1. Introduction
Surface instabilities caused by different forces (mechanical, spinodal dewetting, electric field, and thermal gradient) have received particular attention since they can derive in a variety of surface nano/micro structures. Surfaces instabilities used to pattern polymer surfaces have been reported in literature and include the use of modified substrates [
1,
2,
3], electric fields [
4,
5,
6,
7,
8], dewetting in thin films [
1,
9,
10,
11,
12,
13,
14] or phase separation of polymer blends or block copolymers [
15,
16,
17,
18].
A particularly extended approach to pattern polymer surfaces concerns the use of mechanically induced wrinkled morphologies [
19,
20,
21,
22,
23]. This strategy alone or combined with other patterning technologies has been employed to produce different surface patterns with multiple applications. Wrinkled interfaces have been, for instance, employed in the preparation of polymer actuators and anticorrosive coatings [
24], electroactive [
25]/conductive [
26] to produce high-performance electronics on rubber substrates [
27], and to produce surfaces with controlled wettability, for instance with superhydrophilic [
22] or anisotropic wetting behavior on tunable surfaces [
20,
28].
Several strategies have been successfully employed to produce wrinkled interfaces [
29,
30,
31,
32,
33,
34] including the formation of a rigid layer on a pre-stretched soft film or the gradient polymerization on hydrogels. In effect, the most extended strategy resorts to the use of soft materials with low elastic modulus that can be easily mechanically stretched/compressed. Upon stretching, a rigid layer is either deposited on top of the surface [
25,
35,
36] or created by chemical modification [
37,
38]. Finally, upon removal of the mechanical force the rigid layer at the surface buckles and finally forms surface wrinkles. However, this strategy has several drawbacks including the formation of cracks at the surface as a consequence of the different mechanical resistance of the layer deposited compared to the bulk. In addition, even with the recent studies on the formation of multilayers on these structures the chemistry of such surfaces is restrained to particular metals, oxides resulting of surface treatments or particular high modulus polymers.
Interested in this type of surface patterns, other alternatives have been developed in order to improve the above mentioned drawbacks. In these systems, instead of a soft elastic foundation, photopolymerizable monomers are typically employed. Thus, on the one hand, wrinkles have been obtained from confined hydrogels [
32,
39,
40,
41,
42]. On the other hand, several groups reported the formation of buckling on systems in which the density of crosslinking exhibits a vertical gradient through the film [
42,
43].
Even if, up to now, the latter has been employed only with acrylic monomers this method offers novel chemical possibilities that were limited in the previous approaches. Nevertheless, although with great potential this strategy to fabricate wrinkled surfaces still has remaining issues. These include the need of two distinct steps to be carried out, the precision of the formulation employed that largely affects the final pattern or the presence of residual monomer that must be removed.
In this manuscript, we describe a novel approach to produce wrinkled surfaces. Our strategy relies on the confinement of a phocrosslinkable monomeric mixture between two interfaces with a controlled distance between the two provided by a spacer. We will describe the formation of wrinkled surfaces based on simultaneous heating and UV-irradiation. As will be reported, we employed a mixture of monofunctional (Methyl methacrylate: MMA) and difunctional monomer (Ethylene glycol dimethacrylate: EGDMA) in different proportions to produce wrinkles with variable dimensions. More interestingly, we design the photosensitive mixture to include a variable amount of a functional copolymer additive that will modify the chemical composition of the surface. The functional copolymers employed comprise either hydrophilic moieties (acrylic acid groups: AA) or hydrophobic functional groups (trifluoroethyl methacrylate: TFMA) to produce functional wrinkles with variable chemical composition. The possibility to incorporate polymeric additives offers unique alternatives to chemically fine-tune the interfacial properties of the wrinkled surface. More interestingly, as a proof of concept we will explore the bacterial adhesion of Staphylococcus aureus on the functionalized surfaces as a function of the hydrophilicity of the wrinkled interface.
2. Results and Discussion
The fabrication of the surface wrinkles was carried out using the experimental setup described in
Figure 1A. A photosensitive mixture was confined between two glass slides and simultaneously heated and irradiated with UV-light. This specific setup avoids the monomer evaporation (low boiling point of the component employed) thus permitting the use of a large variety of monomers and assures simultaneously that the composition of the final material is identical to the feed composition. Moreover, a planar cover will limit the thickness variations over the sample.
In particular, the photosensitive mixture employed in this study is composed of a monofunctional monomer (MMA), a crosslinking agent (ethylene glycol dimethacrylate: EGDMA) and a photoinitiator (IRG 651). Initial experiments were conducted using this mixture with variable amounts of MMA and EGDMA to proof the concept. In those experiments, the liquid mixture was irradiated using UV-light able to traverse the cover employed during four minutes of irradiation. It has to be mentioned that, as will be depicted later, wrinkle formation requires simultaneous heating of the photocrosslinkable mixture in order to increase the reaction kinetics.
