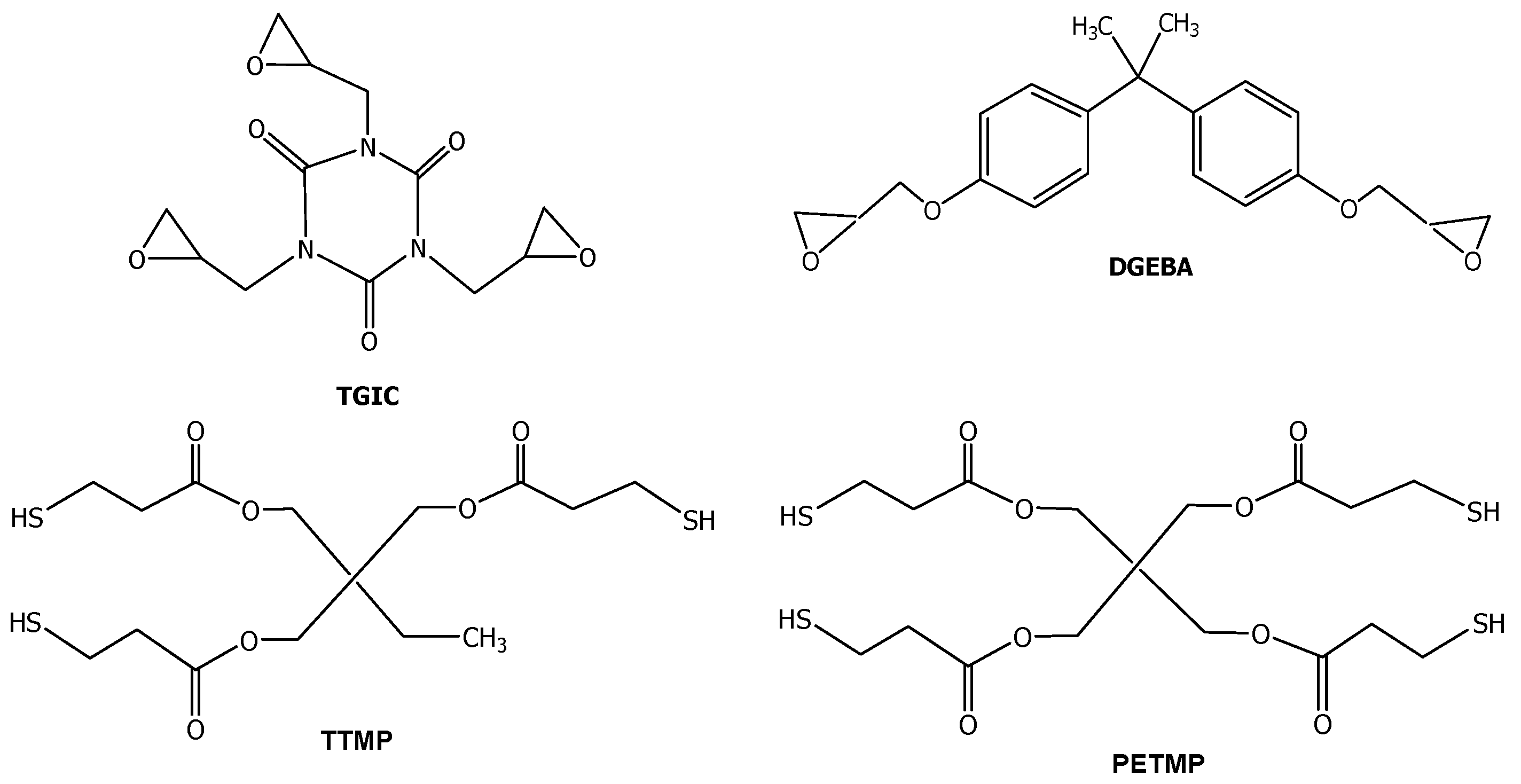
3.1. Study of the Curing Process
To prepare homogenous thermosets with the expected degree of curing from stoichiometric formulations, a good compatibility among reactants is always needed. Thus, the compatibility of resins and curing agents is the key point in the selection of the components in the curing mixture.
The substitution of DGEBA by the trifunctional TGIC to prepare thiol epoxy thermosets with high Tg presents the challenge of low compatibility between both components, which have a very low miscibility. Indeed, the resins needed to be heated to 150 °C—significantly above the melting temperature of TGIC—to allow preparation of homogeneous mixtures. According to that, TGIC/DGEBA mixtures in different proportions (0%, 10%, 20%, 30% and 40% w/w of TGIC in reference to the epoxy resin) were prepared. It should be said that he maximum proportion of the trifunctional resin allowing homogeneous mixture was 40%.
The substitution of trifunctional by tetrafunctional thiol was only studied for the neat DGEBA formulation and the formulation containing a 30% of TGIC (in reference to the resin), because 40% of TGIC led to inhomogeneities in the cured sample.
Table 1 collects the composition of all the formulations studied which also contains a 0.5 phr of LC-80 as the amine precursor. This table also contains the enthalpies per gram and epoxy equivalent evolved during curing.
Table 1.
Notation, composition and calorimetric data for the mixtures studied.
Table 1.
Notation, composition and calorimetric data for the mixtures studied.
| Notation | TGIC (mmol/g) | TTMP (mmol/g) | PETMP (mmol/g) | DGEBA (mmol/g) | ΔH a (J/g) | ΔH b (kJ/eq) | Tpeak c (°C) |
|---|
| Neat (TTMP) | 0 | 1.05 | 0 | 1.59 | 394 | 125 | 137 |
| 10%TGIC/TTMP | 0.19 | 1.10 | 0 | 1.38 | 415 | 125 | 136 |
| 20%TGIC/TTMP | 0.36 | 1.15 | 0 | 1.18 | 427 | 124 | 134 |
| 30%TGIC/TTMP | 0.53 | 1.20 | 0 | 1.00 | 432 | 124 | 131 |
| 40%TGIC/TTMP | 0.68 | 1.24 | 0 | 0.83 | 452 | 123 | 129 |
| Neat (PETMP) | 0 | 0 | 0.82 | 1.64 | 415 | 127 | 123 |
| 30%TGIC/PETMP | 0.55 | 0 | 0.93 | 1.04 | 470 | 126 | 122 |
As we can see, there are some variations in the enthalpy released by gram but values for epoxy equivalent are similar among the different formulations, indicating that the degree of curing is similar. The values obtained for the enthalpy range between 123 and 127 KJ/eq, which indicates that the complete curing of epoxides has been achieved [
16].
To know the kinetic influence of adding TGIC to the curing mixture of the epoxy/thiol formulations, dynamic scanning calorimetry in dynamic and isothermal modes was performed.
