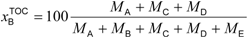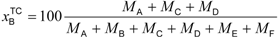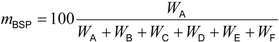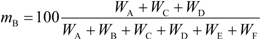1. Introduction
During the early periods of the rubber industry, only natural rubber was used for manufacturing rubber products. Eventually, many types of synthetic rubber such as styrene-butadiene rubber were developed using petroleum resources. In 2005, 58% of global rubber production (21 million tons) used synthetic rubber, which could meet the performance requirements of many types of rubber products.
However, to reduce the use of petroleum, which is a nonrenewable resource, and global warming, by reducing CO
2 emissions, it is important to change the source of rubber products from petroleum to biomass, which is sustainable. To achieve this goal, it is necessary that not only the amount of natural rubber should be increased, but also that synthetic rubbers should be developed from biomass-based monomers. Microbial production of isoprene has been reported [
1,
2]. From such biobased isoprene monomers, polyisoprene could be produced as biobased synthetic rubber. In addition, resources for natural rubber are being diversified. Natural rubber is produced from latex, which is excreted from the rubber tree (
Hevea brasiliensis)—a plantation crop in South Asia. The production of natural rubber from Russian dandelion (
Taraxacum kok-saghyz) and Guayule (
Parthenium argentatum), which can grow in regions of the world, is also being developed [
3,
4].
The carbon content of rubber products is high; thus, rubber product waste such as scrap tires can be used in thermal recycling as fuel for a boiler because of its high energy of combustion. In Japan in 2012, 58% of scrap tires (1 million tons) were used in thermal recycling [
5]. In these cases, CO
2 emitted from the biobased constituents of rubber products during combustion does not increase the amount of CO
2 in the atmosphere, because this emitted CO
2 was fixed by photosynthesis during the growth of plants as biomass resource (the concept of “zero emission”). The content of biomass fuels such as wood pellets or agricultural wastes (e.g., bagasse) in the fuel used in an incinerator or a boiler can be evaluated on the basis of the International Organization for Standardization (ISO) 13833 [
6].
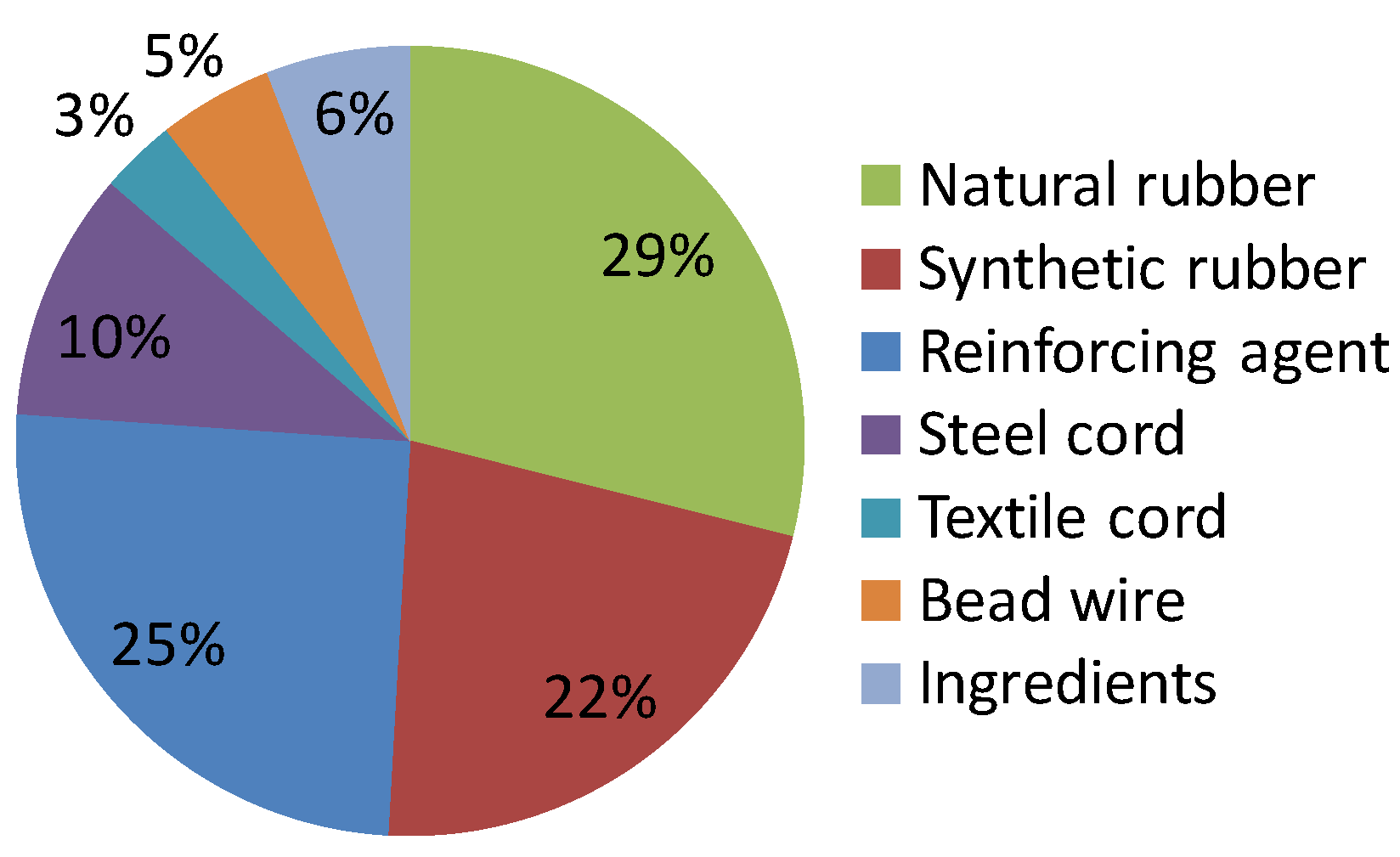
Approximately half of the rubber content of present-day rubber products such as automobile tires comprises fossil-based synthetic rubber, whose ratio is dependent on the product grade or production company, as indicated in
Figure 1 [
5]. Biomass-based additives such as oil or filler are being developed by many companies. A fossil-fuel-free tire has been developed [
7]. Large-sized tires used in buses, trucks, and airplanes include a large amount of natural rubber, primarily because of the sufficient mechanical properties of natural rubber. To promote the use of biobased constituents, a reliable index for the amount of biomass resource used is necessary.
Figure 1.
Composition of main materials used in automobile tires in Japan in 2012 [
5].
Figure 1.