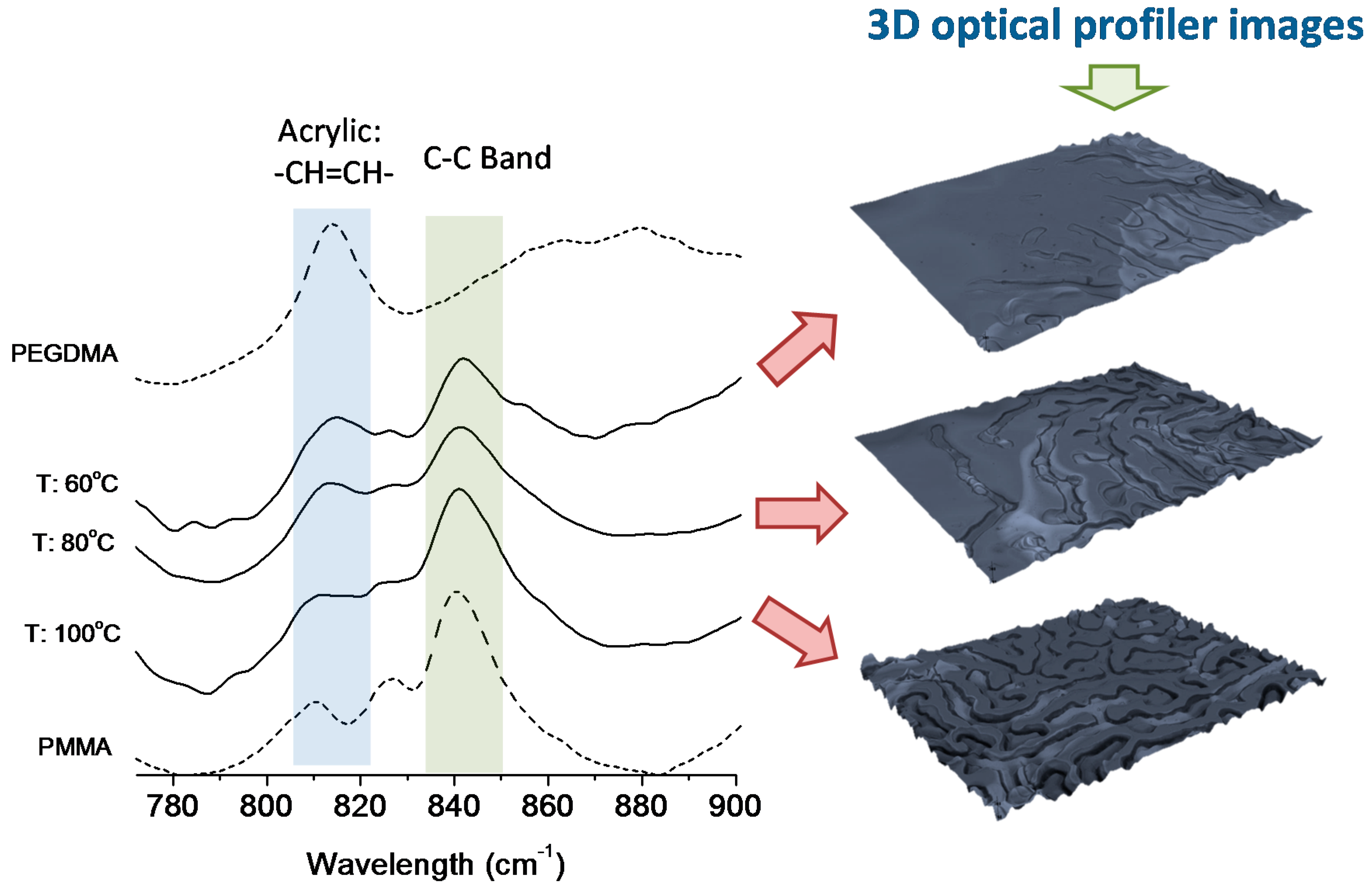
As depicted in
Figure 2, initial experiments were carried out at different temperatures. The 3D optical profiler images show the variation of the surface structure as a function of the temperature employed. In addition, the films resulting of these experiments were characterized by FTIR-ATR (attenuated total reflectance Fourier transform infrared spectroscopy) in order to establish a correlation between the surface patterns observed and the extent of the photocrosslinking reaction. For comparative purposes, also included in
Figure 2 are the FTIR-ATR spectra of the homopolymers obtained from MMA (PMMA) and EGDMA (PEGDMA). A particular signal, identified at 812 cm
−1 relative to residual double bonds of the acrylic functionality (–CH
2=CH– twisting) can be employed to follow the photocrosslinking. In addition, a –C–C– band can be observed at ~840 cm
−1.
Figure 1.
(A) Schematic illustration of the experimental procedure to fabricate wrinkled surfaces. A confined photosensitive mixture is irradiated and heated at the same time. During this process volume contraction and capillary forces act simultaneously, inducing the formation of wrinkled interfaces; (B) Photosensitive mixture employed comprising EGDMA (Ethylene glycol dimethacrylate), MMA (Methyl methacrylate) and IRG651. In addition, the monomer mixture contains either a double hydrophobic copolymer [P(TFMA-co-MMA)] or an amphiphilic copolymer [P(MMA-co-AA)] in order to provide functionality upon surface segregation. (*) indicates the mixtures employed composed of EGDMA, MMA and IRG and either the fluorinated copolymer or the amphiphilic copolymer.
