Food Chemi. Toxicol. 2009, 47, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Meleth, S.; Sharma, S.D. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem. Photobiol. 2008, 84, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H. Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Zaborski, M.; Chrześcijańska, E. Electrochemical oxidation of flavonoid derivatives. Food Chem. 2011, 127, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile redox chemistry complicates antioxidant capacity assessment: Flavonoids as milieu-Dependent anti- and pro-oxidants. Int. J. Mol. Sci. 2013, 14, 11830–11841. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; de Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Kachlicki, P.; Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 47–70. [Google Scholar]
- The regulation of the European Union No. 10/2011. Available online: https://www.fsai.ie/uploadedFiles/Reg10_2011.pdf (accessed on 14 January 2011).
- Kvasnika, F.; Biba, B.; Sevcík, R.; Voldrich, M.; Krátká, J. Analysis of the active components of silymarin. J Chromatogr. A 2003, 990, 239–245. [Google Scholar] [CrossRef]
- PN-EN 728:1999. “Plastic Piping Systems and Ducting Systems. Polyolefin Pipes and Fittings. Determination of Oxidation Induction Time”. Available online: http://www.pkn.pl (accessed on 28 January 1999).
- ISO 11357–6:2002. “Plastics-DSC-Part 6: Determination of Oxygen Induction Time”. 2002. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=42718 (accessed on 21 June 2002).
- Dabkowski, Z. Lifetime prediction for polymer materials using OIT measurements by the DSC method. Polimery 2005, 50, 231–215. [Google Scholar]
- Griesbach, R. Biochemistry and genetics of flower colour. Plant Breed. Rev. 2005, 25, 89–114. [Google Scholar]
- Kevan, P.; Giurfa, M.; Chittka, L. Why are there so many and so few white flowers? Trends in Plant Sci. 1996, 1, 280–284. [Google Scholar] [CrossRef]
- Niyogi, K. Safety values for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–560. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Deroles, S.; Bennett, R.; Davies, K. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol. 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Shiono, M. Structure of the blue cornflower pigment. Nature 2005, 436, 791. [Google Scholar] [CrossRef] [PubMed]
- Młodzińska, E. Survey of plant pigments: molecular and environmental determinants of plant colors. Acta Biol. Crac. Ser. Bot. 2009, 51, 7–16. [Google Scholar]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Rao, V.; Balachandran, B.; Shen, H.; Logan, A.; Rao, L. In vitro and in vivo antioxidant properties of the plant-based supplement greens. Int. J. Mol. Sci. 2011, 12, 4896–4908. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125-1144. https://doi.org/10.3390/polym7061125
Masek A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers. 2015; 7(6):1125-1144. https://doi.org/10.3390/polym7061125
Chicago/Turabian StyleMasek, Anna. 2015. "Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials" Polymers 7, no. 6: 1125-1144. https://doi.org/10.3390/polym7061125
APA StyleMasek, A. (2015). Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers, 7(6), 1125-1144. https://doi.org/10.3390/polym7061125