Composition of main materials used in automobile tires in Japan in 2012 [
5].
Almost all the organic constituents of plastic products are fossil-based. To regulate biobased plastic products, some international standards have been developed for the determination of the biobased content (
Table 1). American Society for Testing and Materials (ASTM) D6866 was the first published method for determining the biobased content in 2004. Conité Européen de Normalisation (CEN) 16137 was published in 2011. Recently, ISO 16620 series for determination of biobased content was discussed in ISO technical committee (TC) 61 “Plastics”. International standards proposed by Japan define four types of biobased contents (
Table 2). These standards will be published soon. Quantification of the biobased content based on the carbon mass (
MX) of the biobased constituents can be calculated as the biobased carbon content in the total organic carbon (
xBTOC) or the biobased carbon content in the total carbon (
xBTC). The method based on the mass (
WX) of the biobased synthetic polymer and biobased constituents can be calculated as the biobased synthetic polymer content (
mBSP) and the biobased mass content (
mB), respectively. Natural rubber is a natural polymer and not a biobased synthetic polymer, so natural rubber products may not be evaluated by ISO 16620-3, where they are defined as biobased synthetic polymer content. It is very important to select the optimal index from the four types of biobased content depending on the intended purpose of the rubber product. Comparing different types of biobased contents is meaningless.
Table 1.
International standards that define biobased contents.
Table 1.
International standards that define biobased contents.
| Organization | Standard |
|---|
| NO | Title |
|---|
| American Society for Testing and Materials (ASTM) | ASTM D 6866-12 | Standard test method for determining biobased content of solid, liquid, and gaseous samples using radiocarbon analysis |
| Conité Européen de Normalisation (CEN) | CEN/TS 16137:2011 | Plastics-determination of biobased carbon content |
| International Organization for Standardization (ISO) | ISO/DIS 16620-1 published soon | Plastics-biobased content—Part 1 General principles |
| ISO/DIS 16620-2 published soon | Plastics-biobased content—Part 2 Determination of biobased carbon content |
| ISO/DIS 16620-3 published soon | Plastics-biobased content—Part 3 Determination of biobased synthetic polymer content |
| ISO/WD 16620-4 under discussion | Plastics-biobased content—Part 4 Determination of biobased mass content |
Several mark certification systems have been developed for biobased products using these biobased contents (
Table 3). Using these systems, consumers can recognize whether these products are biobased and what is the amount of biobased constituents in these products. Solvent-separation methods for determining the biobased synthetic polymer content and biobased mass content of the constituents of plastic products are currently being developed [
8,
9].
Table 2.
Biobased content in plastic products 1,*.
Table 3.
Mark certification system using biobased content.
