Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Plant Material
2.3. Extract Preparation
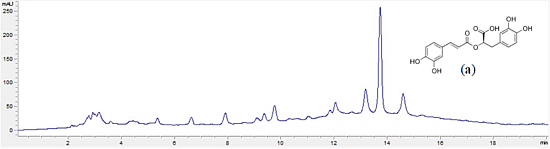
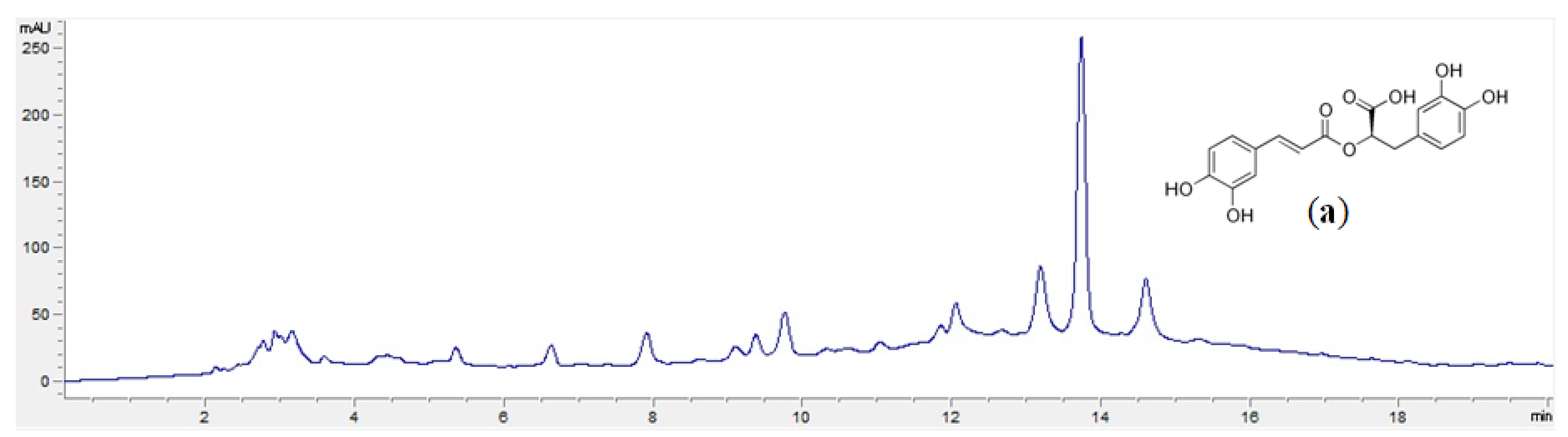
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Microorganisms and Well Diffusion Method
2.6. Determination of Minimum Inhibitory Concentrations (MICs)
2.7. Determination of Antioxidant Activity

2.8. Screening of General Toxicity

2.9. Evaluation of Cytotoxicity against the HaCaT Line (Human Adult Low-Calcium High-Temperature Keratinocytes)
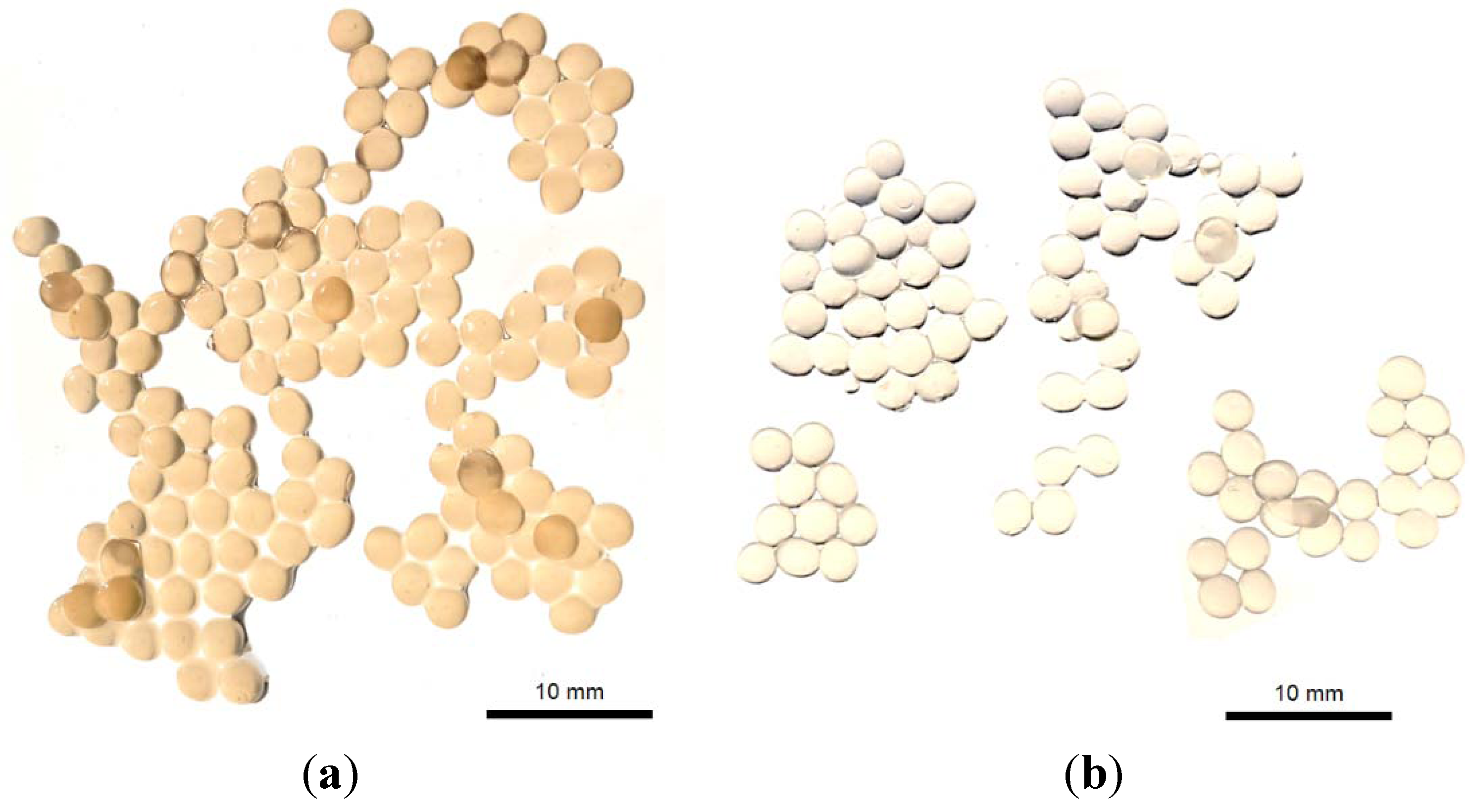
2.10. Production of Plectranthus spp. Extract-Loaded Calcium Alginate Beads
2.11. Characterization of Plectranthus spp. Extract-Loaded Calcium Alginate Beads

2.12. Stability Studies of Plectranthus spp. Extract-Loaded Calcium Alginate Beads
2.12.1. Stability over the Time
2.12.2. Stability against UV Radiation
3. Results and Discussion
3.1. Extraction Yields
| Plant | Extraction method | Weight of dry extract (mg/mL) |
|---|---|---|
| P. barbatus | Decoction | 26 ± 2.0 |
| Infusion | 33 ± 3.0 | |
| Microwave | 22 ± 1.0 | |
| P. hadiensis var. Tomentosus | Decoction | 32 ± 2.0 |
| Infusion | 26 ± 1.0 | |
| Microwave | 17 ± 1.0 | |
| P. madagascarensis | Decoction | 22 ± 1.0 |
| Infusion | 26 ± 4.0 | |
| Microwave | 15 ± 4.0 | |
| P. neochilus | Decoction | 22 ± 2.0 |
| Infusion | 23 ± 10.0 | |
| Microwave | 11 ± 4.0 | |
| P. verticillatus | Decoction | nd |
| Infusion | nd | |
| Microwave | 12 |
3.2. Phytochemical Composition of Extracts
| Plant | Extraction method | Identified polyphenols (w/w% of extract) | Antioxidant activity (AA%) |
|---|---|---|---|
| P. barbatus | Decoction | CA, RA | 10.30 |
| Infusion | CA, RA | 15.70 | |
| Microwave | CA, RA | 10.10 | |
| P. hadiensis var. tomentosus | Decoction | CA, RA | 17.9 |
| Infusion | CA, RA | 40.0 | |
