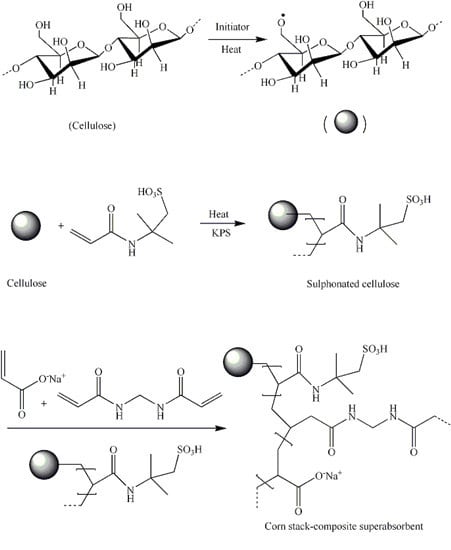
Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sulfonic Cellulose by Pretreating Corn Straws
2.3. Synthesis of Superabsorbent Hydrogels
| Number | Pretreated straw (g) | AA (g) | MBA/AA (%) | KPS/AA (%) | Neutralization value (%) | Temperature (°C) |
|---|---|---|---|---|---|---|
| SH-0 | 0 | 6 | 0.8 | 1.5 | 70 | 40 |
| SH-1 | 0 | 6 | 0.4 | 1.5 | 70 | 40 |
| SH-2 | 1 | 6 | 0.8 | 1.5 | 70 | 40 |
| SH-3 | 1 | 6 | 1.2 | 1.5 | 70 | 40 |
| SH-4 | 1 | 6 | 1.6 | 1.5 | 70 | 40 |
| SH-5 | 1 | 6 | 2.0 | 1.5 | 70 | 40 |
| SH-6 | 1 | 6 | 2.4 | 1.5 | 70 | 40 |
| SH-7 | 1 | 4 | 0.8 | 1.5 | 70 | 40 |
| SH-8 | 1 | 5 | 0.8 | 1.5 | 70 | 40 |
| SH-9 | 1 | 7 | 0.8 | 1.5 | 70 | 40 |
| SH-10 | 1 | 8 | 0.8 | 1.5 | 70 | 40 |
| SH-11 | 1 | 9 | 0.8 | 1.5 | 70 | 40 |
| SH-12 | 1 | 6 | 0.8 | 1.0 | 70 | 40 |
| SH-13 | 1 | 6 | 0.8 | 2.0 | 70 | 40 |
| SH-14 | 1 | 6 | 0.8 | 2.5 | 70 | 40 |
| SH-15 | 1 | 6 | 0.8 | 3.0 | 70 | 40 |
| SH-16 | 1 | 6 | 0.8 | 3.5 | 70 | 40 |
| SH-17 | 1 | 6 | 0.8 | 1.5 | 30 | 40 |
| SH-18 | 1 | 6 | 0.8 | 1.5 | 40 | 40 |
| SH-19 | 1 | 6 | 0.8 | 1.5 | 50 | 40 |
| SH-20 | 1 | 6 | 0.8 | 1.5 | 60 | 40 |
| SH-21 | 1 | 6 | 0.8 | 1.5 | 80 | 40 |