Figure 1.
(A) Schematic illustration of the experimental procedure to fabricate wrinkled surfaces. A confined photosensitive mixture is irradiated and heated at the same time. During this process volume contraction and capillary forces act simultaneously, inducing the formation of wrinkled interfaces; (B) Photosensitive mixture employed comprising EGDMA (Ethylene glycol dimethacrylate), MMA (Methyl methacrylate) and IRG651. In addition, the monomer mixture contains either a double hydrophobic copolymer [P(TFMA-co-MMA)] or an amphiphilic copolymer [P(MMA-co-AA)] in order to provide functionality upon surface segregation. (*) indicates the mixtures employed composed of EGDMA, MMA and IRG and either the fluorinated copolymer or the amphiphilic copolymer.
Figure 2.
FTIR-ATR spectra of the crosslinked films obtained by simultaneous heating and UV irradiation of the monomer mixture and the resulting 3D images of the surface patterns formed by photocrosslinking. The photosensitive mixture employed was MMA/EGDMA (85/15) v/v% and 2 wt% of IRG 651. The irradiation experiments were carried out at three different temperatures: 60, 80 and 100 °C, using a spacer of 50 μm.
Figure 2.
FTIR-ATR spectra of the crosslinked films obtained by simultaneous heating and UV irradiation of the monomer mixture and the resulting 3D images of the surface patterns formed by photocrosslinking. The photosensitive mixture employed was MMA/EGDMA (85/15) v/v% and 2 wt% of IRG 651. The irradiation experiments were carried out at three different temperatures: 60, 80 and 100 °C, using a spacer of 50 μm.
From the IR (Infrared) spectra of
Figure 2 obtained from the films photopolymerized at room temperature a large amount of unreacted double bonds can be easily distinguished. This clearly indicated that the reaction was not completed in the period of time employed (~4 min). Longer photopolymerization times carried out also at room temperature did not produce the formation of a wrinkled surface in spite of a clear reduction of the residual double bonds due to the photochemical reaction progress. Upon increasing the temperature, the –C=C– signal gradually decreased while the –C–C– band was in comparison larger (observed at ~840 cm
−1) for the same reaction time. This indicates both a significant increase of the reaction kinetics and that the crosslinking reaction occurs to a larger extent. By comparison with the 3D profile images of the surfaces we observed that an increase of the reaction temperature up to 80 °C initiates the wrinkle formation whereas at 100 °C the film exhibits a rather regular wrinkled pattern at the surface.
The optimal experimental conditions to obtain a rather homogeneous wrinkle formation were further analyzed by varying both the UV-light intensity employed or the amount of photoinitiator (in this case Irgacure) introduced in the mixture. In the first case a decrease of the light intensity below 50% leaded to the formation of rather irregular wrinkles with some planar surface areas. Using light intensities above 75% produced surfaces with randomly distributed wrinkles homogeneous covered. As an excess of light exposure may not be required, the rest of the experiments were carried out at 75% light intensity.
We equally explored the influence of the photoinitiator concentration on the wrinkle formation. For this purpose, we varied the amount of Irgacure employed between 0.5 wt% and 5 wt% while maintaining 75% of light intensity and a temperature of 100 °C. In this series of experiments we observed that 0.5 wt% of Irgacure provide heterogeneous surfaces with only partial formation of wrinkles. An increasing amount of Irgacure was then added in following samples and the results indicated that the minimum amount of Irgacure required to produced homogeneous wrinkles was 2 wt%. Larger amounts of photoinitiator also produced wrinkled surface. However, herein for the following experiments we employed 2 wt% in order to prepare the functional microwrinkled films.
Thus, the formation of wrinkled surface requires fast reactions. According to our experiments we can propose the mechanism of formation shown in
Figure 1A. Before the photopolymerization started, the volume comprised between the substrate and the glass cover was filled by the monomer mixture. When the photopolymerization starts, an initial wrinkle formation appears as a consequence of the volume contraction forming cavities while the rest of the film due to capillarity remains in contact with the glass cover. The polymerization continues and the volume contraction induces the enlargement of the cavities. As a result of this process, the cavities interconnect before the completion of the reaction and produce a completely wrinkled film.
The approach illustrated in
Figure 1A, has been later employed with photosensitive mixtures containing functional copolymers as additives with the aim of producing simultaneously microstructured and functional surfaces in the polymer blends. In particular, we have explored two different additives, a double hydrophobic copolymer: P(TFMA-
co-MMA) or an amphiphilic copolymer: P(MMA-
co-AA).