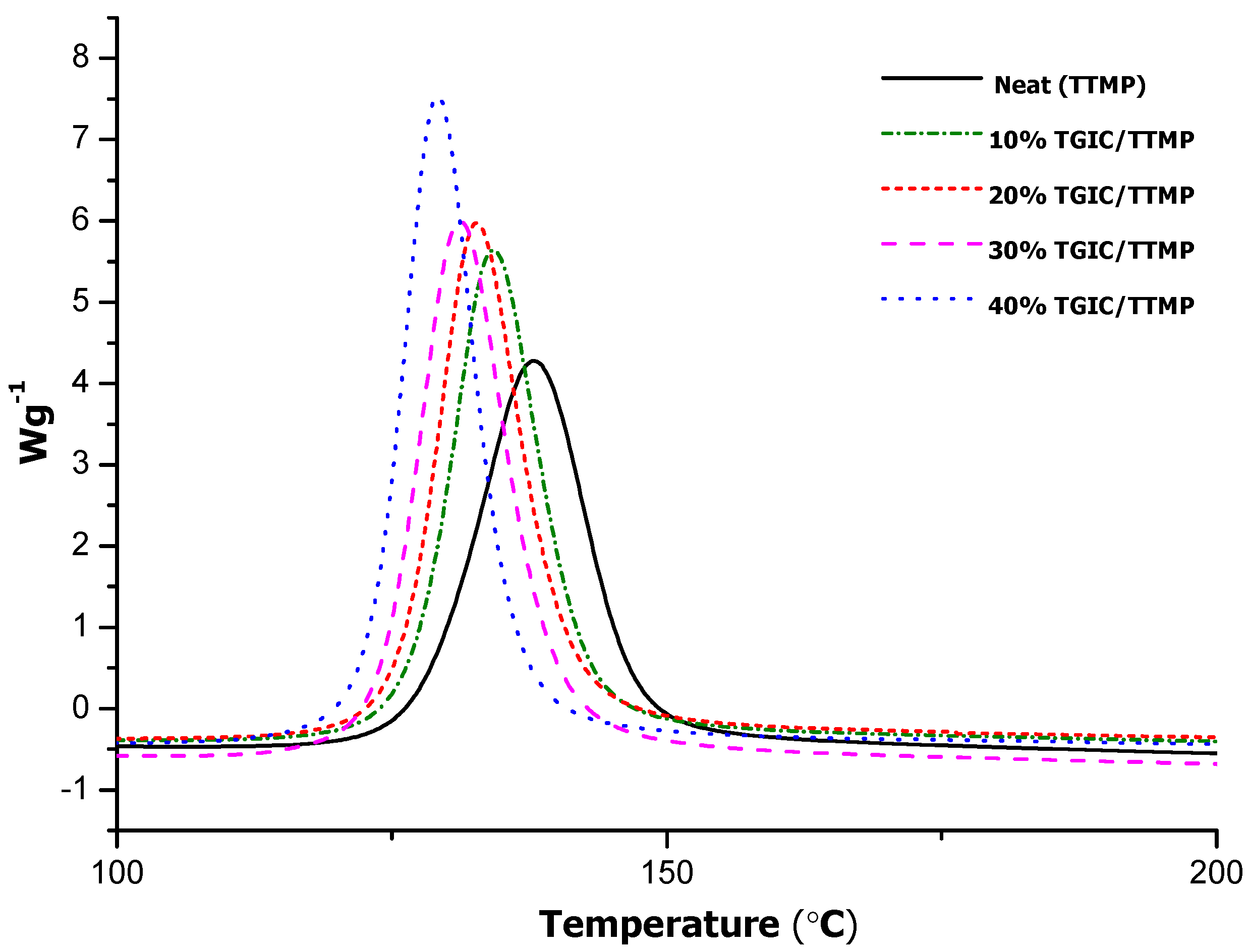
Figure 1 shows the DSC thermograms of the dynamic curing studies at 10 °C/min for mixtures DGEBA/TTMP with different proportions of TGIC.
As can be observed, the curing of the different formulations starts at temperatures higher than 110 °C. It should be taken into account that LC-80 has a melting onset temperature of 60 °C determined by DSC, although it is described as a melting point at about 100 °C in its data sheet [
17]. After melting, the encapsulated imidazole is released, but the activation of the reaction is not immediate, leading to the initiation of the curing at about 110 °C. On increasing the proportion of TGIC in the formulation, the curing exotherm is shifted to lower temperatures and increases the curing rate. The increase in reaction rate seems to be connected with the increase in the amount of reactive epoxy and thiol groups in the mixture, as deduced from the data shown in
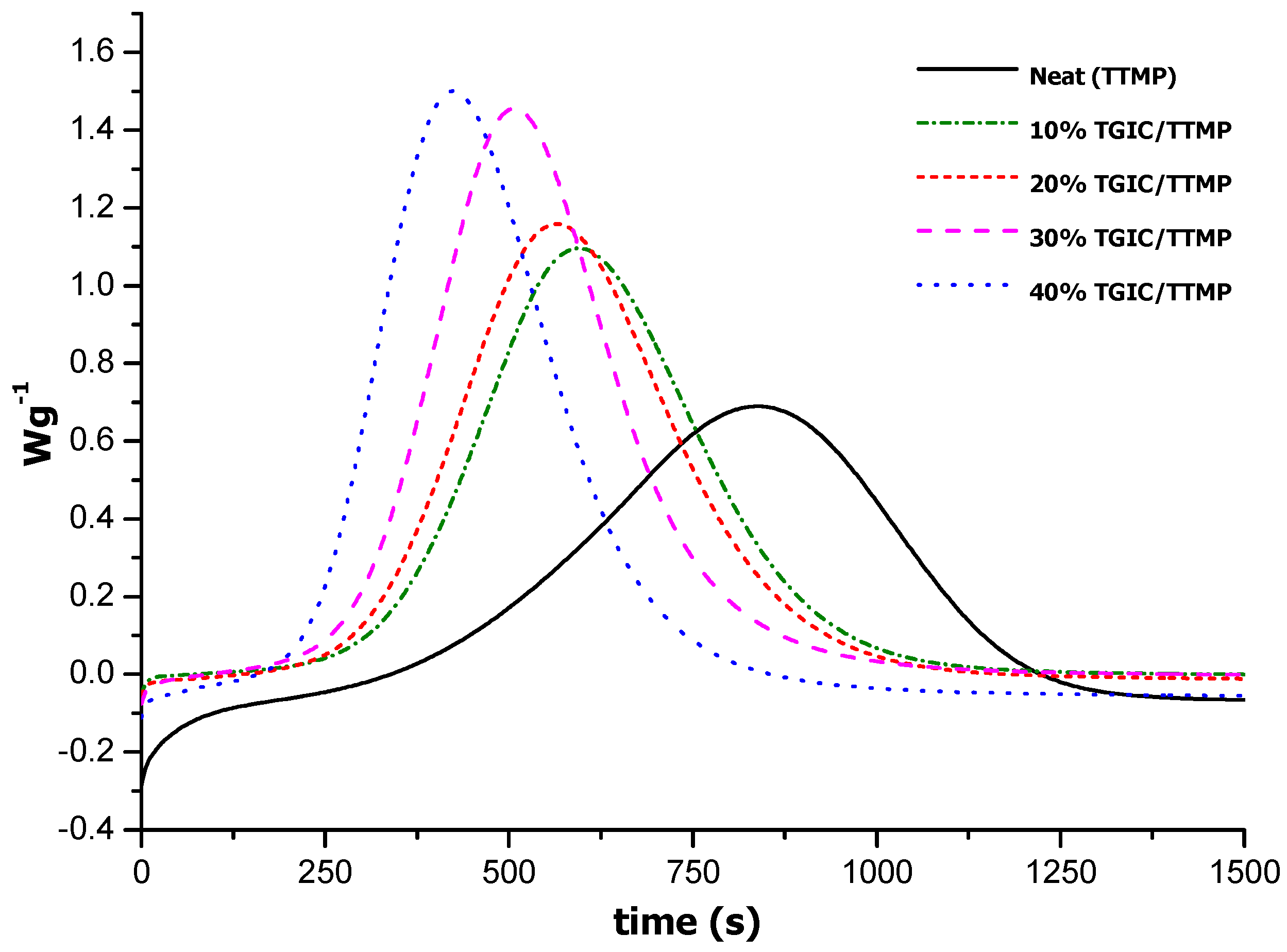
Table 1, but it might be that TGIC is more active than DGEBA in the reaction with thiolates, allowing it to cure in a shorter time. The trend observed in the dynamic curing experiments was confirmed by isothermal curing at 100 °C, as shown in
Figure 2.