| SH-22 | 1 | 6 | 0.8 | 1.5 | 70 | 35 |
| SH-23 | 1 | 6 | 0.8 | 1.5 | 70 | 45 |
| SH-24 | 1 | 6 | 0.8 | 1.5 | 70 | 50 |
| SH-25 | 1 | 6 | 0.8 | 1.5 | 70 | 55 |
| SH-26 | 1 | 6 | 0.8 | 1.5 | 70 | 60 |
2.4. Characterization of Hydrogels
2.4.1. Measurement of Swelling Ratio Q
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Hydrogels
2.4.3. Measuring the Swelling Dynamics of Hydrogels
2.4.4. Salt Resistance of Hydrogels
2.4.5. Microstructure Analysis of Hydrogels
2.4.6. X-ray Diffraction (XRD) Characterization of the Composite Hydrogel
2.4.7. Thermal Gravity Analysis (TG)/Differential Thermal Gravity (DTG) Analysis of Hydrogels
2.4.8. Water Retaining Analysis
2.4.9. Water Reabsorption of Hydrogels
2.4.10. Rheological Properties of Hydrogels
3. Results and Discussion
3.1. FTIR Analysis
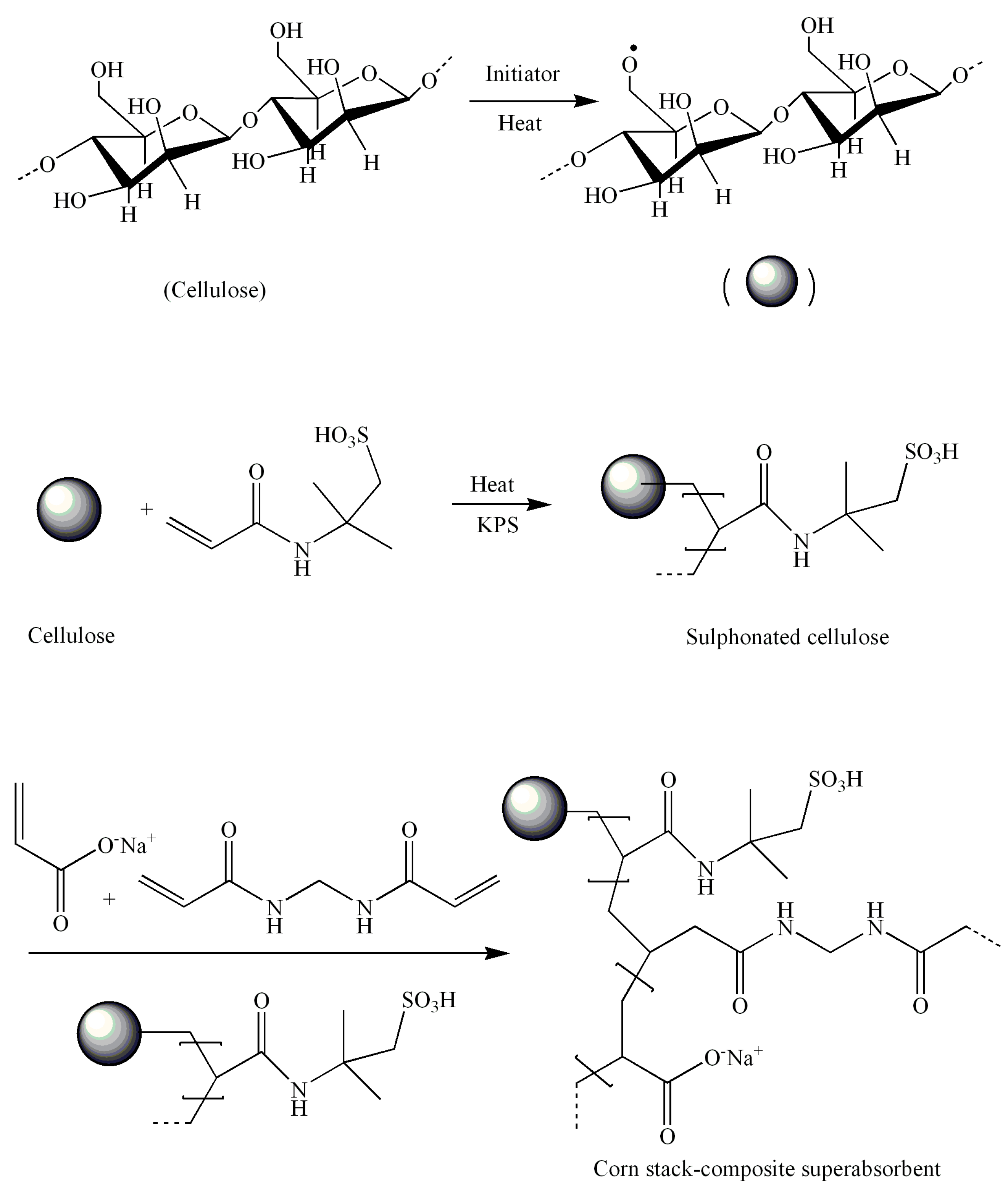

3.2. XRD Characterization
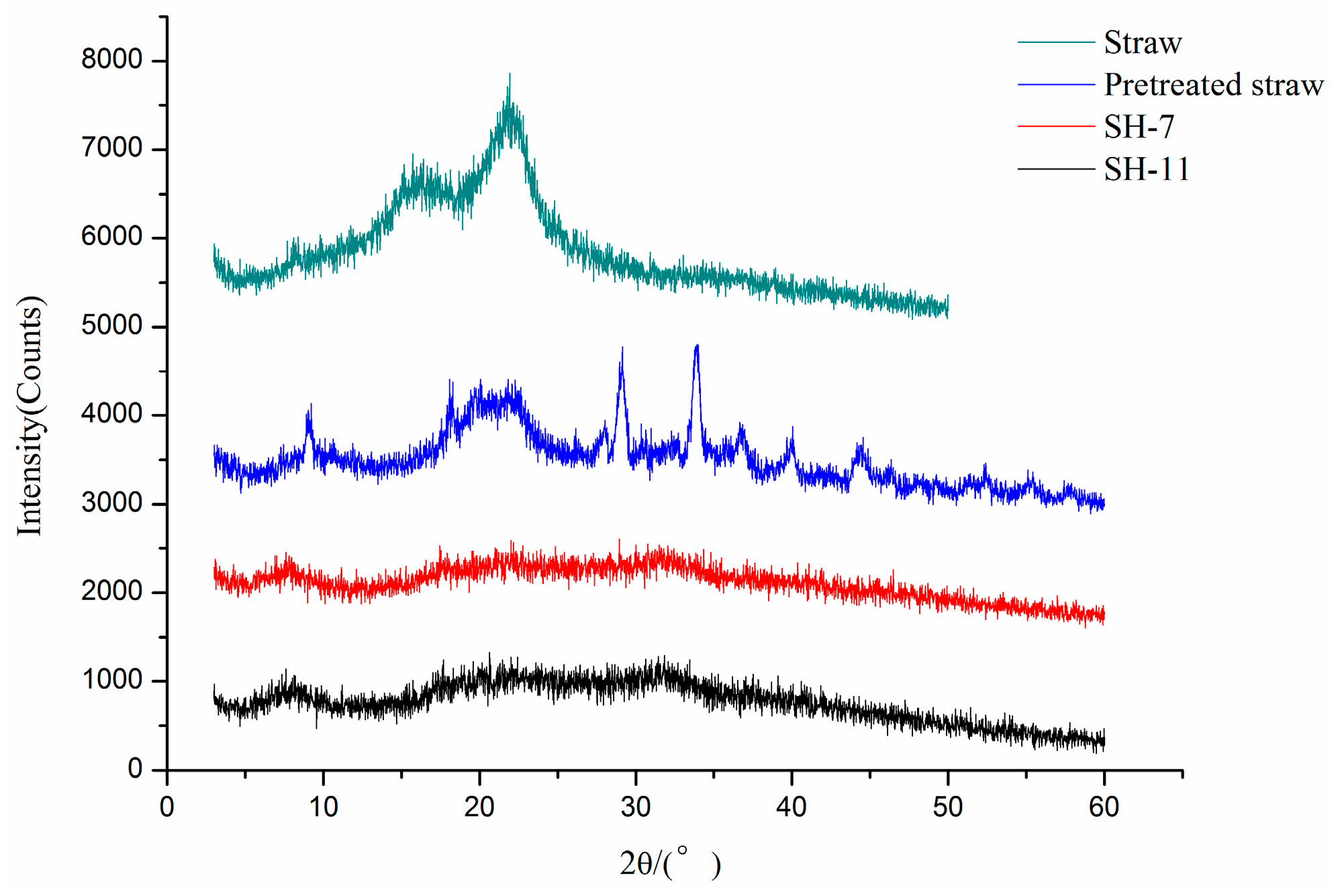
3.3. Effect of MBA on Q Value Of Hydrogel
3.4. Effect of AA Content on the Swelling Ratio of Hydrogel
3.5. Effect of KPS on the Swelling Ratio of Hydrogel
3.6. The Effect of Neutralization Degree of AA on Swelling Ratio
3.7. Effect of Temperature on Swelling Ratio of Hydrogel
