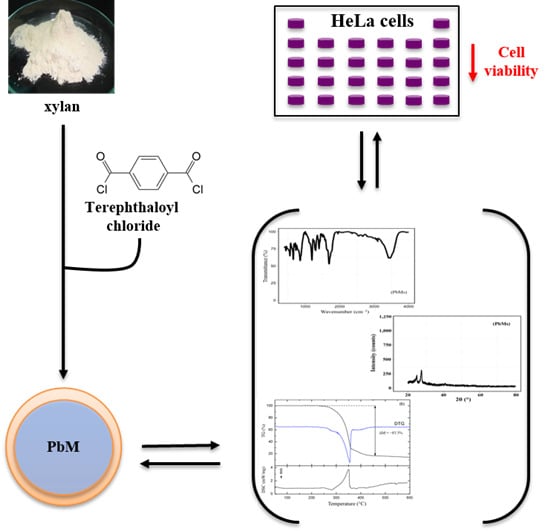
Leads from Physical, Chemical, and Thermal Characterization on Cytotoxic Effects of Xylan-Based Microparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Microparticle Production

2.3. Xylan Dispersions for Biocompatibility Assay
| Tested product | Dilutions (Product:DMEM, v/v) |
|---|---|
| Xylan dispersion in water (49.8 mg·mL−1 of xylan) | 1:1 (corresponding to 24.8 mg·mL−1) |
| 1:3 (corresponding to 12.4 mg·mL−1) | |
| 1:7 (corresponding to 6.2 mg·mL−1) | |
| 1:15 (corresponding to 3.1 mg·mL−1) | |
| PbMs suspension in water (1.488 mg·mL−1 of xylan) | 1:1 (corresponding to 0.744 mg·mL−1) |
| 1:3 (corresponding to 0.372 mg·mL−1) | |
| 1:7 (corresponding to 0.186 mg·mL−1) | |
| 1:15 (corresponding to 0.093 mg·mL−1) | |
| 1:31 (corresponding to 0.0465 mg·mL−1) |
2.4. Morphology and Particle Size Distribution Analysis
2.5. Powder X-ray Diffraction (XRD)
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Thermal Analysis
2.8. In Vitro Biocompatibility Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Characterization
3.1.1. Morphology and Size Distributions
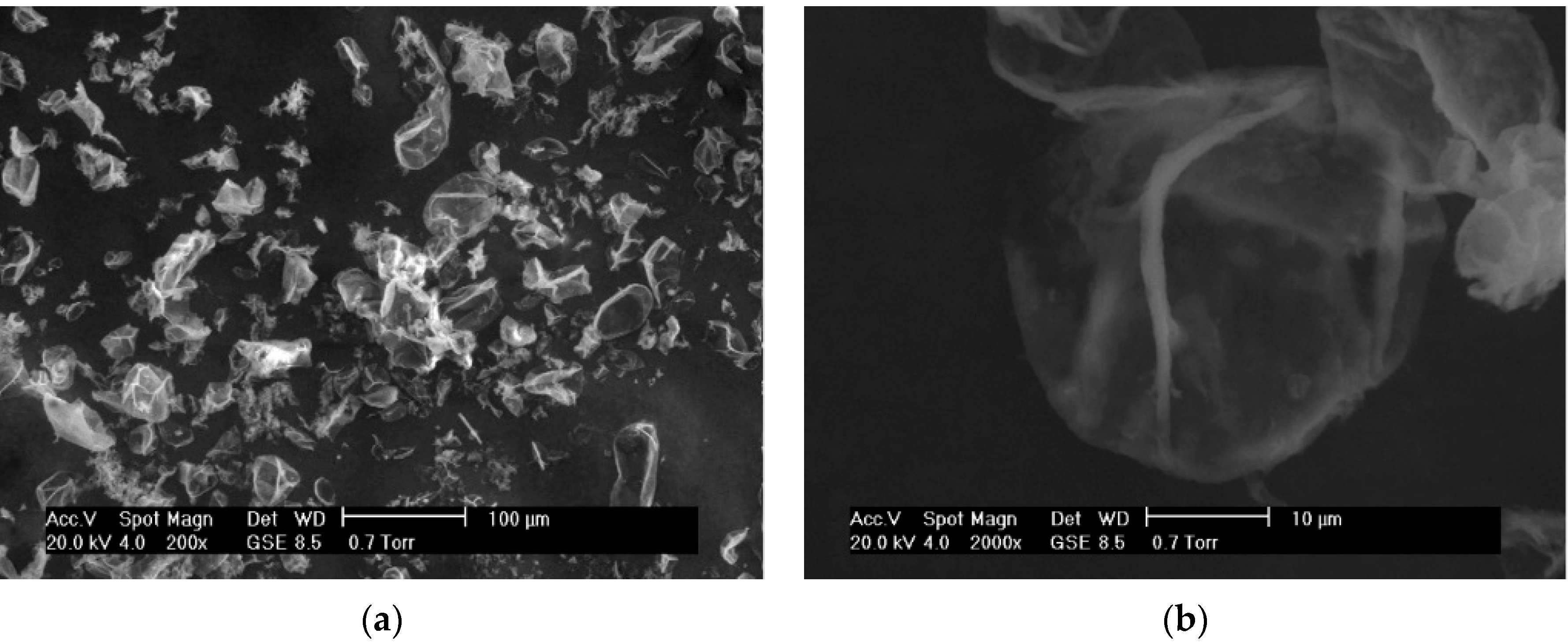