As we can see, on increasing the proportion of TGIC, the curing time is notably reduced at 100 °C, but a first induction time, related to the slow release of the catalyst from its encapsulation and activation of the curing process, is observed for all formulations. The most notable difference among the curves is the longer time needed to complete the cure for the neat formulation, which takes more than 20 min.
Figure 1.
DSC thermograms corresponding to the dynamic curing at 10 °C/min of mixtures with TTMP and different proportions of TGIC catalyzed by 0.5 phr of LC-80.
Figure 1.
DSC thermograms corresponding to the dynamic curing at 10 °C/min of mixtures with TTMP and different proportions of TGIC catalyzed by 0.5 phr of LC-80.
Figure 2.
DSC thermograms of the isothermal curing at 100 °C of the mixtures with TTMP and different proportions of TGIC catalyzed by 0.5 phr of LC-80.
Figure 2.
DSC thermograms of the isothermal curing at 100 °C of the mixtures with TTMP and different proportions of TGIC catalyzed by 0.5 phr of LC-80.
As we commented above, the increase in
Tg could be accomplished on increasing the functionality of thiol. There are not many commercially available thiols but we can increase the functionality from 3 to 4 by taking PETMP instead of TTMP, both of which are commercially available. In the case of tetrathiol, we have only studied the neat formulation with DGEBA and the formulation containing 30% TGIC.
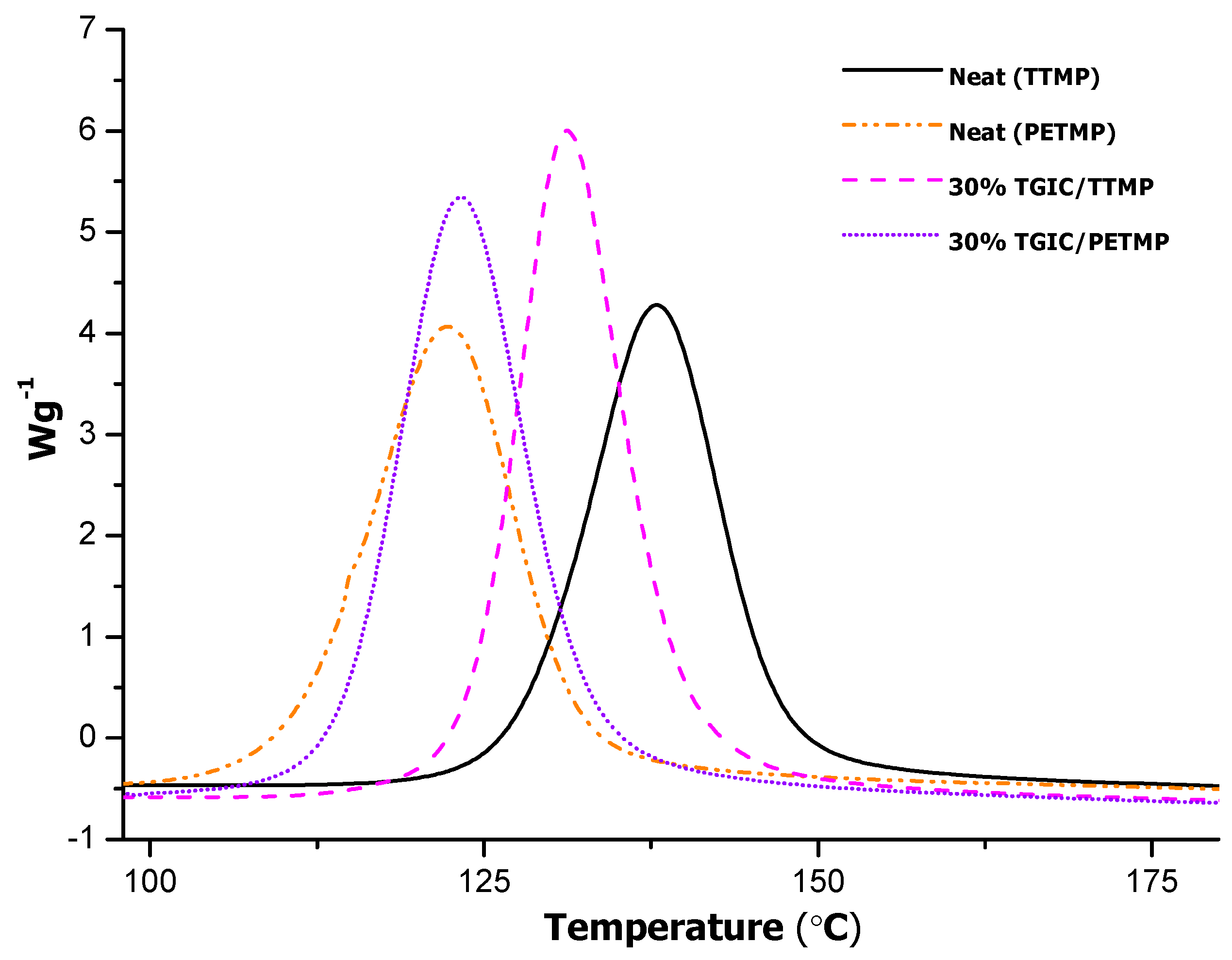
Figure 3 shows the calorimetric dynamic curves of these formulations.