3.8. Effect of pH on Swelling Ratios of Hydrogels
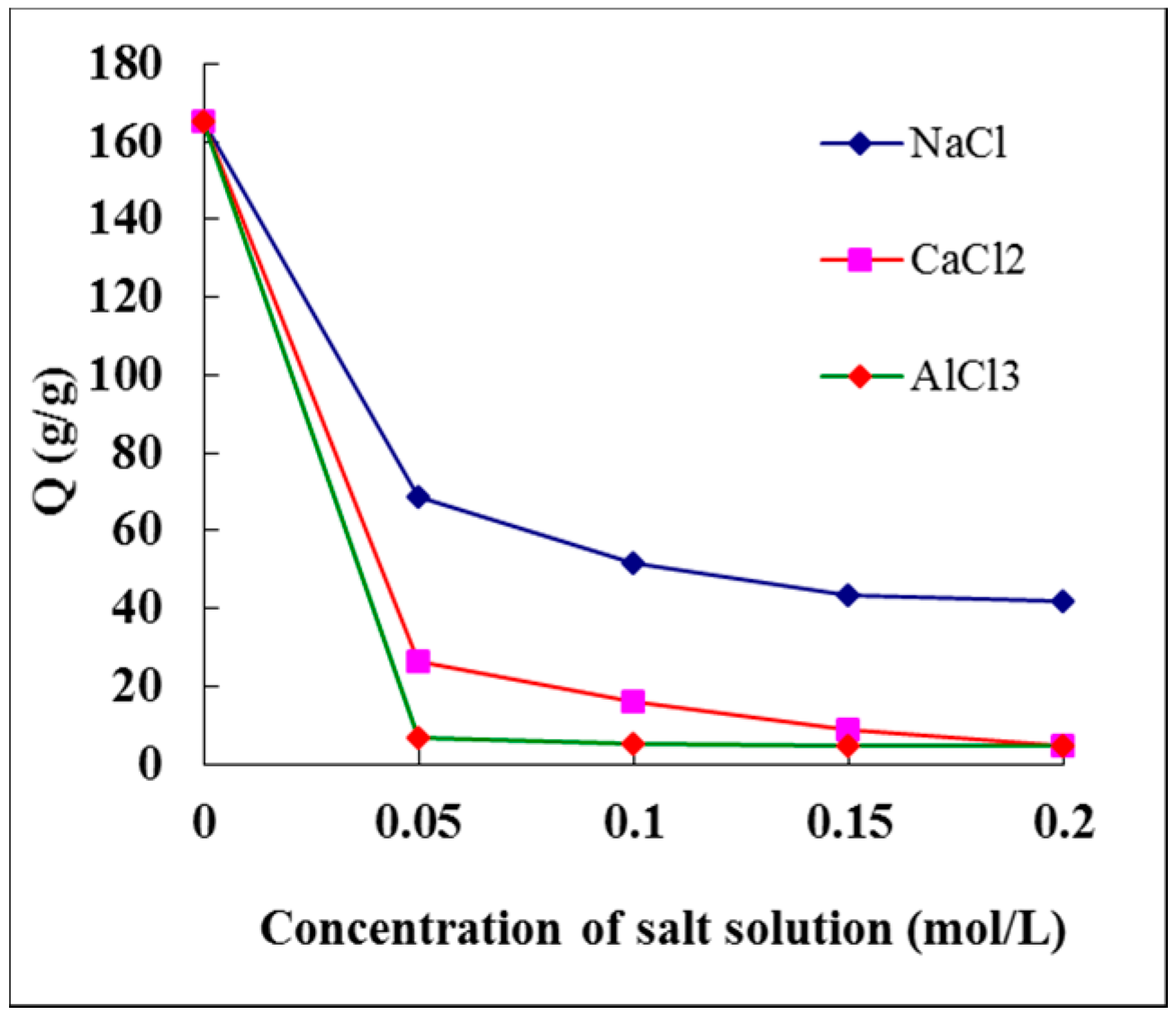
3.9. Effect of Salt Solution on the Swelling Ratio of Hydrogel
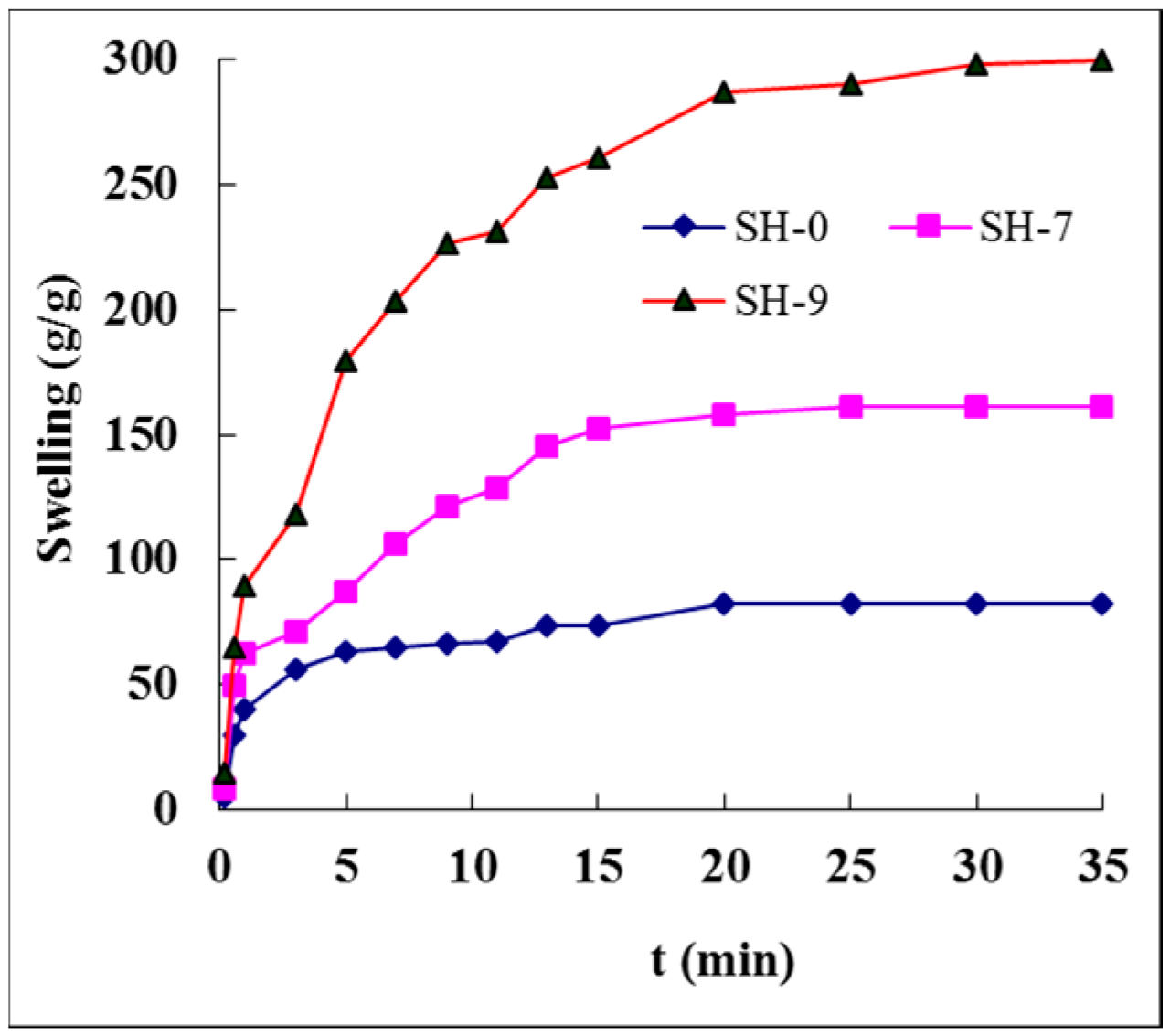
3.10. Swelling Dynamics of Hydrogels
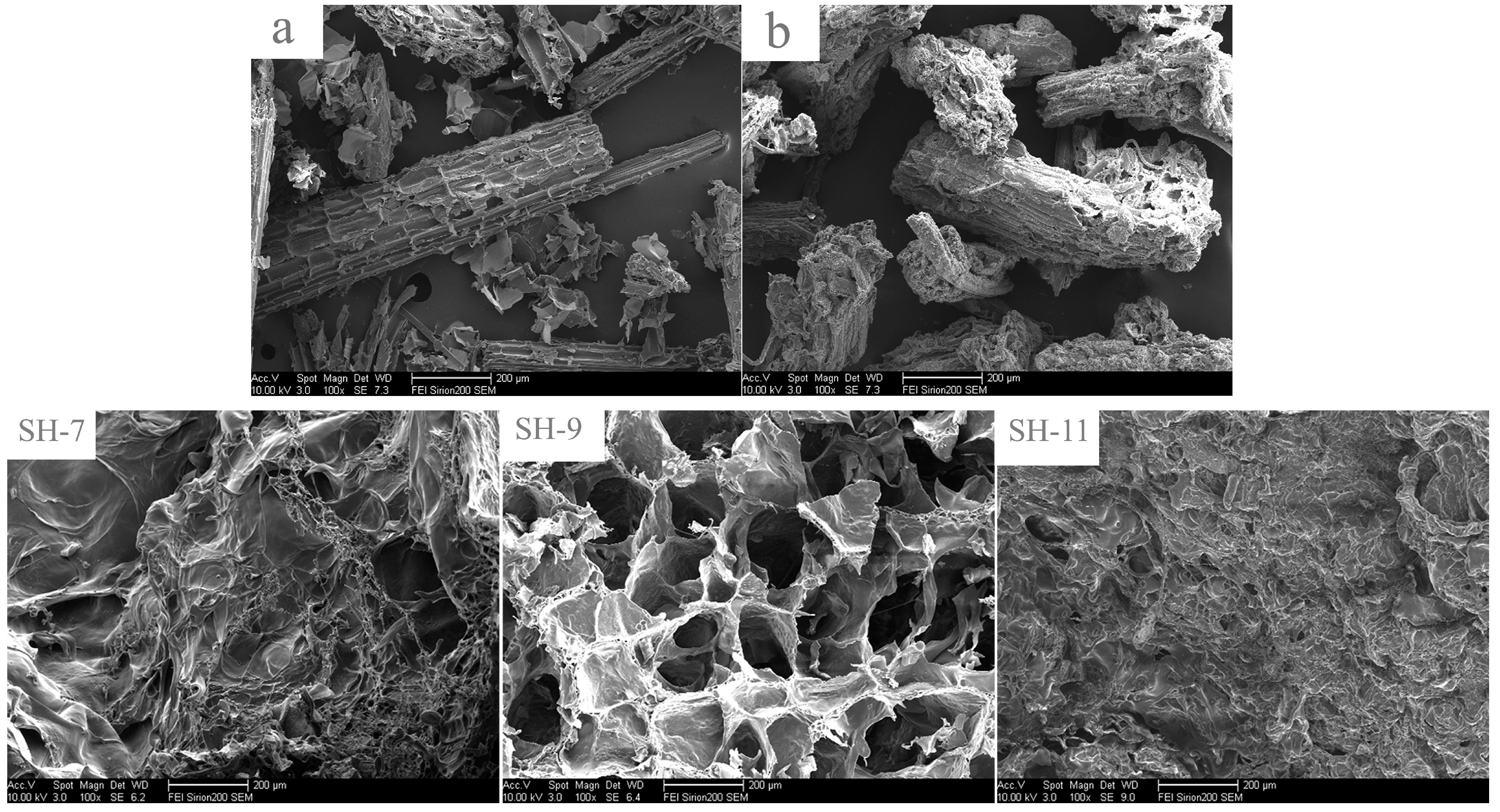
3.11. Microstructure Analysis of Hydrogels
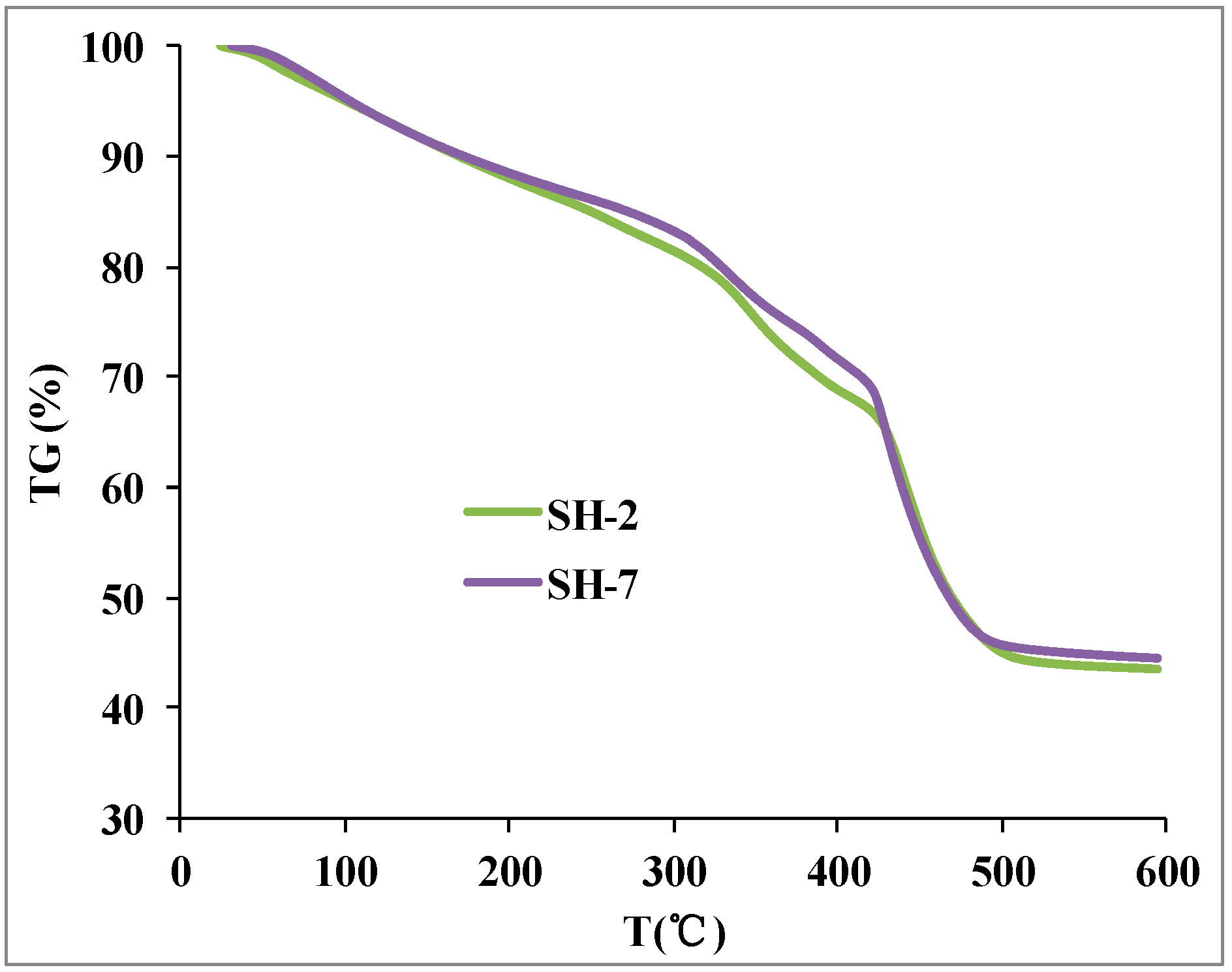
3.12. Thermal Stability
3.13. Storage Modulus Analysis of Hydrogels
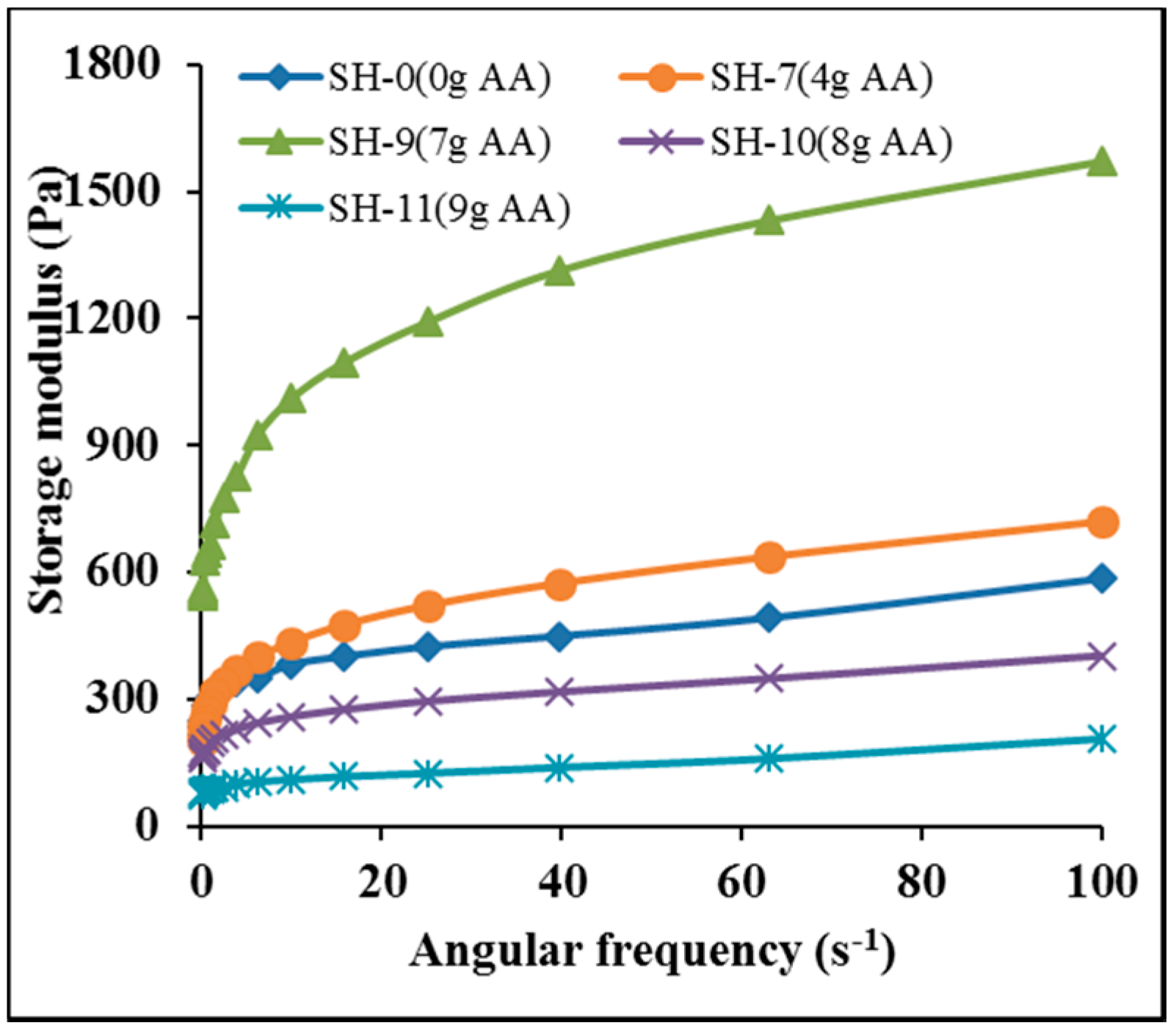
3.14. Water Retainability
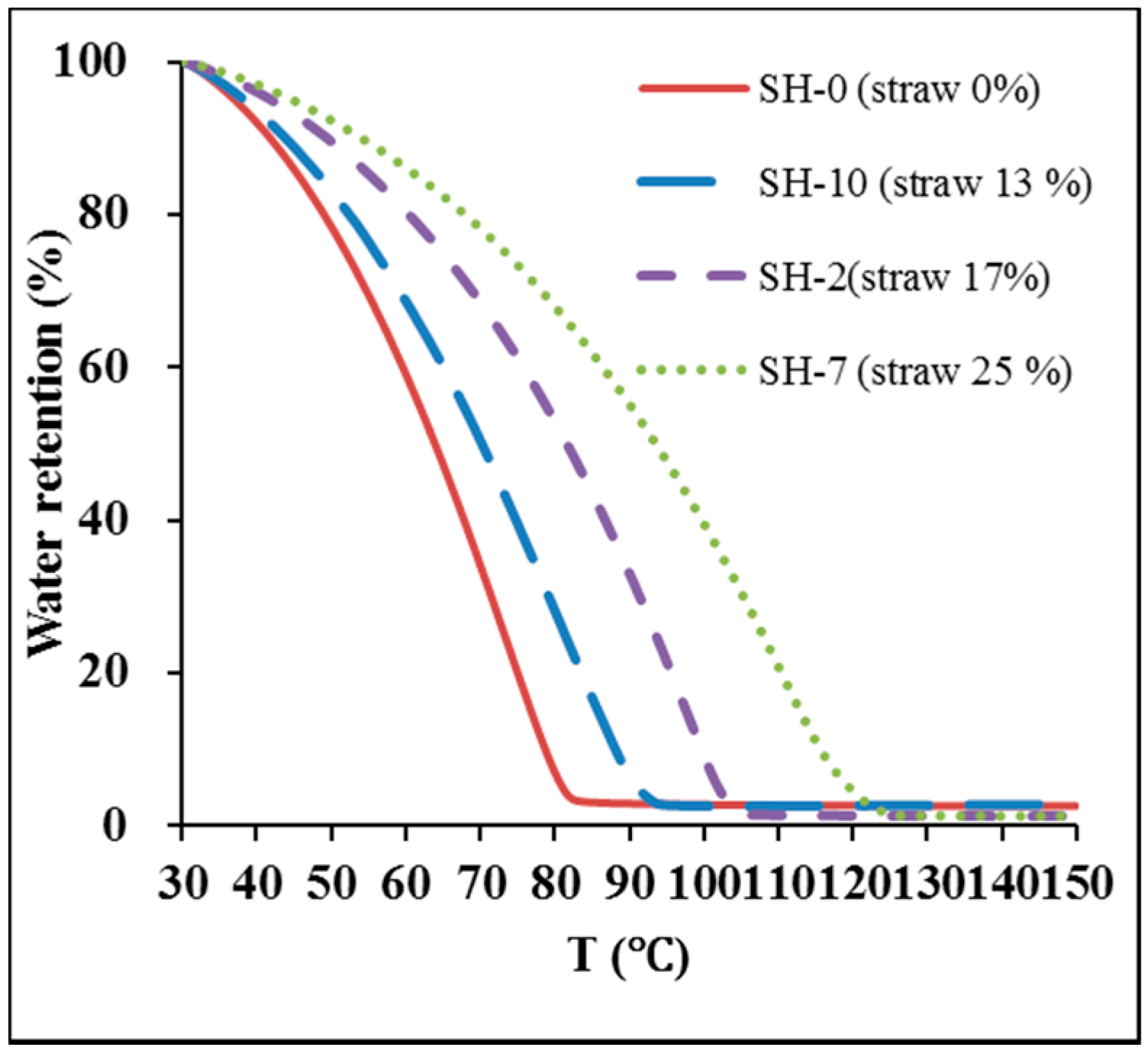
3.15. Recyclability of Hydrogels
| Number | Repeat number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| SH-2 | Water absorption (g/g) | 263 | 270 | 251 | 219 | 196 |
| r | 1 | 1.03 | 0.95 | 0.81 | 0.75 | |
| SH-9 | Water absorption (g/g) | 326 | 332 | 305 | 283 | 256 |
| r | 1 | 1.02 | 0.94 | 0.87 | 0.81 |
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.R.; Rahneshin, N. Synthesis and characterization of novel stimuli-responsive hydrogels based on starch and l-aspartic acid. Carbohydr. Polym. 2013, 98, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Wang, A.Q. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91. [Google Scholar] [CrossRef]