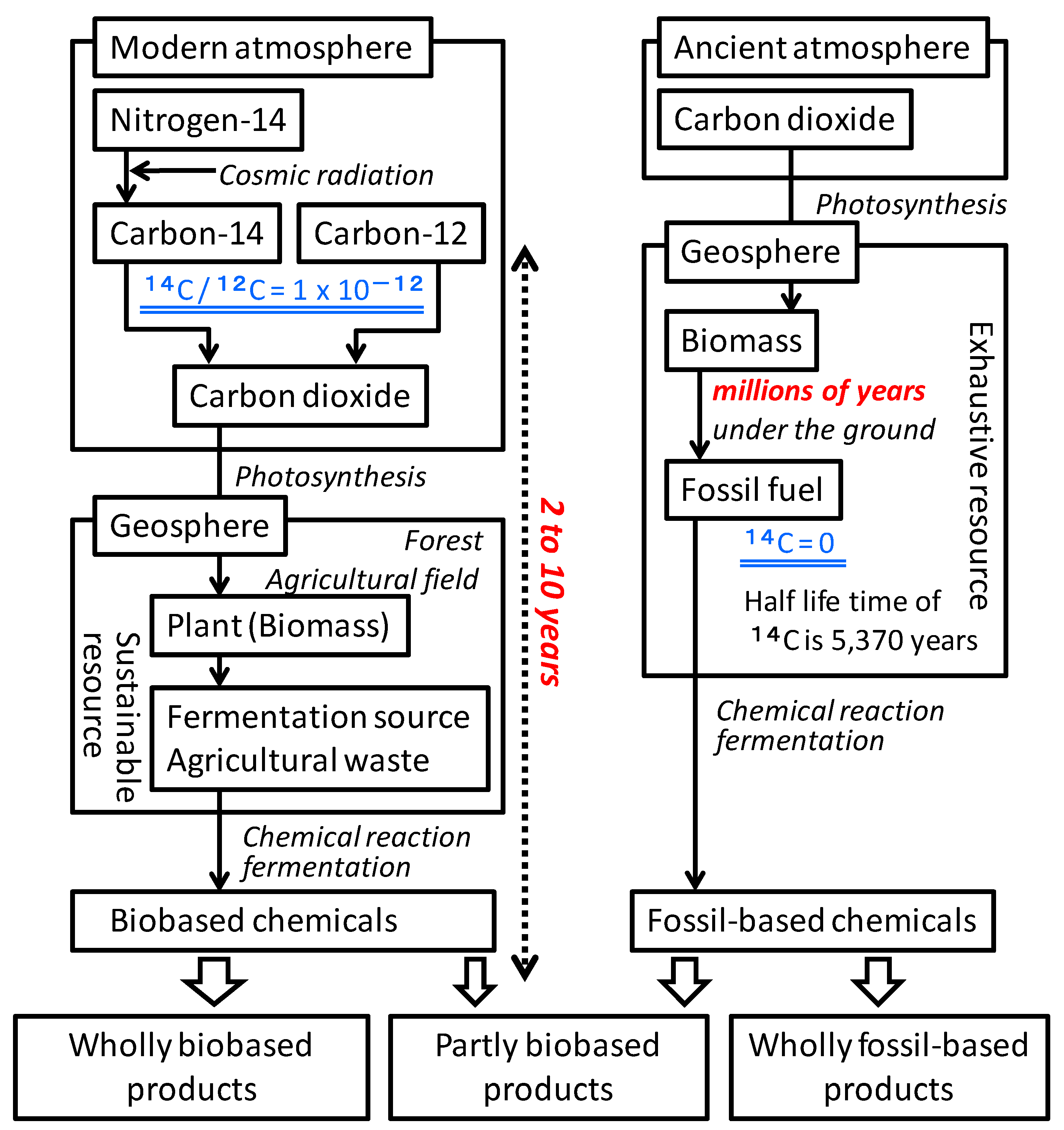
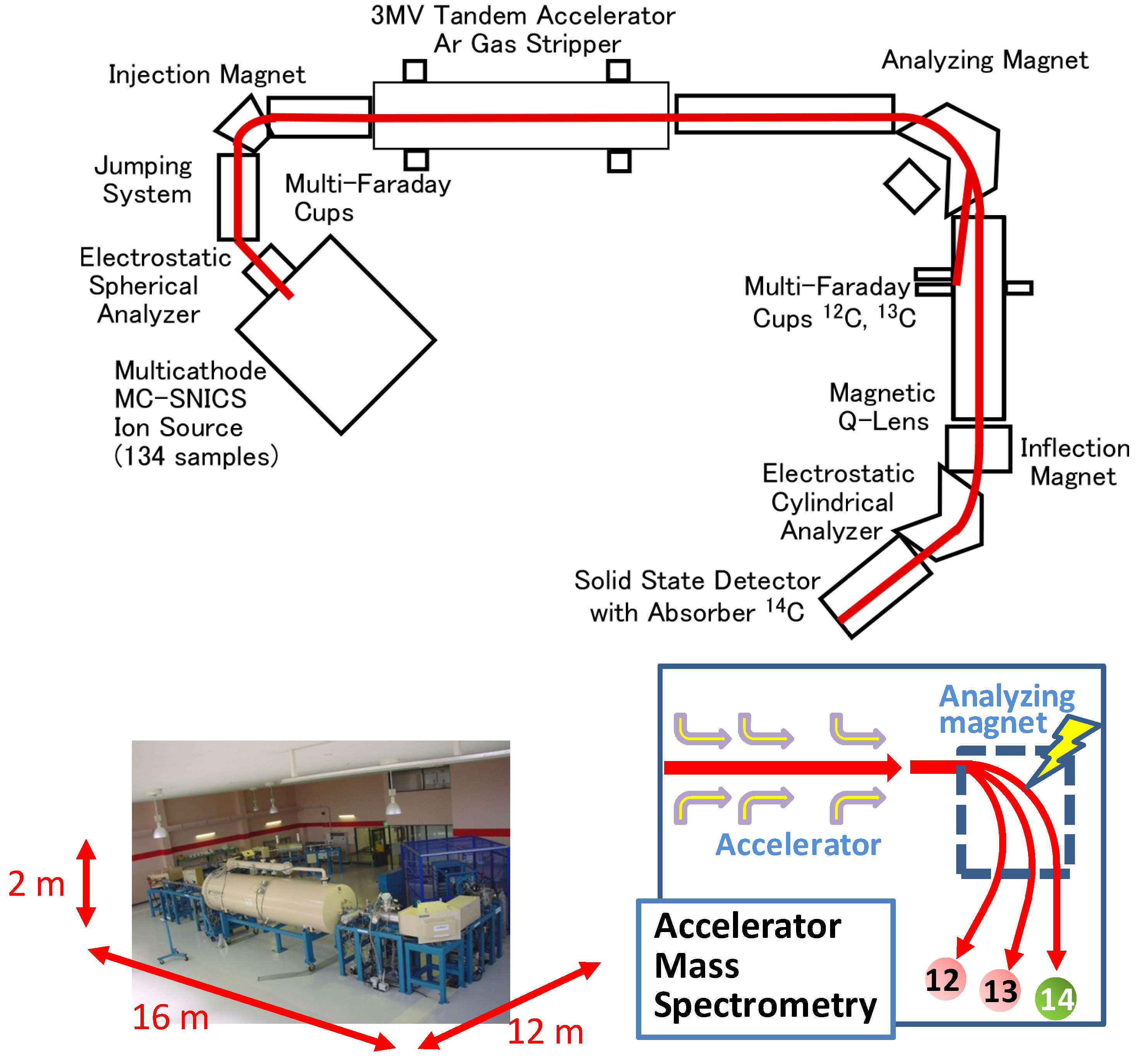
The difference between biobased carbon and fossil-based carbon in plastic or rubber products is determined by measuring the difference in the ratio of carbon isotopes (
12C,
13C, and
14C) present in these products. Biobased carbon includes a very small amount of
14C, produced from
14N irradiated by cosmic radiation in the upper region of the atmosphere (
Figure 2). Fossil-based carbons included
14C initially, but all
14C, which is an unstable radiocarbon with a half-life of 5730 years, decayed during the long period of time spent underground by such carbons. The ratio of
14C to
12C in biobased carbon is 1 × 10
−12. The percent of modern carbon (
pMC) of biobased carbon from biomass harvested in 2010 is 104%, which varied by ±2% depending on the place and its situation. In addition, the value of
pMC is decreasing gradually at approximately 0.5%/year and will gradually reach 100%. The
pMC value of fossil-based carbon is 0%. The biobased carbon content for plastic products can be calculated by dividing the
pMC value of the sample material by 1.04, on the basis of CEN/TS 16137, or by multiplying the
pMC value by 0.95, on the basis of ASTM D6866-12 [
14,
15,
16,
17].
Figure 2.
Mechanisms of 14C insertion into biobased products.
Figure 2.
Mechanisms of 14C insertion into biobased products.