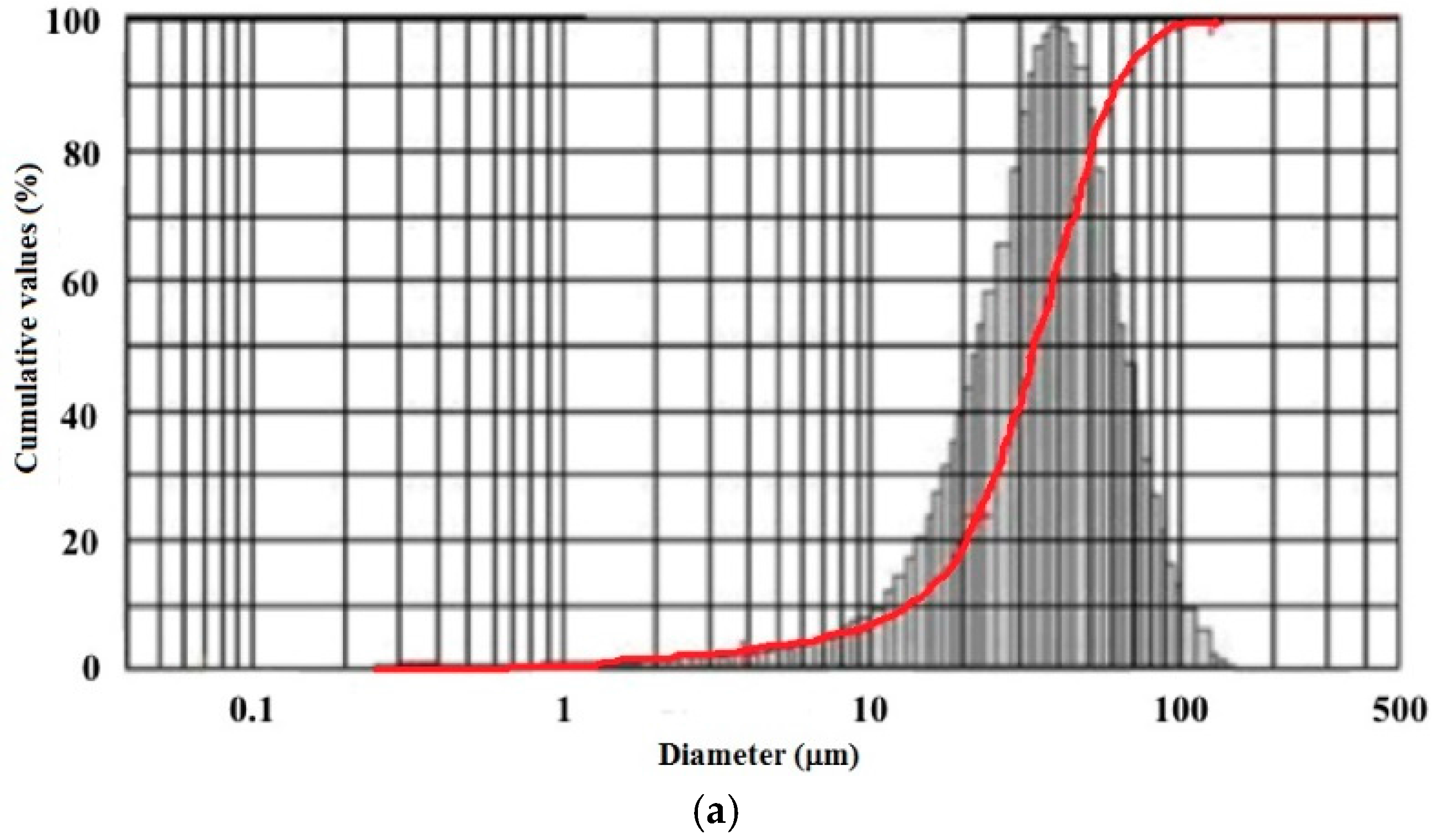
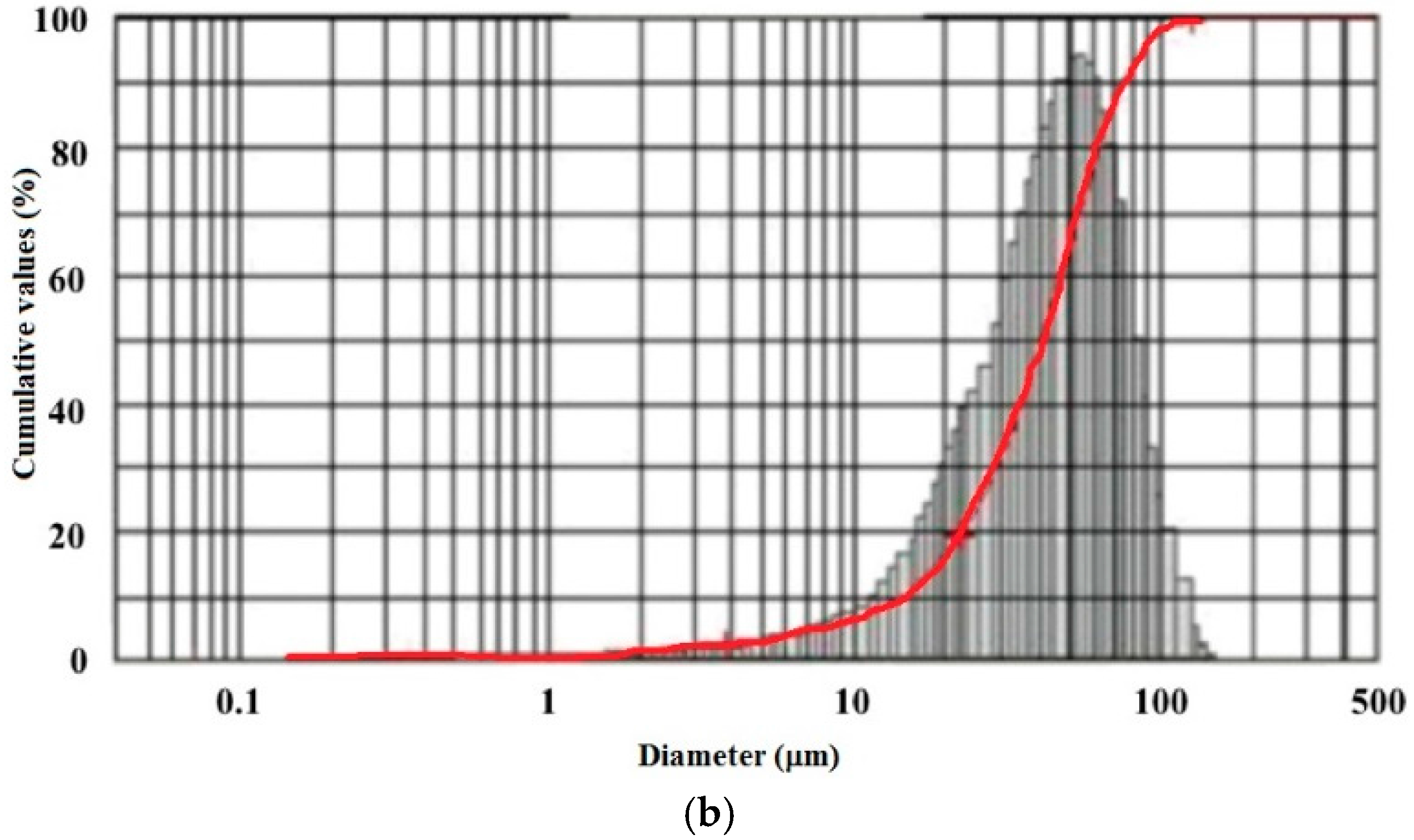
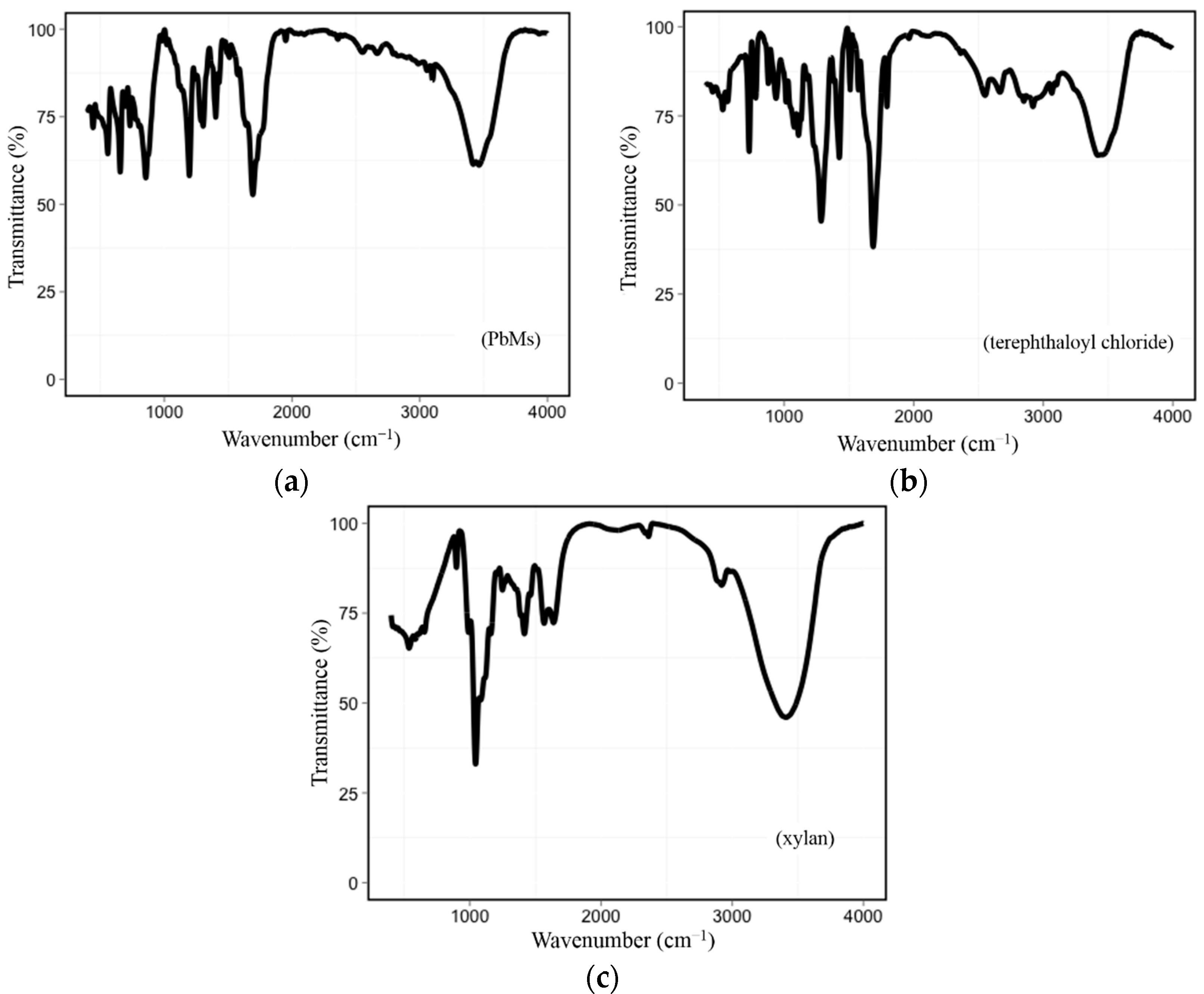
3.1.2. FT-IR Spectroscopy
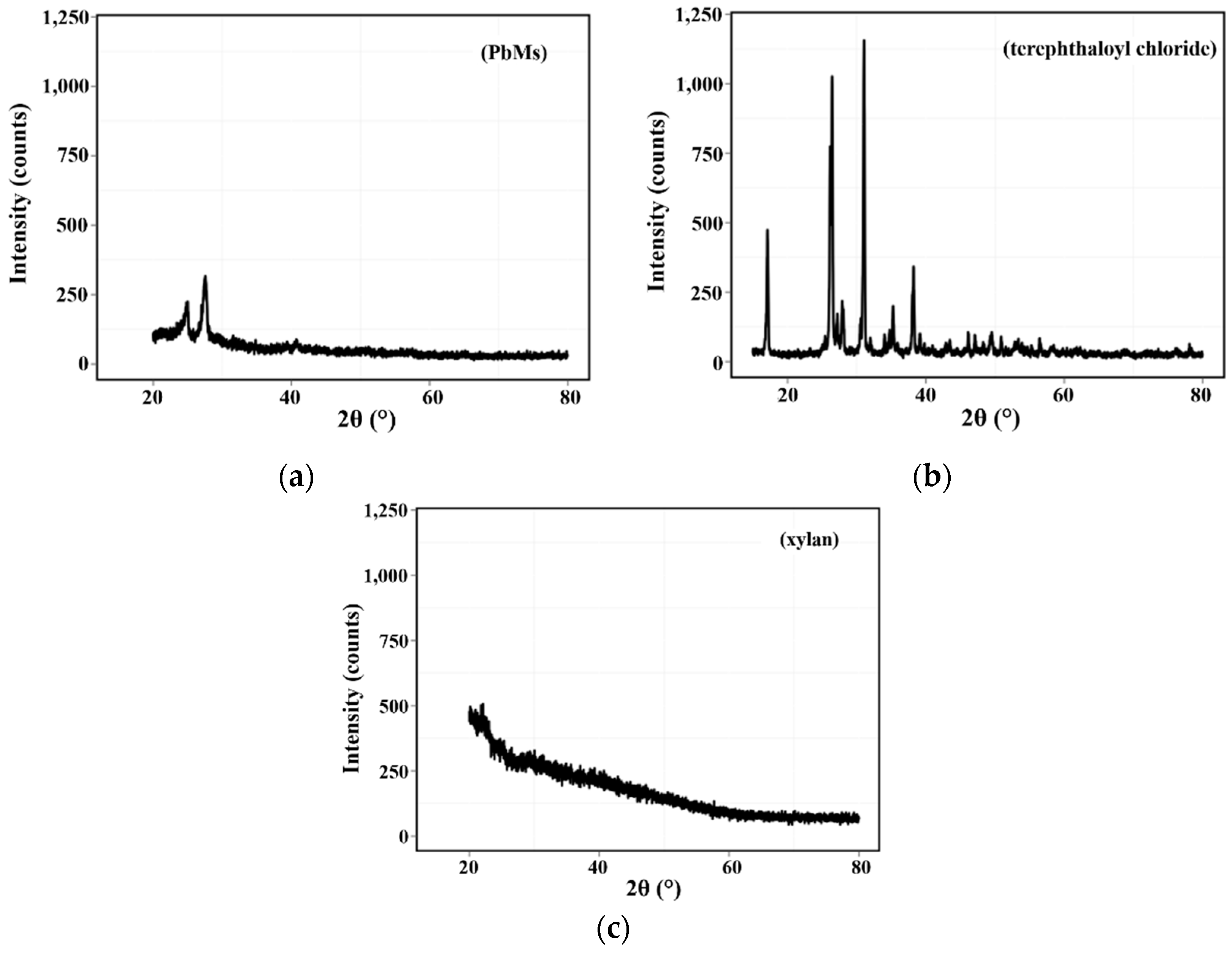
3.1.3. XRD Analysis
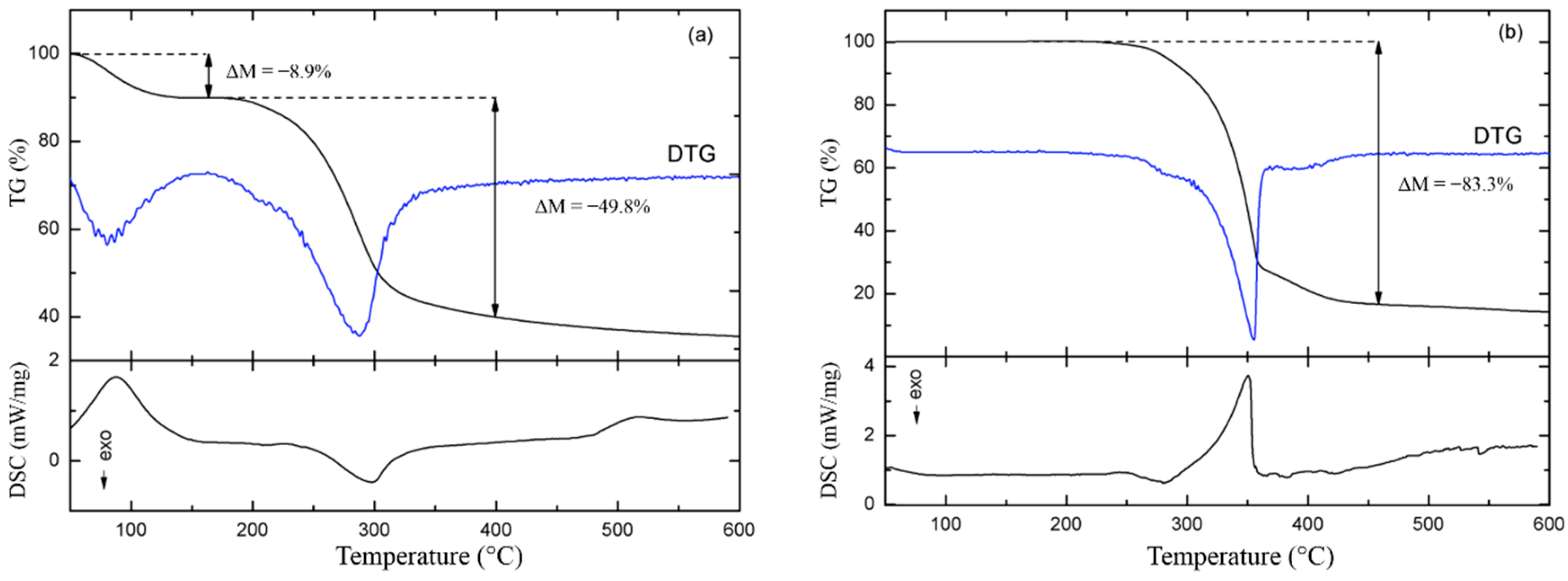
3.1.4. Thermal Analysis
3.2. Cell Viability through MTT Assay
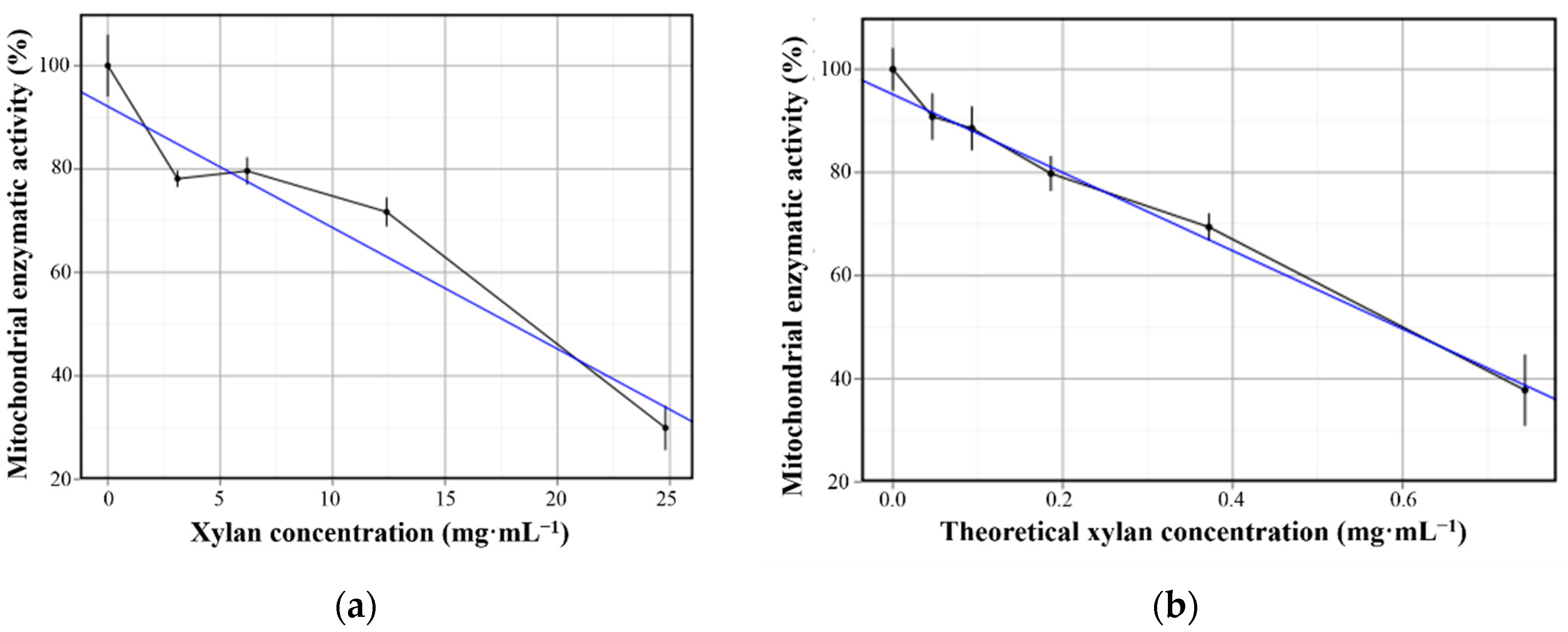
| Sample | Slope (Angular Coefficient) | Intercept (Linear Coefficient) | R2 (Determination Doefficient) |
|---|---|---|---|
| xylan | −2.35 | 92.12 | 0.895 |
| PbMs | −75.79 | 95.13 | 0.944 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rothman, U.; Arfors, K.E.; Aronsen, K.F.; Lindell, B.; Nylander, G. Enzymatically degradable microspheres for experimental and clinical use. In Sixth Meeting of the Nordic Microcirculation Group; Microvascular Research: Geilo, Norway, 1976; Volume 11, pp. 421–430. [Google Scholar]
- Lévy, M.C.; Andry, M.C. Microcapsules prepared through interfacial cross-linking of starch derivatives. Int. J. Pharm. 1990, 62, 27–35. [Google Scholar] [CrossRef]