- Ji, D.Y.; Kuo, T.F.; Wu, H.D.; Yang, J.C.; Lee, S.Y. A novel injectable chitosan/polyglutamate polyelectrolyte complex hydrogel with hydroxyapatite for soft-tissue augmentation. Carbohydr. Polym. 2012, 89, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.X.; Deng, J.; Xing, X.M.; Yuan, C.Q.; Yu, X.B.; Xu, Q.C.; Yue, J. Biocompatibility of a chitosan-based injectable thermosensitive hydrogel and its effects on dog periodontal tissue regeneration. Carbohydr. Polym. 2010, 82, 1153–1160. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5712. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H.; El-Hadi, O.; El-Dewiny, C.Y. Evaluation of rice straw-based hydrogels for purification of wastewater. Polym. Plast. Technol. 2013, 52, 1074–1080. [Google Scholar] [CrossRef]
- Jin, S.P.; Liu, M.Z.; Zhang, F.; Chen, S.L.; Niu, A.Z. Synthesis and characterization of pH-sensitivity semi-IPN hydrogel based on hydrogen bond between poly(N-vinylpyrrolidone) and poly(acrylic acid). Polymer 2006, 47, 1526–1532. [Google Scholar] [CrossRef]
- Yu, J.; Pan, Y.P.; Lu, Q.F.; Yang, W.; Gao, J.Z.; Li, Y. Synthesis and swelling behaviors of P(AMPS-co-AAc) superabsorbent hydrogel produced by glow-discharge electrolysis plasma. Plasma Chem. Plasma Process. 2013, 33, 219–220. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Ermolaev, A.M.; Myakshina, T.N. Ecological strategies of soil microbial communities under plants of meadow ecosystems. Biol. Bull. 2004, 31, 620–627. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H. Cross-linked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel super-absorbent polymer. Carbohydr. Polym. 2006, 341, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, A.; Wang, A. Synthesis and characterization of multifunctional poly(acrylic acid-co-acrylamide)/sodium humate super-absorbent composite. React. Funct. Polym. 2006, 66, 747–756. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite super-absorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4158. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Fast-swelling auperabsorbent hydrogels from poly(2-hydroxy ethyl acrylate-co-sodium acrylate) grafted on starch. Starch Stärke 2008, 60, 79–86. [Google Scholar] [CrossRef]
- Wan, T.; Xiong, L. Synthesis and swelling properties of corn stalk-composite superabsorbent. J. Appl. Polym. Sci. 2013, 130, 698–703. [Google Scholar] [CrossRef]