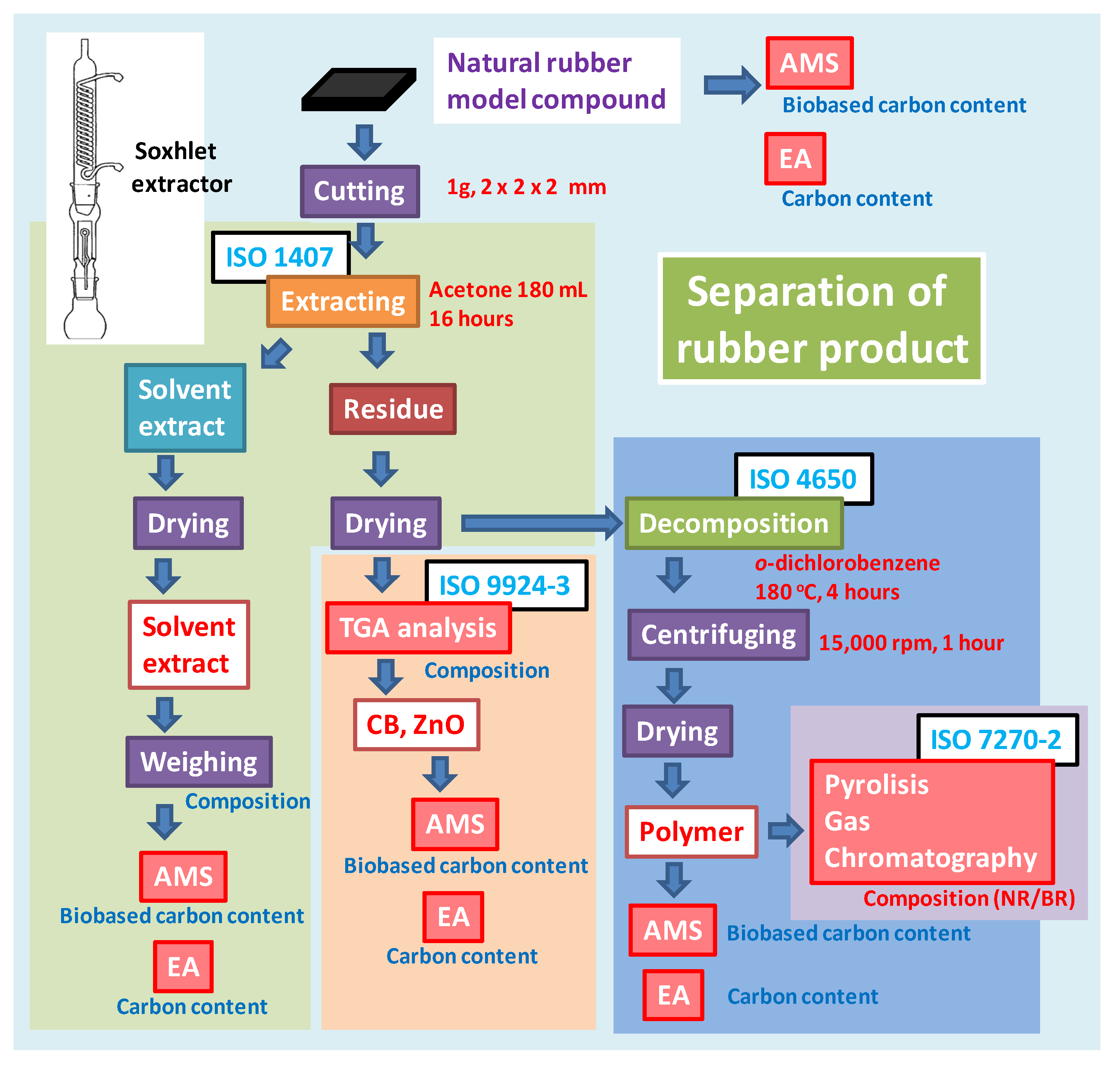
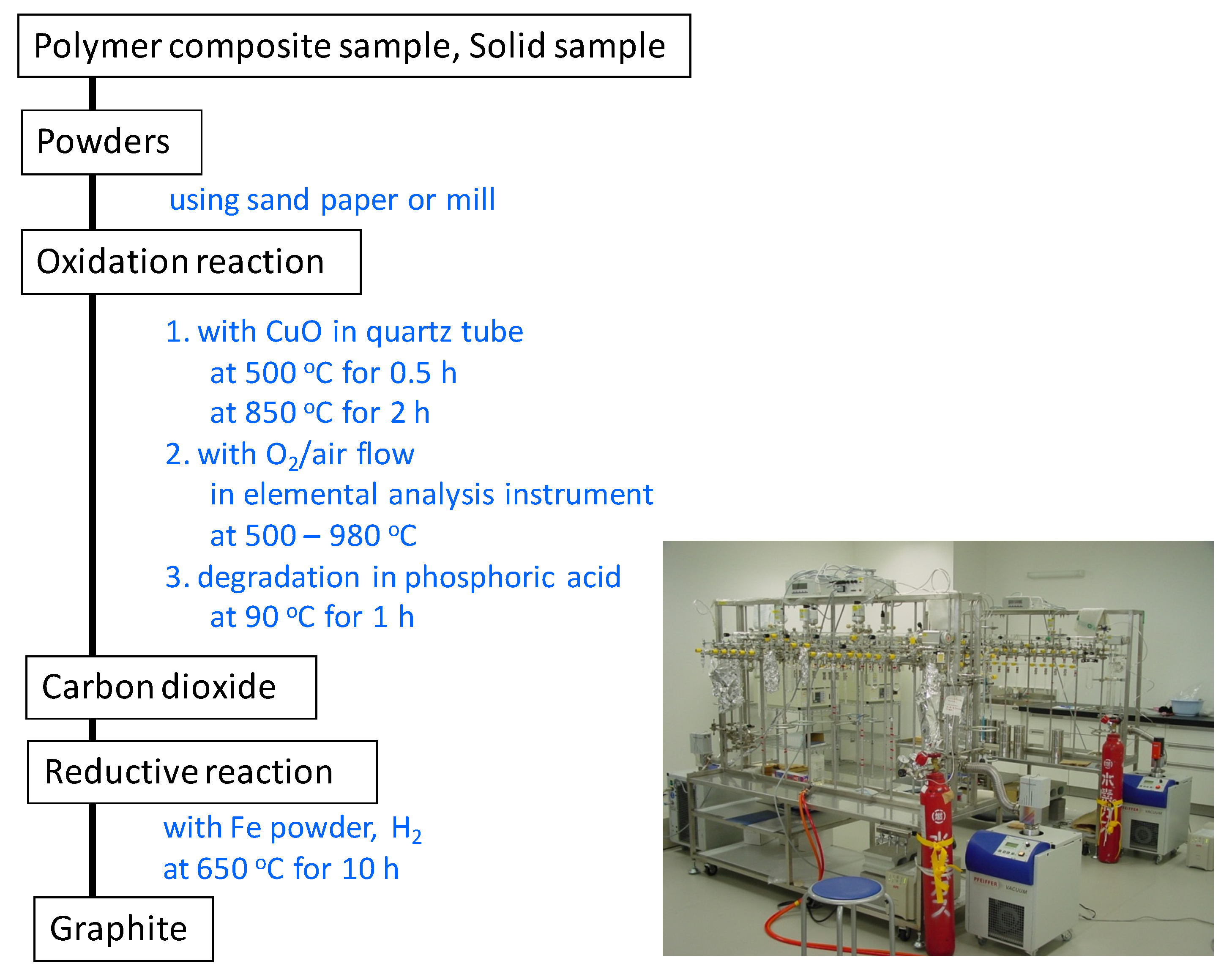
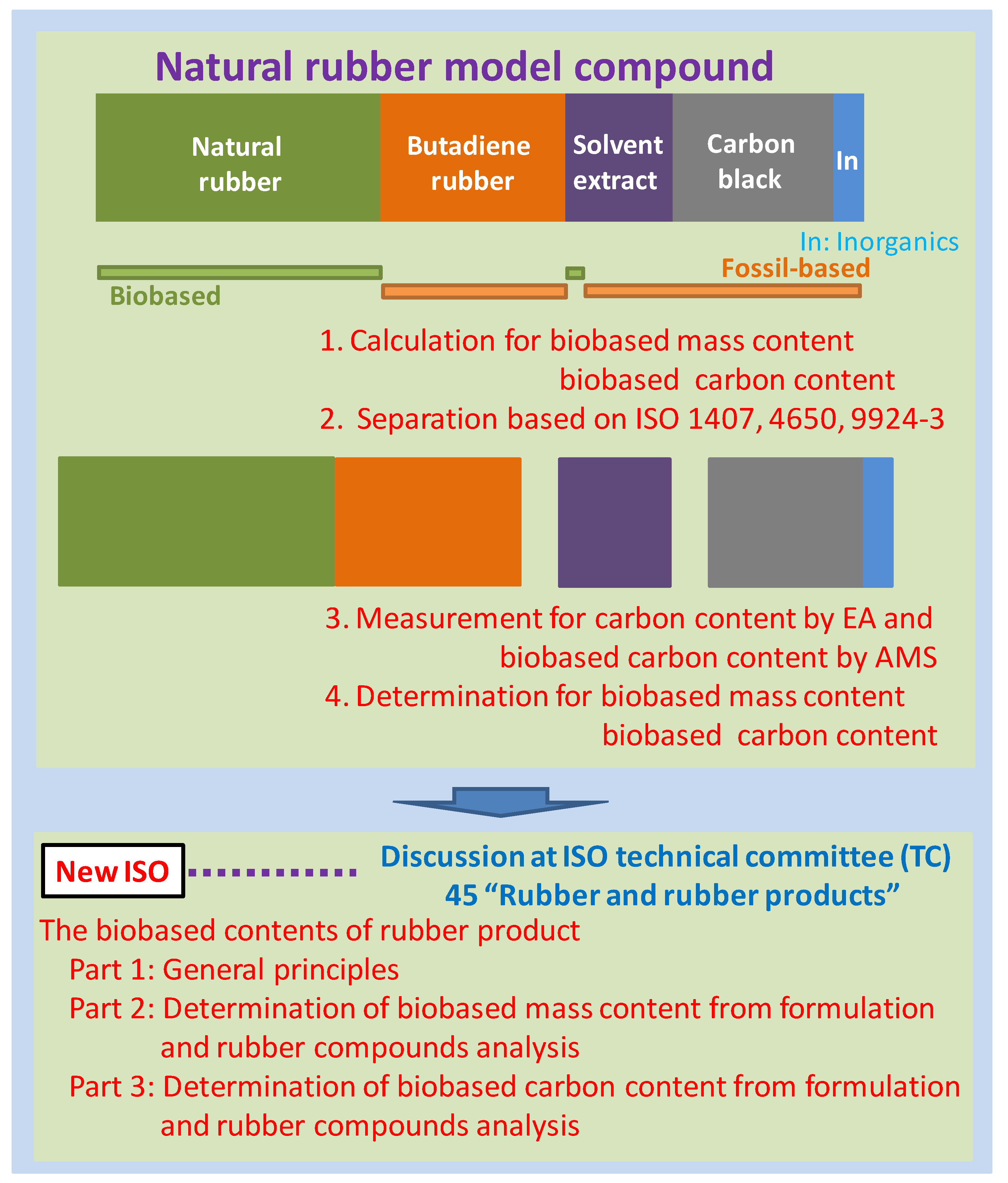
Some problems are associated with using the ISO 16620 series for rubber products. Rubber products include many types of ingredients such as silica and steel. One of the main components in rubber products is carbon black (CB), which improves the rubber’s performance. For plastic products, the inorganic carbon components are assumed to be mainly calcium carbonate. Calcium carbonate in plastic products is treated by acid degradation, e.g., with hydrochloric or phosphoric acid, for evaluating the biobased content on the basis of ISO 16620 for plastics. However, this method is not applicable for CB in rubber products, because CB is stable under acidic conditions and cannot be transformed to CO
2 by acid degradation. The biobased mass content and biobased carbon content for rubber products can be calculated from the composition of the feed constituents similar to that in plastic products. In addition, some composition analysis methods have been defined in the ISO standards (