| Microwave | CA, RA | 32.6 | |
| P. madagascarensis | Decoction | CA, RA (15.72) | 20.60 |
| Infusion | CA, RA (10.93) | 10.20 | |
| Microwave | CA, RA (18.64) | 17.20 | |
| P. neochilus | Decoction | CA, RA | 12.1 |
| Infusion | CA, RA | 14.7 | |
| Microwave | CA, RA | 14.0 | |
| P. verticillatus | Microwave | CA, RA | 19.5 |
3.3. Antibacterial Activity
3.4. Antioxidant Activity
3.5. General Toxicity
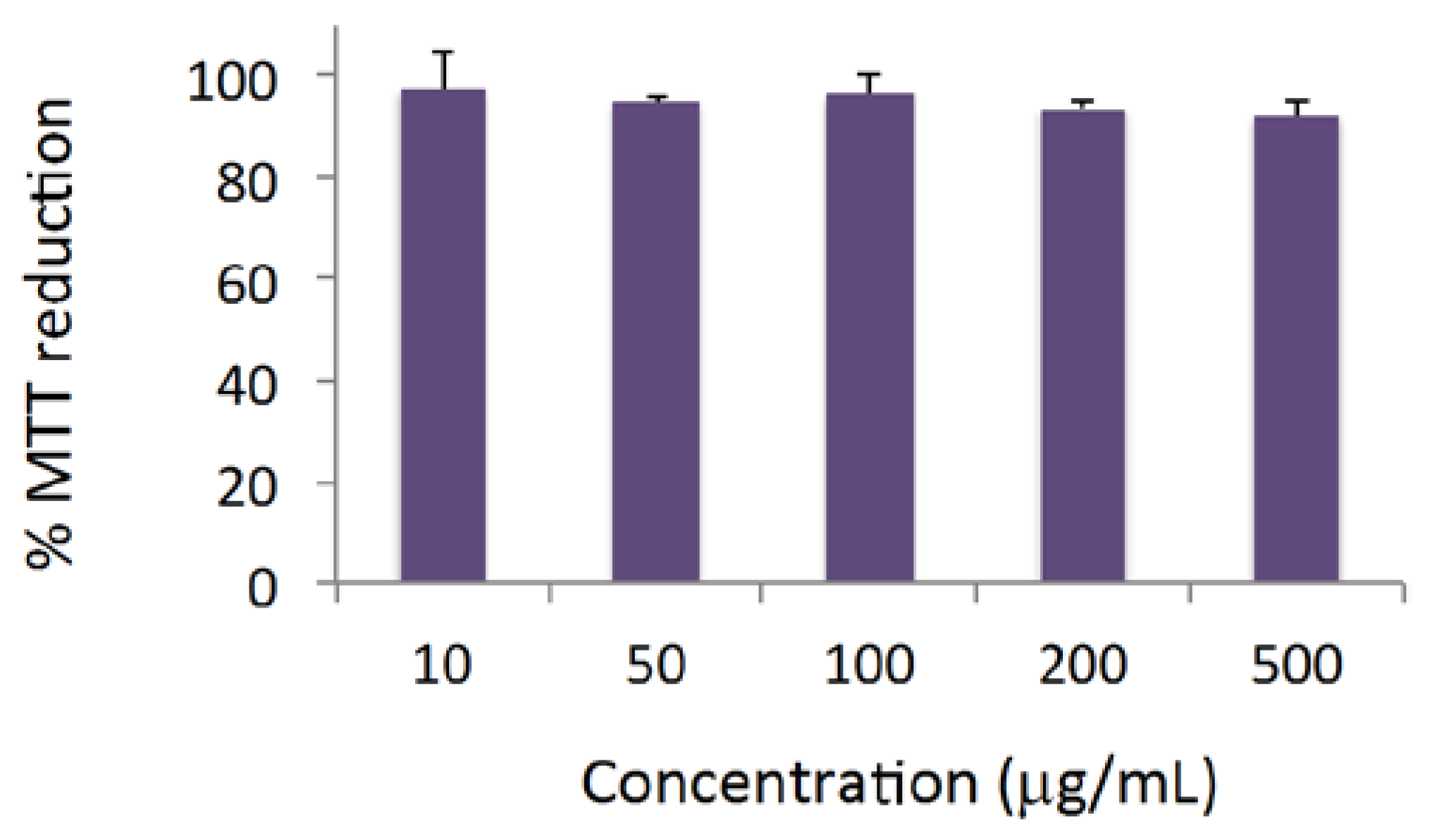
3.6. Cytotoxicity
3.7. Production and Characterization of Plectranthus spp. Extract-Loaded Calcium Alginate Beads
3.8. Stability Studies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Rice, L.J.; Brits, G.J.; Potgeiter, C.J.; van Staden, J. Plectranthus: A plant for the future? South Afr. J. Bot. 2011, 77, 947–959. [Google Scholar] [CrossRef]
- Rijo, P.; Gaspar-Marques, C.; Simões, M.F.; Duarte, A.; Apreda-Rojas, M.C.; Cano, F.H.; Rodriguez, B. Neoclerodane and labdane diterpenoids from Plectranthus ornatus. J. Nat. Prod. 2002, 65, 1387–1390. [Google Scholar] [CrossRef]
- Simões, M.F.; Rijo, P.; Duarte, A.; Barbosa, D.; Matias, D.; Delgado, J.; Cirilo, N.; Rodriguez, B. Two diterpenoids from Plectranthus species. Phytochem. Lett. 2010, 3, 221–225. [Google Scholar] [CrossRef]
- Rijo, P.; Simões, M.F.; Francisco, A.P.; Rojas, R.; Gilman, R.H.; Vaisberg, A.J.; Rodriguez, B.; Moiteiro, C. Antimycobacterial metabolites from Plectranthus: Roylone derivates against mycobacterium tuberculosis strains. Chem. Biodivers. 2010, 7, 922–932. [Google Scholar] [CrossRef]
- Murata, Y.; Nakada, K.; Miyamoto, E.; Kawashima, S.; Seo, S.-H. Influence of erosion of alcium-induced alginete gel matrix on the release of Brilliant Blue. J. Control. Rel. 1993, 23, 21–26. [Google Scholar] [CrossRef]