As depicted above, wrinkle formation largely relies on the experimental conditions employed for the film preparation. In order to get further insight into the role of the latter on the formation of structured surfaces, preliminary studies were carried out without adding the copolymer. On the one hand, film thickness can be controlled by the spacer that is employed. On the other hand, the relative composition of MMA/EGDMA of the photosensitive mixture on the final surface morphology was then analyzed. From these initial experiments we found that the formation of wrinkles for a particular spacer (50 μm) was observed in samples with monomer composition with at least 5 v/v% of EGDMA. The volume contraction is rather low in the sample having only 1 v/v% and appears to be insufficient to initiate the nucleation process. However, samples containing both 5 v/v% and 15 v/v% of EGDMA produce wrinkled interfaces. In addition, whereas the monomer composition for a particular thickness does not appeared to influence the pattern dimensions, variations on the spacer play an important role. An increasing spacer leads to larger average wrinkle periods. For either, 100 μm spacers or 150 μm spacers average wrinkle dimensions between 460 and 580 μm and above 670 μm were obtained respectively. Within this context we selected the use of 50 μm spacer with a relative ratio MMA/EGDMA of 85/15 v/v%.
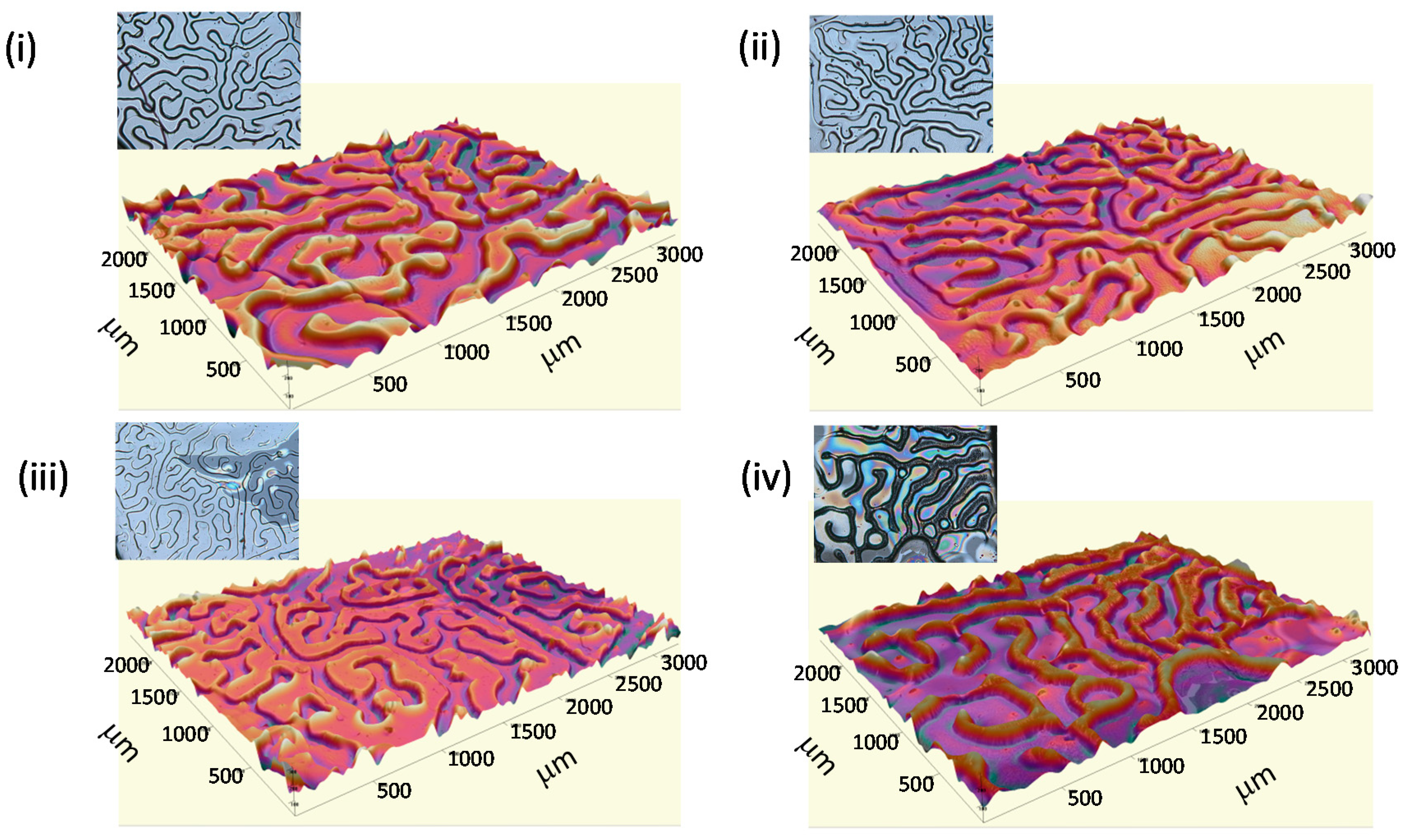
As an illustrative example, in
Figure 3 is depicted the optical profiler images on the surfaces prepared incorporating 2, 5, 10 and 20 wt% of functional copolymer within the photosensitive mixture. Remaining in this range of copolymer employed (2–20 wt%), the wrinkle periodicity remains in a region between 280 and 360 μm whereas to height varies between 7 and 14 μm.
Figure 3.
3D images of the wrinkled surfaces prepared form blends having (i) 2, (ii) 5, (iii) 10, and (iv) 20 wt% of copolymer P(TFMA-co-MMA). Inset: Optical images of the surfaces.
Figure 3.