- Aloulou, F.; Boufi, S.; Labidi, J. Modified cellulose fibres for adsorption of organic compound in aqueous solution. Sep. Purif. Technol. 2006, 52, 332–342. [Google Scholar] [CrossRef]
- Bertrand, I.; Prevot, M.; Chabbert, B. Soil decomposition of wheat internodes of different maturity stages: Relative impact of the soluble and structural fractions. Bioresour. Technol. 2009, 100, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Liu, M.Z.; Ni, B.L.; Zhang, X.; Wang, Y.F. Slow-release nitrogen and boron fertilizer from a functional superabsorbent formulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.H.; Yue, Q.Y. Synthesis, characterization and swelling behavior of superabsorbent wheat straw graft copolymers. Bioresour. Technol. 2012, 118, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Su, Y.; Yue, Q.Y.; Gao, B.Y. Characterization and swelling-deswelling properties of wheat straw cellulose based semi-IPNs hydrogel. Carbohydr. Polym. 2014, 107, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Miao, Y.G.; Wang, Z.Y.; Yin, G.G. Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr. Polym. 2009, 77, 131–135. [Google Scholar] [CrossRef]
- Swantomo, D.; Rochmadi, R.; Basuki, K.T.; Sudiyo, R. Synthesis and characterization of graft copolymer rice straw cellulose-acrylamide hydrogels using gamma irradiation. At. Indones. 2013, 39, 57–64. [Google Scholar] [CrossRef]
- El-Saied, H.; Basta, A.H.; Waly, A.I.; El-Hady, O.A.; El-Dewiny, C.Y.; Abo-Sedera, S.A. Evaluating the grafting approaches for utilizing the rice straw as environmental friendly and potential low cost hydrogels. Emir. J. Food Agric. 2013, 25, 211–224. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.J.; Zhu, M.F.; Zhang, X.Y. A study of the synthesis and properties of AM/AMPS copolymer as superabsorbent. Polym. Mater. Sci. Eng. 2004, 289, 1074–1078. [Google Scholar] [CrossRef]
- Muniz, E.C.; Geuskens, G. Polyacrylamide hydrogels and semiinterpenetrating networks (IPNs) with poly(N-isopropylacrylamide): Mechanical properties by measure of compressive elastic modulus. J. Mater. Sci. Mater. Med. 2001, 12, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Castal, D.; Ricard, A.; Audebert, R. Swelling of anionic and cationic starch-based superabsorbents in water and saline solution. J. Appl. Polym. Sci. 1990, 39, 11–29. [Google Scholar] [CrossRef]