- Martinsen, A.; Skjak Braek, G.; Smidsrod, O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate beads. Biotechnol. Bioeng. 1989, 33, 79–89. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Bayer, I.; Pompa, P.; Cingolani, R.; Athanassiou, A. Controlled antiseptic release by alginate polymer films and beads. Carbohydr. Polym. 2013, 92, 176–183. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.; Pompa, P.; Bayer, I.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2013. [Google Scholar] [CrossRef]
- Dalmoro, A.; Barba, A.; Lamberti, G.; d’Amore, M. Intensifying the microencapsulation process: Ultrasonic atomization as an innovative approach. Eur. J. Pharm. Biopharm. 2012, 80, 471–477. [Google Scholar] [CrossRef]
- Dalmoro, A.; d’Amore, M.; Barba, A. Droplet size prediction in the production of drug delivery microsystems by ultrasonic atomization. Transl. Med. 2013, 7, 6–11. [Google Scholar]
- Chan, E.-S.; Lee, B.-B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of Ca-alginate macrobeads produced through extrusion-dripping method. J. Colloid Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
- Quong, D.; Neufeld, R.J.; Skjak-Braek, G.; Poncelet, D. External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnol. Bioeng. 1998, 57, 438–446. [Google Scholar] [CrossRef]
- Reis, C.P.; Ribeiro, A.J.; Veiga, F.; Neufeld, R.J.; Damgé, C. Polyelectrolyte biomaterial interactions provide nanoparticulate carrier for oral insulin delivery. Drug Deliv. 2008, 15, 127–139. [Google Scholar] [CrossRef]
- Falé, P.L.; Borges, C.; Madeira, J.P.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Roberto, A.; Caetano, P.P. A high-throughput screening method for general cytotoxicity part I chemical toxicity. Revista Lusófona de Ciências e Tecnologias de Saúde 2005, 2, 95–100. [Google Scholar]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Rueff, J.; Castro, M.; Batinic-Haberle, I.; Costa, J.; Oliveira, N.G. Protective role of ortho-substituted Mn(III) N-alkylpyridylporphyrins against the oxidative injury induced by tert-butylhydroperoxide. Free Radic. Res. 2010, 44, 430–440. [Google Scholar] [CrossRef]