3D images of the wrinkled surfaces prepared form blends having (i) 2, (ii) 5, (iii) 10, and (iv) 20 wt% of copolymer P(TFMA-co-MMA). Inset: Optical images of the surfaces.
2.1. Wettability of the Functional Wrinkled Surfaces
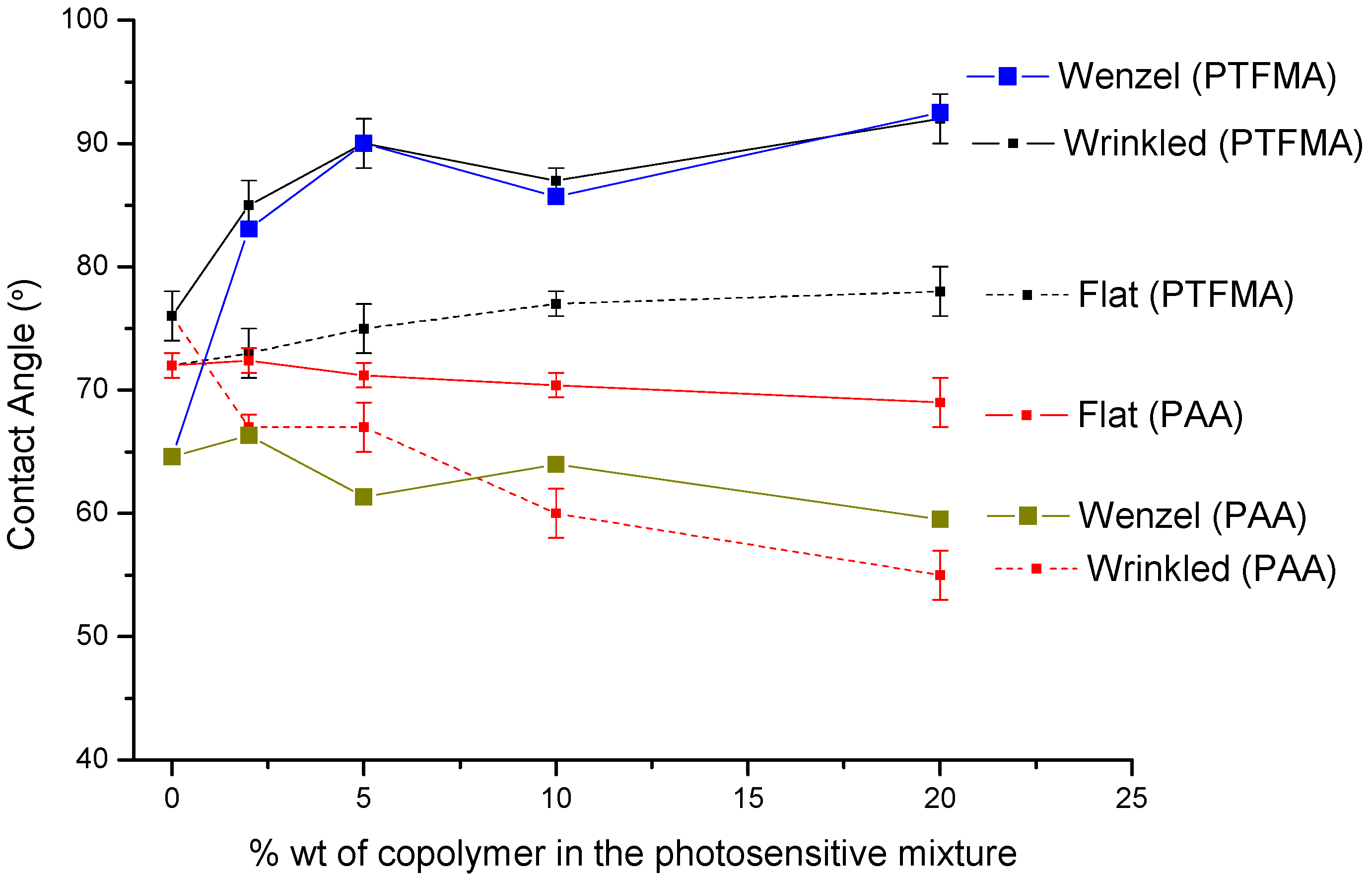
Thus, in principle, the incorporation of functional copolymer did not alter the wrinkle formation. However, as they are designed with either hydrophobic or hydrophilic functional groups the surface wettability should be affected. The contact angle of the wrinkled surface prepared using either the hydrophilic or the hydrophobic copolymers as additive are depicted in
Figure 4.