- Jain, A.K.; Khar, R.K.; Ahmed, F.J.; Diwan, P.V. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Euro. J. Pharm. Biopharm. 2008, 69, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008, 4, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Mackie, R.I.; Cann, I.K. Xylan degradation, a metabolic property shared by rumen and human colonic bacteroidetes. Mol. Microbiol. 2011, 79, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.L.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Daus, S.; Heinze, T. Xylan-based nanoparticles: Prodrugs for ibuprofen release. Macromol. Biosci. 2010, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Petzold-Welcke, K.; Schwikal, K.; Daus, S.; Heinze, T. Xylan derivatives and their application potential—Mini-review of own results. Carbohydr. Polym. 2014, 100, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Nagashima-Junior, T.; Oliveira, E.E.; da Silva, A.E.; Marcelino, H.R.; Gomes, M.C.S.; Aguiar, L.M.; de Araujo, I.B.; Soares, L.A.; Oliveira, A.G.; Egito, E.S.T. Influence of the lipophilic external phase composition on the preparation and characterization of xylan microcapsules—A technical note. Aaps PharmSciTech 2008, 9, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.E.; Oliveira, E.E.; Gomes, M.C.S.; Marcelino, H.R.; Silva, K.C.; Souza, B.S.; Nagashima-Junior, T.; Ayala, A.P.; Oliveira, A.G.; Egito, E.S.T. Producing xylan/Eudragit® s100-based microparticles by chemical and physico-mechanical approaches as carriers for 5-aminosalicylic acid. J. Microencapsul. 2013, 30, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jerome, C.; Brayden, D.J.; Schneider, Y.J.; Preat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.E.; Silva, A.E.; Nagashima-Junior, T.; Gomes, M.C.S.; Aguiar, L.M.; Marcelino, H.R.; Araujo, I.B.; Bayer, M.P.; Ricardo, N.M.; Oliveira, A.G.; et al. Xylan from corn cobs, a promising polymer for drug delivery: Production and characterization. Bioresour. Technol. 2010, 101, 5402–5406. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.S.; Telles, C.B.; Dantas-Santos, N.; Oliveira Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Devy, J.; Balasse, E.; Kaplan, H.; Madoulet, C.; Andry, M.C. Hydroxyethylstarch microcapsules: A preliminary study for tumor immunotherapy application. Int. J. Pharm. 