Figure 3.
DSC thermograms corresponding to the dynamic curing at 10 °C/min of the mixtures epoxy resin-thiol tri- and tetrafunctional thiol catalyzed for 0.5 phr of LC-80.
Figure 3.
DSC thermograms corresponding to the dynamic curing at 10 °C/min of the mixtures epoxy resin-thiol tri- and tetrafunctional thiol catalyzed for 0.5 phr of LC-80.
The curing of the neat formulation with PETMP takes place at a much lower temperature indicating a higher reactivity of the curing system, due to a lower stability of the amine precursor LC-80 in the formulation. Since it is an encapsulated imidazole, little changes in the polarity or in the concentration of reactive groups in the mixture can lead to an easier release of the encapsulated imidazole, which acts as a base favoring the attack of the thiol groups to the epoxides.
In mixtures with PETMP, the addition of TGIC does not change significantly within curing temperature range. In contrast, with TTMP, the addition of TGIC to PETMP slightly increases the temperature range of the curing. This seems to support the effect of the chemical environment in the stability of the encapsulated LC-80, but the effect is quite complex. The thiol groups in both TTMP and PETMP have similar stereoelectronic effects and therefore the different reactivity cannot be attributed to the sole structure of the curing agents, but to other factors. In a previous paper, we observed that on increasing the proportion of LC-80, the curing took place at a much lower temperature, even lower than 100 °C or in a shorter amount of time, and in latency tests performed at 35 °C we could observe that with 2 phr of LC-80, the curing was complete in 14 days [
13]. This means that the capsules can be opened in thiol media even without reaching the melting temperature of the capsule.
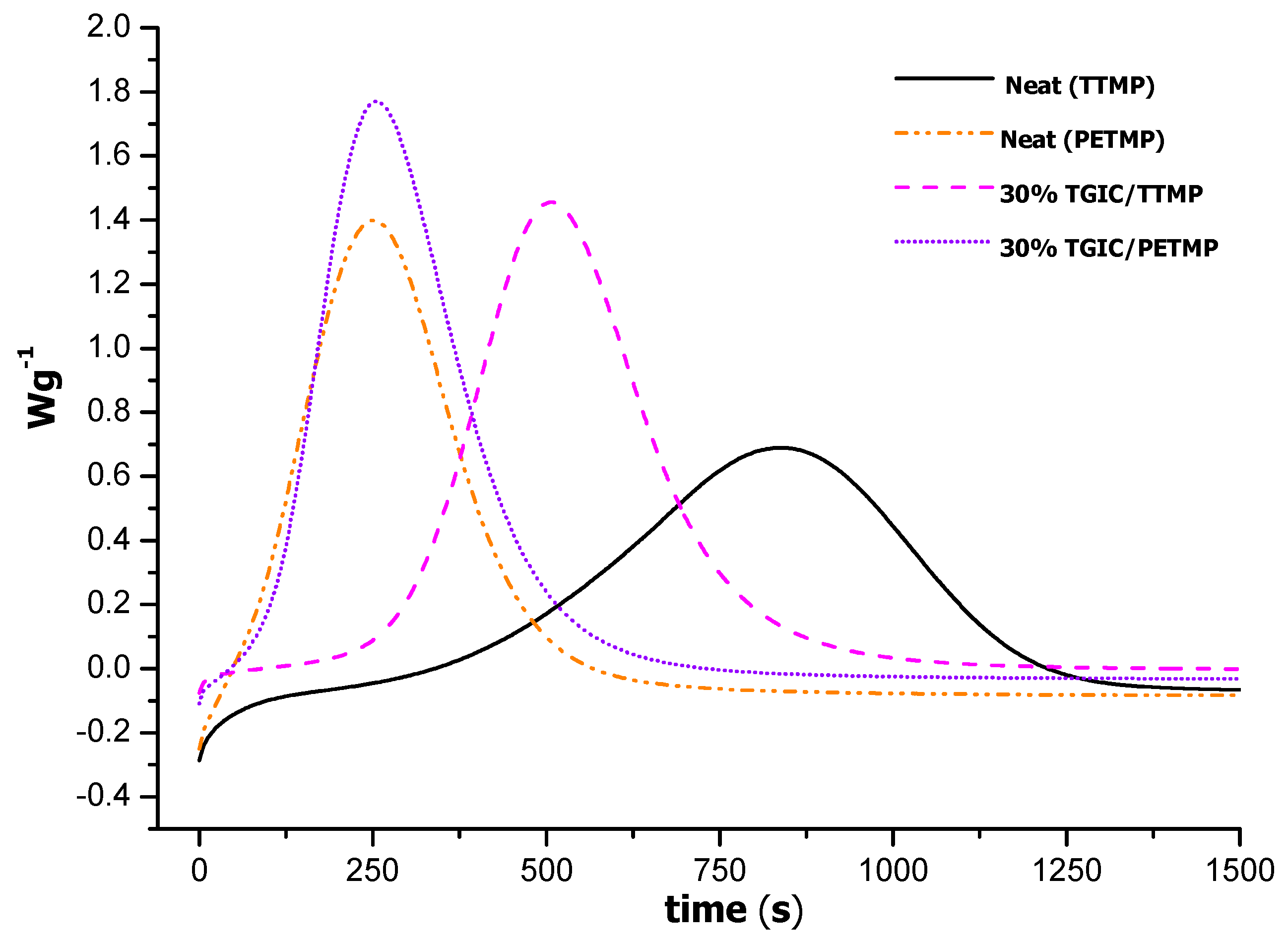
The acceleration caused by the presence of PETMP was confirmed by an isothermal study of these formulations. In
Figure 4, we can see that at 100 °C the induction time in PETMP formulations is dramatically shortened and the formulations begin to cure just when the samples were introduced in the furnace. Thus, the higher reactivity of the formulations with the tetrathiol as the curing agent can be confirmed.
Figure 4.
DSC thermograms of the isothermal curing at 100 °C of neat and TGIC mixtures with tri- and tetrathiol as curing agent.
Figure 4.
DSC thermograms of the isothermal curing at 100 °C of neat and TGIC mixtures with tri- and tetrathiol as curing agent.
3.2. Study of the Latency
In a previous work [
13], the latent character of the DGEBA/TTMP catalysed by 0.5 phr of LC-80 was studied and a period of save storage of 21 days at 35 °C could be determined. In the present study, DSC analysis showed that PETMP formulations were more active than TTMP formulations, and therefore the latency of PETMP formulations was in doubt. Latency has been determined by rheometry, measuring the viscosities after several times of storage at 35 °C. The evolution of the viscosity for the DGEBA formulations with both thiols is represented in
Figure 5.
Figure 5.
Plot of viscosities and degree of curing achieved of the different DGEBA formulations with tri- and tetrathiol against storage time. Filled symbols represent the viscosity and open symbols represent the degree of conversion.
Figure 5.