In addition, in
Figure 4 the values obtained by using the Wenzel state model are equally plotted (see Table S1 of further details). The Wenzel model assumes a complete wetting when a drop is placed on a rough surface. The model is represented by Equations (1) where θ
i is the ideal contact angle on a flat surface,
r is the roughness factor defined as the ratio of actual surface area divided by the projected area [
44]:
The incorporation of P(MMA-
co-AA) increased the surface wettability of the films, and the decrease of the measured contact angle is directly related to the amount of copolymer incorporated in the photosensitive mixture. On the contrary, hydrophobic trifluoro-functional groups decreased the surface wettability and provided contact angles above 90°. In addition, in
Figure 4 are included the contact angle values measured for both flat surfaces obtained by spin coating. Independently of the surface structure, the tendency exhibited is similar. Thus, surfaces prepared with the acrylic acid (AA) containing copolymer decreased the contact angle values while the copolymer having trifluoroethyl methacrylate (TFMA) units increased the hydrophobicity of the surface. Nevertheless, significant differences were observed between the samples with or without wrinkles. Wrinkled interfaces appeared to enlarge the tendency observed for flat surfaces. Thus, whereas the planar surfaces prepared with P(MMA-
co-AA) as additive slightly decreased the contact angles down to ~75°, the wrinkled surfaces reduced the measured contact angles up to ~55°. On the contrary, the incorporation of the fluorinated additive significantly reduced the surface wettability. Whereas in the flat films a slight increase of the contact angle from ~72° to ~78° was observed, the structuration further improved the hydrophobicity of the interface. By incorporation of 20 wt% of copolymer the contact angle increased from ~78° observed in flat films and up to ~92° measured in the wrinkled surfaces with the same composition.
More interestingly, as observed in
Figure 4 the values obtained for the wrinkled surfaces independently of the chemical composition are in good agreement with the contact angle values calculated by using the Wenzel model. This wetting model allows us to affirm that the surface is wetted both on top and at the bottom of the wrinkles.
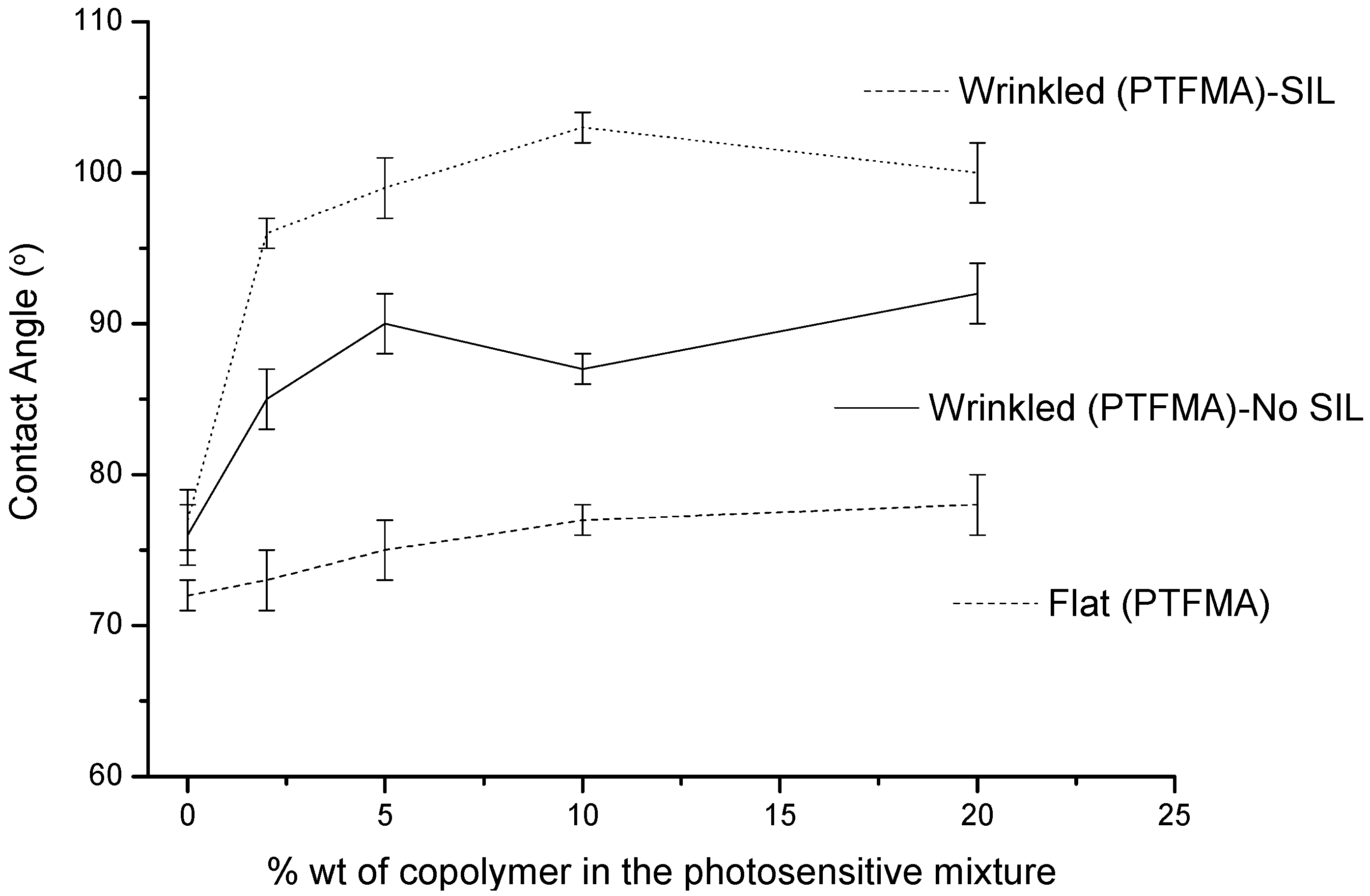
As described above, the setup of the wrinkle fabrication requires a cover in order both to prevent monomer evaporation during the irradiation step and simultaneously to avoid film irregularities. However, the hydrophilicity of the glass cover significantly affects the interfacial migration of the additive and therefore the contact angle observed. The contact angle values measured for wrinkled interfaces prepared using a hydrophilic glass cover and a silanized (hydrophobic) glass cover and blends with the hydrophobic additive P(TFMA-
co-MMA) are represented in
Figure 5. As expected, the contact angle values observed for the wrinkled interfaces prepared using a silanized glass cover are higher than those measured for wrinkled surfaces prepared using a hydrophilic cover. As illustrated in
Figure 5, before the film rigidifies as a consequence of the crosslinking reaction the additive with a larger affinity by the hydrophobic silanized cover migrates towards this surface and enriched the interface in fluorinated copolymer.