- Omidian, H.; Hashemi, S.A.; Sammes, P.G.; Meldrum, I. A model for the swelling of superabsorbent polymers. Polymer 1998, 39, 6697–6704. [Google Scholar] [CrossRef]
- Marandi, G.B.; Mahdavinia, G.R.; Ghafary, S. Collagen-g-poly(sodium acrylate-co-acrylamide)/sodium montmorillonite superabsorbent nanocomposites: Synthesis and swelling behavior. J. Polym. Res. 2011, 18, 1487–1499. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-M.; Hu, X.-M.; Wang, D.-M.; Liu, G.-H. Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel. Polymers 2015, 7, 2431-2445. https://doi.org/10.3390/polym7111522
Cheng W-M, Hu X-M, Wang D-M, Liu G-H. Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel. Polymers. 2015; 7(11):2431-2445. https://doi.org/10.3390/polym7111522
Chicago/Turabian StyleCheng, Wei-Min, Xiang-Ming Hu, De-Ming Wang, and Guo-Hua Liu. 2015. "Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel" Polymers 7, no. 11: 2431-2445. https://doi.org/10.3390/polym7111522
APA StyleCheng, W.-M., Hu, X.-M., Wang, D.-M., & Liu, G.-H. (2015). Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel. Polymers, 7(11), 2431-2445. https://doi.org/10.3390/polym7111522