Plot of viscosities and degree of curing achieved of the different DGEBA formulations with tri- and tetrathiol against storage time. Filled symbols represent the viscosity and open symbols represent the degree of conversion.
As we can see, the viscosity of the formulation with PETMP is somewhat higher than the one with TTMP. The viscosities are quite stable up to 19 days for TTMP formulations and 13 days for PETMP. The viscosity began to increase steadily and PETMP mixtures became hard after 19 days and TTMP mixtures after 23 days. After the different periods of storage, the enthalpy released in a DSC scan was also measured to determine the degree of curing achieved, also represented in
Figure 5. TTMP reached 12% conversion after 21 days of storage, while PETMP reached the same conversion after 16 days of storage. According to the figure, the increase in viscosity—about 1–2 orders of magnitude—is more noticeable than the evolution of the conversion degree during storage, and therefore should be taken as reference for the evaluation of latency. The reported latency data agrees with the higher reactivity of PETMP shown before.
The difference between TTMP and PETMP formulations can be related to the difference in the polarity of the mixture, higher in the case of PETMP because of the higher proportion of S–H polar groups. The higher polarity should affect the stability of the capsule of LC-80, leading to an earlier release of the reactive imidazole. We demonstrated in a previous work that imidazole begins to react at low temperature in a short time [
13]. However, in the present case, the latency period is enough for technological applications, because of the long pot-life achieved even at 35 °C. Lower temperatures can help to keep prepared formulations for a much longer time, but these formulations are not stable enough to prepare one-component epoxy formulations.
3.3. Thermal Characterization of the Materials
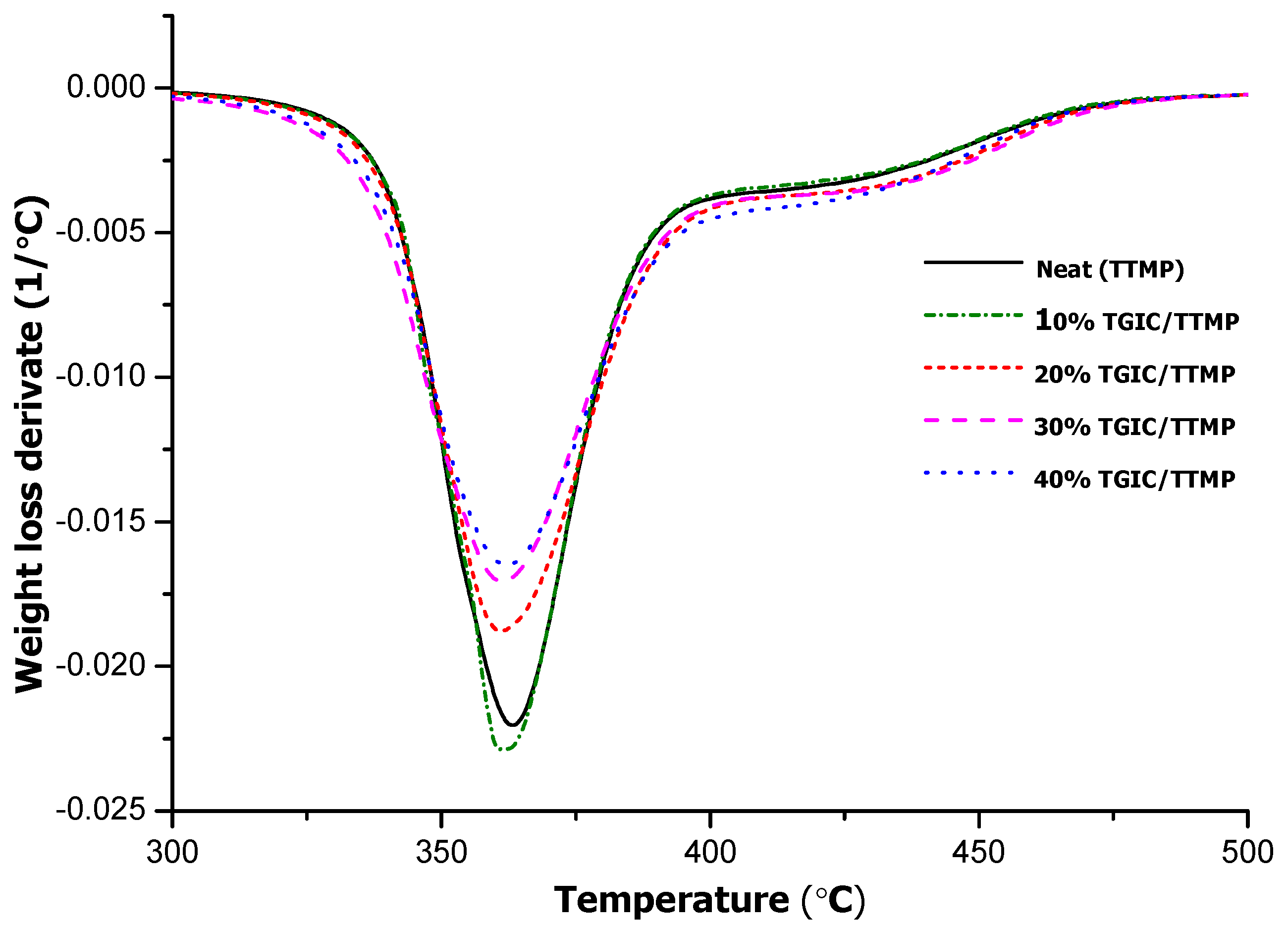
The materials obtained from the different formulations were studied by TGA, DMTA and DSC. The thermal stability of the materials was determined by thermogravimetry under inert atmosphere and the derivative of the thermograms of some formulations is represented in
Figure 6. In
Table 2, the most characteristic thermal data are given.
Figure 6.
DTG (derivative of the thermogravimetric) curves registered under N2 at 10 K/min of thermosets obtained from TTMP/TGIC/DGEBA mixtures.
Figure 6.
DTG (derivative of the thermogravimetric) curves registered under N2 at 10 K/min of thermosets obtained from TTMP/TGIC/DGEBA mixtures.
Table 2.
Thermal data of the thermosets prepared.
Table 2.