- Ostberg, L.; Graffner, C. Calcium alginate matrices for oral multiple unit administration: II. Effect of process and formulation factors on matrix properties. Int. J. Pharm. 1993, 97, 183–193. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of C(2+)-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Gray, C.J.; Dowsett, J. Retention of insulin in alginate gel beads. Biotechnol. Bioeng. 1988, 31, 607–612. [Google Scholar] [CrossRef]
- Chen, L.; Subirade, M. Effect of preparation conditions on the nutrient release properties of alginate-whey protein granular microspheres. Eur. J. Pharm. Biopharm. 2007, 65, 354–362. [Google Scholar] [CrossRef]
- Reis, C.; Veiga, F.; Ribeiro, A.J.; Neufeld, R.J.; Damgé, C. Nanoparticulate biopolymers deliver insulin orally eliciting pharmacological response. J. Pharm. Sci. 2008, 97, 5290–5305. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers 2014, 6, 479-490. https://doi.org/10.3390/polym6020479
Rijo P, Matias D, Fernandes AS, Simões MF, Nicolai M, Reis CP. Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers. 2014; 6(2):479-490. https://doi.org/10.3390/polym6020479
Chicago/Turabian StyleRijo, Patrícia, Diogo Matias, Ana S. Fernandes, M. Fátima Simões, Marisa Nicolai, and Catarina Pinto Reis. 2014. "Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin" Polymers 6, no. 2: 479-490. https://doi.org/10.3390/polym6020479
APA StyleRijo, P., Matias, D., Fernandes, A. S., Simões, M. F., Nicolai, M., & Reis, C. P. (2014). Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers, 6(2), 479-490. https://doi.org/10.3390/polym6020479