Figure 4.
Variation of the contact angle as a function of the wt% of functional copolymer incorporated within the photosensitive mixture. Whereas PAA refers to the copolymer P(MMA-co-AA) introduced, PTFMA refers to the P(TFMA-co-MMA). Flat refers to planar surfaces, wrinkled to the surfaces with wrinkled interfaces and Wenzel to the contact angle values calculated using the Wenzel model.
Figure 4.
Variation of the contact angle as a function of the wt% of functional copolymer incorporated within the photosensitive mixture. Whereas PAA refers to the copolymer P(MMA-co-AA) introduced, PTFMA refers to the P(TFMA-co-MMA). Flat refers to planar surfaces, wrinkled to the surfaces with wrinkled interfaces and Wenzel to the contact angle values calculated using the Wenzel model.
Figure 5.
Variation of the contact angle as a function of the wt% of P(TFMA-co-MMA) copolymer incorporated within the photosensitive mixture prepared using either silanized glass covers (SIL) or cleaned glass covers (No SIL).
Figure 5.
Variation of the contact angle as a function of the wt% of P(TFMA-co-MMA) copolymer incorporated within the photosensitive mixture prepared using either silanized glass covers (SIL) or cleaned glass covers (No SIL).
2.2. Bacterial Assays on the Functional and Wrinkled Surfaces
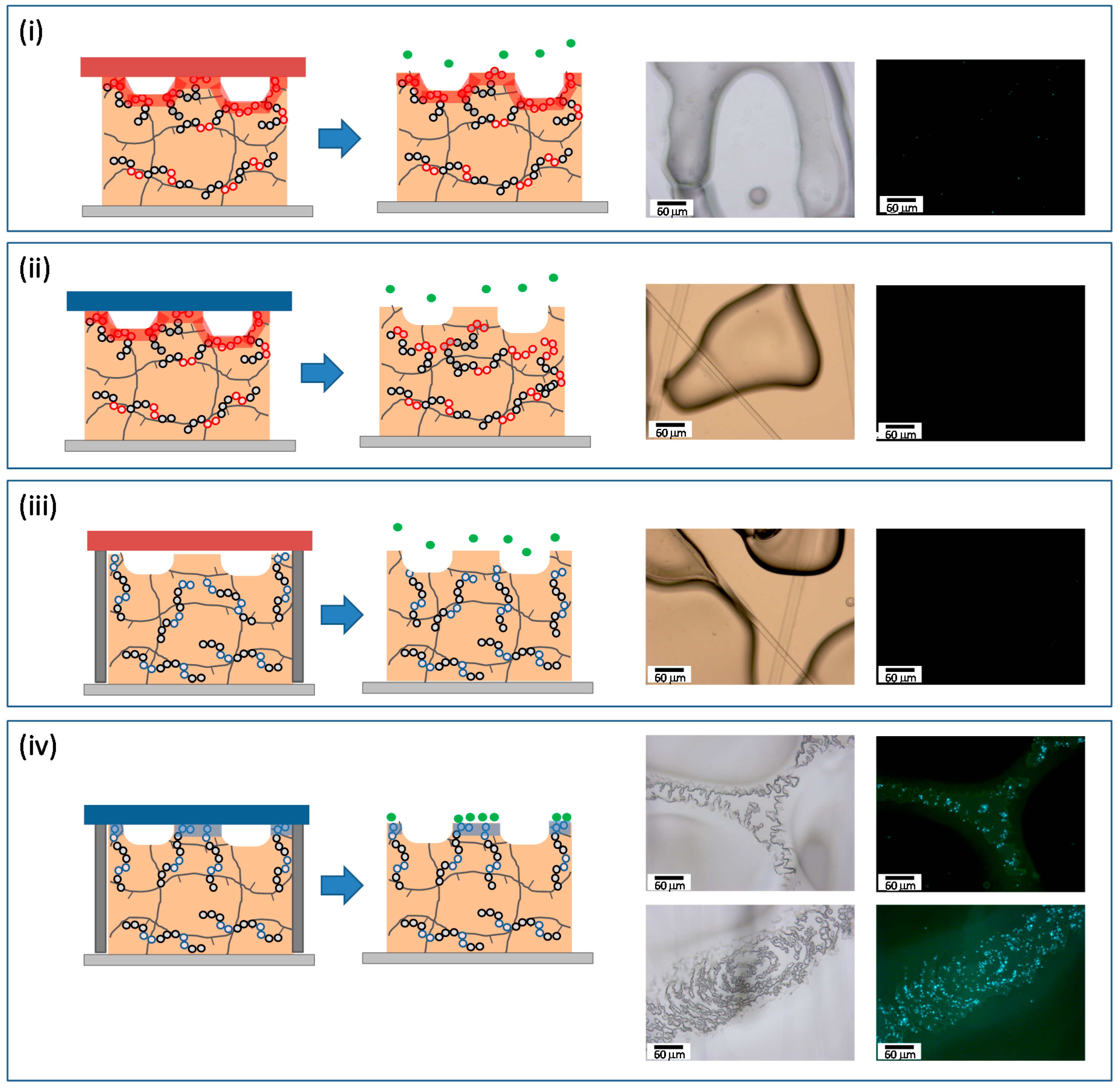
As demonstrated above both the formation of a wrinkled surface and the presence of either hydrophilic or hydrophobic functional groups at the interface as a result of the segregation of the functional copolymer affected the surface wettability. The hydrophilicity/hydrophobicity of polymer surfaces is related with the final application of the material. Herein, as a proof of concept we explored the bacterial adhesion onto these wrinkled surfaces depending on the surface chemical composition. For that purpose, we incubated S. aureus onto wrinkled surfaces prepared using two different glass covers and formed form blends of either the hydrophilic or the hydrophobic copolymer.
On the one hand, we prepared wrinkled surfaces from blends having 20 wt% of P(MMA-co-AA) copolymer using a hydrophilic cover (cleaned using Ultraviolet Ozone (UVO) treatments). The hydrophilic interface will induce the segregation of the hydrophilic PAA groups towards the interface. On the other hand, blends of the photosensitive monomers and 20 wt% of P(MMA-co-TFMA) were prepared using a silanized glass cover. In this case the fluorinated groups of the copolymer are expected to migrate to the polymer/glass interface.