Thermal data of the thermosets prepared.
| Notation | TGA | DSC | DMTA |
|---|
| T5% a (°C) | T50% b (°C) | Tmax c (°C) | Tg d (°C) | Ttanδ (°C) | Er e (Pa) | FWHM f (°C) | Artanδ g (°C) | E h (MPa) |
|---|
| Neat (TTMP) | 342 | 370 | 363 | 35 | 51 | 7.89 | 10.0 | 16.0 | 391.4 |
| 10%TGIC/TTMP | 342 | 370 | 361 | 37 | 55 | 7.92 | 10.6 | 16.3 | 551.7 |
| 20%TGIC/TTMP | 341 | 373 | 361 | 40 | 59 | 9.04 | 11.1 | 15.6 | 678.2 |
| 30%TGIC/TTMP | 335 | 369 | 361 | 44 | 62 | 10.82 | 10.9 | 15.7 | 736.1 |
| 40%TGIC/TTMP | 336 | 374 | 362 | 44 | 63 | 9.99 | 12.2 | 16.0 | 720.8 |
| Neat (PETMP) | 341 | 371 | 361 | 55 | 68 | 9.77 | 8.5 | 12.8 | 1,069 |
| 30%TGIC/PETMP | 341 | 376 | 360 | 63 | 83 | 19.30 | 9.9 | 12.0 | 1,250 |
In
Figure 6, we can see that the derivative curves show a main degradation process at about 360 °C, without much difference in the maximum of the peak for all the materials, as is detailed in
Table 2. In addition to the main degradation peak, there is a shoulder at higher temperatures. In general, the presence of TGIC gradually decreases the decomposition rate because the higher crosslinking density provided by the isocyanurate rings reduces the likelihood of producing volatile fragments during the thermal decomposition process. There is an only slight decrease in the initial degradation temperature (taken as T
5%) on adding TGIC to the reactive mixture, because the thermal stability of the bonds is not changed. Thus, the addition of TGIC to DGEBA/TTMP systems provides no clear advantages in terms of thermal stability of the thermosets.
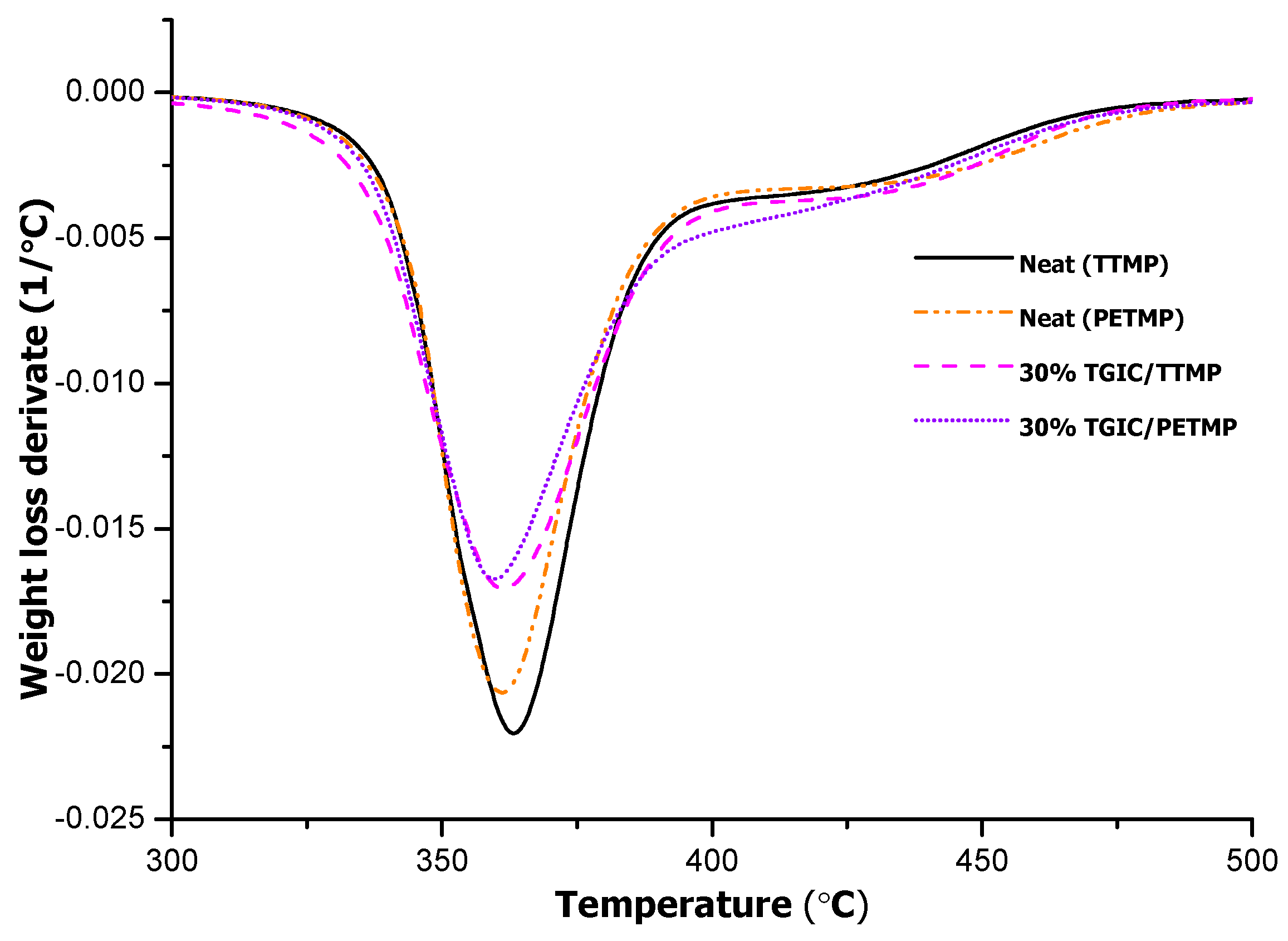
When TTMP was replaced by PETMP in the thermosets, a similar behavior was observed with a main degradation process in the same range (see
Figure 7).
Figure 7.
DTG curves registered under N2 at 10 K/min of thermosets obtained by using TTMP and PETMP as curing materials.
Figure 7.
DTG curves registered under N2 at 10 K/min of thermosets obtained by using TTMP and PETMP as curing materials.
Tgs were determined by DSC from the samples prepared for DMTA measurements. As we can see in
Table 2, on increasing the proportion of TGIC in the formulation or increasing the functionality of the thiol, this value also increases, reaching a
Tg of 63 °C for the 30%TGIC/PETMP material. The values in the table demonstrate that the change from TTMP to PETMP, which increases the thiol crosslinker functionality, produces a significant enhancement in
Tg. The use of TGIC introduces an increasing amount of rigid isocyanurate rings acting as crosslinks in the cured material. The increase in crosslinking density should contribute to increase the rigidity of the network structure and, consequently, the
Tg. However, the inherent rigidity of the material may decrease, because the overall concentration of aromatic rings from DGEBA and the isocyanurate rings from TGIC decrease upon addition of TGIC. Both factors—crosslinking density and network rigidity—act in opposite directions but the overall effect should depend on their relative strength. Indeed,
Table 2 and
Figure 8 show that at low proportions of TGIC the
Tg increases gradually but, above 30%, the effect is not clear.