2006, 307, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Pariot, N.; Edwards-Levy, F.; Andry, M.C.; Levy, M.C. Cross-linked β-cyclodextrin microcapsules: Preparation and properties. Int. J. Pharm. 2000, 211, 19–27. [Google Scholar] [CrossRef]
- Larionova, N.V.; Kazanskaya, N.F.; Larionova, N.I.; Ponchel, G.; Duchene, D. Preparation and characterization of microencapsulated proteinase inhibitor aprotinin. Biochemistry 1999, 64, 857–862. [Google Scholar] [PubMed]
- Larionova, N.V.; Ponchel, G.; Duchene, D.; Larionova, N.I. Biodegradable cross-linked starch/protein microcapsules containing proteinase inhibitor for oral protein administration. Int. J. Pharm. 1999, 189, 171–178. [Google Scholar] [CrossRef]
- Krasota, A.; Belousova, R.; Duchene, D.; Larionova, N. In vitro inhibition of bovine herpes virus 1 reproduction with native and microencapsulated proteinase inhibitor aprotinin. J. Control. Release 2002, 85, 117–124. [Google Scholar] [CrossRef]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Krenzlin, S.; Siepmann, J. How porosity and size affect the drug release mechanisms from pliga-based microparticles. Int. J. Pharm. 2006, 314, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, F.; Tsapis, N.; Deyme, M.; Vasconcelos, T.G.; Gueutin, C.; Guterres, S.S.; Pohlmann, A.R.; Fattal, E. Spray-dried chitosan-metal microparticles for ciprofloxacin adsorption: Kinetic and equilibrium studies. Soft Matter 2011, 7, 7304. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Guan, J.; Ferrell, N.; James Lee, L.; Hansford, D.J. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials 2006, 27, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Z.; Wang, L.-J.; Li, D.; Bhandari, B.; Li, S.-J.; Lan, Y.; Chen, X.D.; Mao, Z.-H. Fabrication of starch-based microparticles by an emulsification-crosslinking method. J. Food Eng. 2009, 92, 250–254. [Google Scholar] [CrossRef]
- Li, B.-Z.; Wang, L.-J.; Li, D.; Chiu, Y.L.; Zhang, Z.-J.; Shi, J.; Chen, X.D.; Mao, Z.-H. Physical properties and loading capacity of starch-based microparticles crosslinked with trisodium trimetaphosphate. J. Food Eng. 2009, 92, 255–260. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Luo, Z.; Wen, L.; Cen, K. Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front. Energy Power Eng. China 2007, 1, 413–419. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Cross-linking with acid chlorides improves thermal and mechanical properties of collagen based biopolymer material. Thermochim. Acta 2011, 525, 50–55. [Google Scholar] [CrossRef]
- Sargent, J.M. The use of the mtt assay to study drug resistance in fresh tumour samples. In Chemosensitivity Testing in Oncology; Springer Berlin Heidelberg: Berlin, Germany, 2003; Volume 161, pp. 13–25. [Google Scholar]
- Silva, A.E.; Marcelino, H.R.; Gomes, M.C.S.; Oliveira, E.E.; Nagashima-Junior, T.; Egito, E.S.T. Xylan, a promising hemicellulose for pharmaceutical use. In Products and Applications of Biopolymers; Verbeek, C.J.R., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1, p. 220. [Google Scholar]
- Ebringerova, A.; Heinze, T. Xylan and xylan derivatives—Biopolymers with valuable properties. 1. Naturally occurring xylans structures, procedures and properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Sorg, O. Oxidative stress: A theoretical model or a biological reality? Comptes Rendus Biol. 2004, 327, 649–662. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelino, H.R.; Da Silva, A.E.; Gomes, M.C.S.; Oliveira, E.E.; Nagashima-Junior, T.; Pinheiro, G.S.; Da Silva, A.E.; Timoteo, A.R.d.S.; Agnez-Lima, L.F.; Ayala, A.P.; et al. Leads from Physical, Chemical, and Thermal Characterization on Cytotoxic Effects of Xylan-Based Microparticles. Polymers 2015, 7, 2304-2315. https://doi.org/10.3390/polym7111515
Marcelino HR, Da Silva AE, Gomes MCS, Oliveira EE, Nagashima-Junior T, Pinheiro GS, Da Silva AE, Timoteo ARdS, Agnez-Lima LF, Ayala AP, et al. Leads from Physical, Chemical, and Thermal Characterization on Cytotoxic Effects of Xylan-Based Microparticles. Polymers. 2015; 7(11):2304-2315. https://doi.org/10.3390/polym7111515
Chicago/Turabian StyleMarcelino, Henrique Rodrigues, Acarília Eduardo Da Silva, Monique Christine Salgado Gomes, Elquio Eleamen Oliveira, Toshiyuki Nagashima-Junior, Gardênia Sousa Pinheiro, Acarízia Eduardo Da Silva, Ana Rafaela de Souza Timoteo, Lucymara Fassarela Agnez-Lima, Alejandro Pedro Ayala, and et al. 2015. "Leads from Physical, Chemical, and Thermal Characterization on Cytotoxic Effects of Xylan-Based Microparticles" Polymers 7, no. 11: 2304-2315. https://doi.org/10.3390/polym7111515