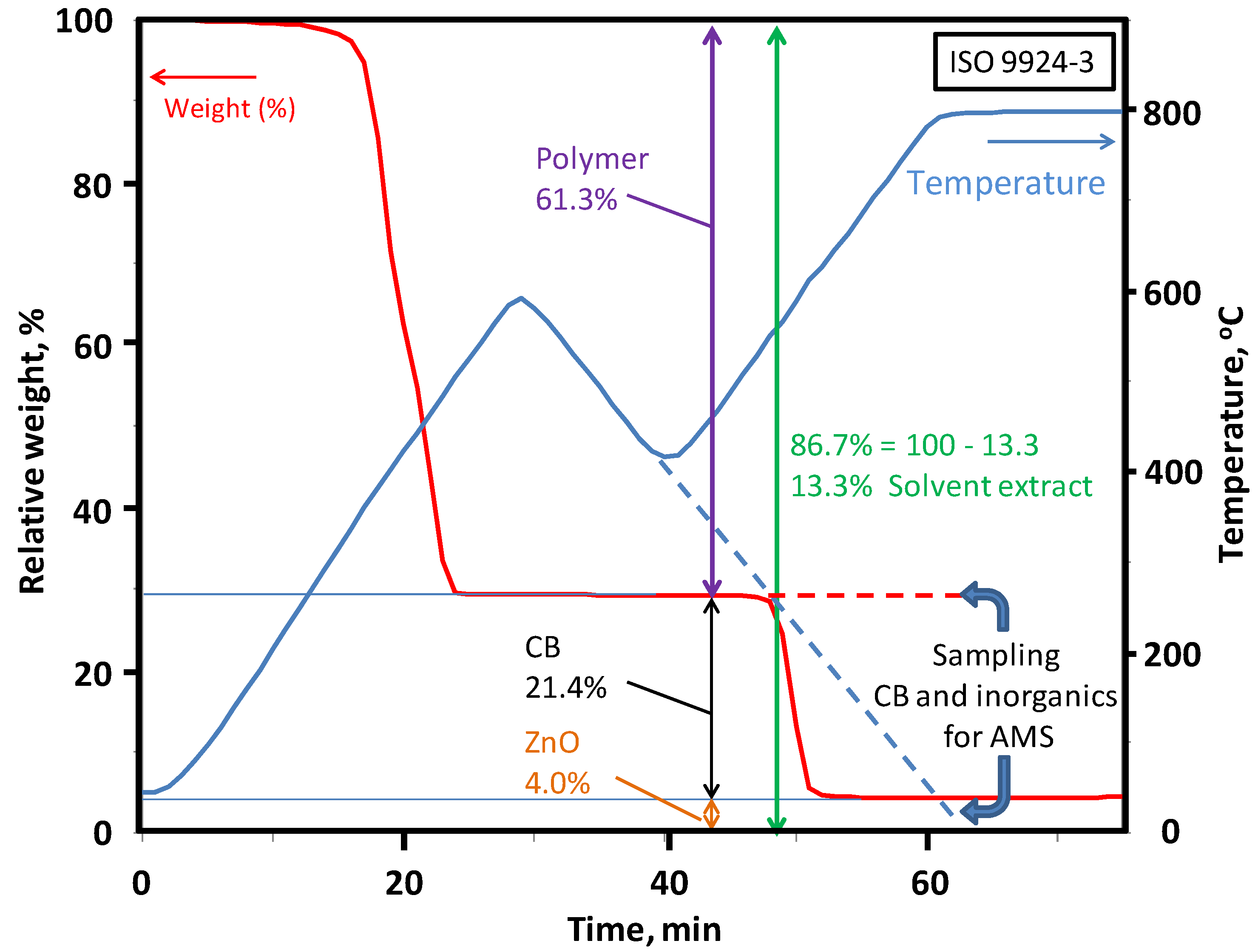
Table 4). It is possible to separate the defined constituents using these ISO standards. The biobased content of these separated constituent may be calculated using the biobased carbon content from the
pMC value, which can be measured using the accelerator mass spectrometry (AMS) and a liquid scintillation counter (LSC); the carbon content by elemental analyses confirms the calculated values for the composition of rubber products.
Table 4.
ISO standards related to composition analysis by separation of vulcanized rubber products published in ISO technical committee (TC) 45 “Rubber and rubber products”.
Table 4.
ISO standards related to composition analysis by separation of vulcanized rubber products published in ISO technical committee (TC) 45 “Rubber and rubber products”.
| Method | ISO number | Title of standard |
|---|
| Content of extract | ISO 1407 | Rubber–Determination of solvent extract |
| Separation of polymer from vulcanized rubber | ISO 4650 | Rubber–Identification–Infrared spectrometric method |