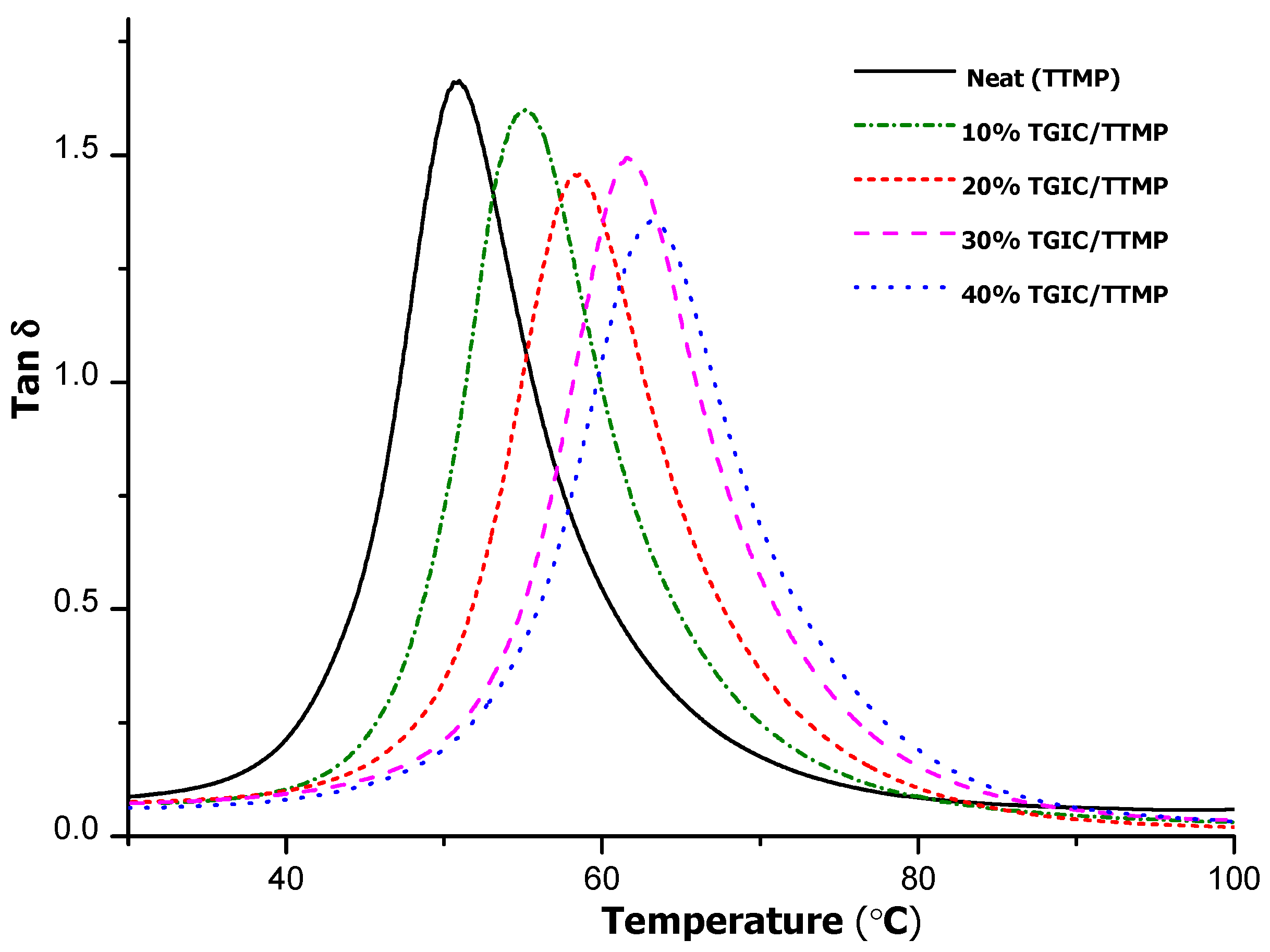
The viscoelastic properties of the cured materials were analysed by DMTA.
Figure 8 represents the curves corresponding to the variation of loss tangent against temperature for the materials obtained with TTMP and different proportions of TGIC. The maximum of tanδ is the temperature of α relaxation of the network and is related to the
Tg of the material. In the figure, we can observe that as the TGIC proportion increases, the tanδ peak is shifted to higher temperatures. In
Table 2, we can see that the temperature of the maximum of the peak goes from 51 to 63 °C for the maximum percentage of TGIC in the formulation. Similarly to the evolution of
Tg determined by DSC, above 30% of TGIC there is not a clear increase in the tanδ peak temperature. On increasing the proportion of TGIC, there is a general increase in the storage modulus in the rubbery state up to the material 30%TGIC/TTMP, but further increase in the proportion of TGIC led to a decrease in this value.
Figure 8.
Plots of tanδ against temperature for the thermosets obtained for TTMP with different proportions of TGIC.
Figure 8.
Plots of tanδ against temperature for the thermosets obtained for TTMP with different proportions of TGIC.
According to the rubber elasticity theory, the relaxed modulus should be proportional to the crosslinking density or, more specifically, to the density of network strands. The theoretical crosslinking density of the formulations was calculated taking into account the contribution of the thiol curing agents and TGIC, and the theoretical network strand density was calculated taking into account that TTMP and TGIC which lead to tri-functional crosslinks, but PETMP leads to tetra-functional crosslinks and every strand is shared by two crosslinks. The results of these calculations are shown in
Table 3.
Table 3.
Crosslinking density of the different cured materials calculated according to the formulation composition.
Table 3.
Crosslinking density of the different cured materials calculated according to the formulation composition.
| Notation | Crosslinking Density (eq/kg) | Network Strands (eq/kg) |
|---|
| TTMP | PETMP | TGIC | Total | DGEBA | TGIC | Total |
|---|
| Neat (TTMP) | 1.06 | - | - | 1.06 | 1.59 | - | 1.59 |
| 10%TGIC/TTMP | 1.11 | - | 0.19 | 1.30 | 1.38 | 0.59 | 1.95 |
| 20%TGIC/TTMP | 1.16 | - | 0.36 | 1.52 | 1.19 | 1.09 | 2.28 |
| 30%TGIC/TTMP | 1.20 | - | 0.53 | 1.73 | 1.01 | 1.58 | 2.59 |
| 40%TGIC/TTMP | 1.24 | - | 0.68 | 1.92 | 0.84 | 2.05 | 2.88 |
| Neat (PETMP) | - | 0.82 | - | 0.82 | 1.64 | - | 1.64 |
| 30%TGIC/PETMP | - | 0.93 | 0.55 | 1.48 | 1.05 | 1.65 | 2.69 |
In accordance with the values shown in the table, the addition of TGIC to the formulation results in an increase in the number of TTMP crosslinks. However, the modulus increase reported in
Table 2 is not strictly proportional to the increase network strand density, especially above 30% of TGIC. While this may be due, in part, to the uncertainties associated with the measurement of oscillatory mechanical properties in the relaxed state using the DMTA apparatus, because of their very small stiffness, these results are in agreement with the observed trends in
Tg. One can therefore use the same argumentation here to justify these results. It must be taken into account that the mechanical response of crosslinked polymers is highly complex and departures from the ideal elastomeric behaviour can be expected [
18,
19]. As an example, Xie and Rousseau [
20] showed that it was possible to increase the number of crosslinks by replacing DGEBA with a shorter and flexible diepoxide, but leading to an actual decrease of the
Tg and of the relaxed modulus due to the enhanced mobility of the network structure. In the present case, it is verified that the constraining effect of the increased crosslinking density and the presence of rigid isocyanurate crosslinks dominate, at least in the formulations with a low amount of TGIC but, upon increasing the TGIC content, furthermore, the resulting effect could be a balance between different factors. However, there is still the possibility that the curing has not been completed by topological reasons in formulations with 40% TGIC, which helps to explain the low modulus in the rubbery region and the lack of increase in
Tg value.