| Content of polymer, carbon black, and inorganics | ISO 9924-3 | Rubber and rubber products–Determination of the composition of vulcanizates and uncured compounds by thermogravimetry—Part 3: Hydrocarbon rubbers, halogenated rubbers, and polysiloxane rubbers after extraction |
| Blend ratio of polymer | ISO 7270-2 | Rubber–Analysis by pyrolytic gas-chromatographic methods—Part 2: Determination of styrene/butadiene/isoprene ratio |
The Japan Rubber Manufacturers Association (JRMA) plans to make the new ISOs applicable for determining the amount of biobased contents in rubber products. JRMA is responsible for maintaining the quality of Japanese rubber products worldwide [
18]. In addition, JRMA is discussing ISO with the role of the sub committee (SC)’s chairman and secretariat of ISO/TC 45 (Rubber and rubber products)/SC 2 (Testing and analysis) and Japanese Industrial Standards (JIS) related to rubber products. To develop a new ISO standard for determining the biobased contents, a working team was organized by JRMA for a detailed discussion. The biobased contents for some types of rubber products as described in this paper were measured, and the methods of determination were developed by this working team. JRMA proposed these new ISO standards at the 61st annual meeting of TC 45, held in Bali (Indonesia), October 2013. The determination methods for biobased contents described in this paper will be discussed at the ISO TC 45 for new ISO documentation that will be used worldwide to promote and certify rubber products with higher biobased contents.
In this paper, a natural rubber model compound as a simulated tire rubber product was produced. The biobased contents of this model compound were calculated from the feed composition of the constituents and were determined by the pMC value calculated from the 14C concentration using AMS and the carbon content from the elemental analyses of the separated constituents of this model compound. The calculated biobased contents were compared with those obtained from the feed composition. The international standardizations of these calculations and measuring methods at technical committee (TC) 45 “Rubber and rubber products” were discussed.