The width of the tan δ peak can be related to the degree of inhomogeneity of the distribution of the comonomers and crosslinking points in the network structure. On increasing the proportion of TGIC in the formulation, this parameter is not increased significantly, indicating a random and regular distribution of crosslinks and network strands in the network structure, up to 40% of TGIC content, because of the similar reactivity of the epoxy groups of both DGEBA and TGIC. The area of the peak accounts for the damping characteristics of the network structure, but there is no significant variation in this characteristic on adding TGIC to the formulation.
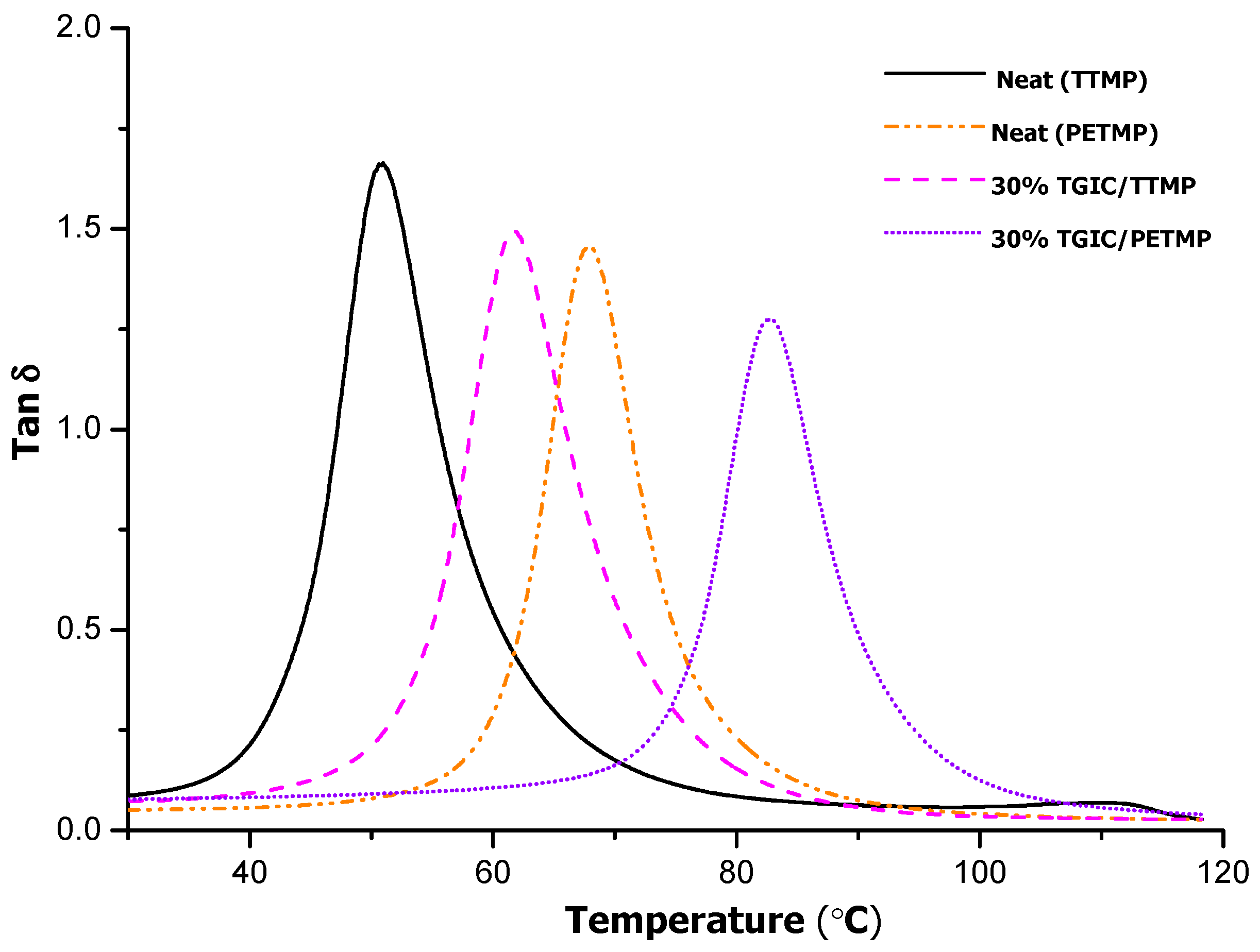
Figure 9 plots the variation in tanδ curves on changing the curing agent, from TTMP to PETMP for neat and TGIC containing materials. As we can see, there is a remarkable shift of the curves when increasing the functionality of the curing agent, as expected, and a temperature of the maximum of the peak at 83 °C has been reached in the sample with a 30% of TGIC. Thus, the cooperative effect of increasing the functionality of the thiol and increasing the proportion of TGIC in the formulation leads to an increase of 32 °C in the
Tg of the material. Although
Table 3 shows that the total number of crosslinks decreases when PETMP is used instead of TTMP, it should be taken into account that they are tetrafunctional so that the total number of network strands is similar. This should constrain the network mobility, resulting in an increase in
Tg, and of the relaxed modulus as well, given the similar amount of network strands. This is indeed what takes place and, overall, there is an additive effect on this parameter by increasing the functionality of the curing agent and the functionality of the epoxy resin. The lower values of FWHM indicate higher network homogeneity, while the lower area reflects the immobilization caused by the presence of tetrafunctional cross-links coming from PETMP in comparison with the trifunctional ones coming from TTMP.
Figure 9.
Plots of tanδ against temperature for the thermosets obtained by using TTMP and PETMP as curing agent.
Figure 9.
Plots of tanδ against temperature for the thermosets obtained by using TTMP and PETMP as curing agent.
Young’s modulus was measured by three-point bending in the DMTA device. On increasing the proportion of TGIC in the formulation, the material becomes more rigid at room temperature up to the material containing a 30% of TGIC. The modulus is higher for PETMP materials. Thus, the functionality of the crosslinking agent affects more notably this parameter.
The materials obtained were colorless and transparent but the high transparency of DGEBA/TTMP and DGEBA/PETMP formulations were slightly decreased on adding TGIC at the naked eye.