Bacterial attachment is directly related, at least during the early stages, with the hydrophilicity and structure of the contact surface. In general, hydrophilic surfaces enhance the affinity of bacteria for these surfaces and favor the bacterial immobilization, whereas hydrophobic interfaces repel the adhesion of microorganisms. Based on this initial hypothesis we explored the immobilization of
S. aureus labelled with green fluorescent protein that allowed for their detection using fluorescence microscopy in both hydrophilic and hydrophobic surfaces upon 1 h of incubation. The resulting bright field and fluorescence images are depicted in
Figure 6. More precisely we explored the bacterial adhesion onto wrinkled surfaces prepared using the hydrophobic copolymer (i and ii) and the amphiphilic copolymer (iii and iv) using either a silanized cover (ii and iv) or a hydrophilic (UVO cleaned) cover (i and iii). As anticipated, independently of the copolymer employed, the surfaces prepared using the hydrophobic copolymer and a silanized interface did not exhibit bacterial adhesion upon 1 h of incubation. In the case of the wrinkles prepared using P(MMA-
co-TFMA) the presence of trifluoro-groups prevented the attachment of
S. aureus on the surface (
Figure 6i). In the case of P(MMA-
co-AA), the hydrophilic groups are depleted from the surfaces in order to reduce the thermodynamically unfavorable contact with the hydrophobic cover (
Figure 6iii). As a result, the surface chemical composition is mainly composed of PMMA that appears (at least under the experimental conditions employed) to avoid bacterial adhesion. Similar behavior was observed in those wrinkled films prepared using P(MMA-
co-AA) and a cleaned (hydrophilic) cover (
Figure 6ii). In this case, the hydrophobic groups of the TFMA units may direct the migration of the additive towards the bulk of the film. Thus, the surface may have similar surface composition as the previous case.
Figure 6.
Immobilization of S. aureus onto wrinkled surfaces with the copolymers either P(MMA-co-TFMA) (i and ii) or P(MMA-co-AA) (ii and iv). Wrinkled surfaces with P(MMA-co-TFMA) were prepared using either a hydrophobic (silanized glass covers: SIL) (i) or a hydrophilic cover (cleaned glass covers: No SIL) (ii). Equally, wrinkled surfaces with P(MMA-co-AA) were prepared using either a hydrophobic (iii) or a hydrophilic cover (iv).
Figure 6.
Immobilization of S. aureus onto wrinkled surfaces with the copolymers either P(MMA-co-TFMA) (i and ii) or P(MMA-co-AA) (ii and iv). Wrinkled surfaces with P(MMA-co-TFMA) were prepared using either a hydrophobic (silanized glass covers: SIL) (i) or a hydrophilic cover (cleaned glass covers: No SIL) (ii). Equally, wrinkled surfaces with P(MMA-co-AA) were prepared using either a hydrophobic (iii) or a hydrophilic cover (iv).
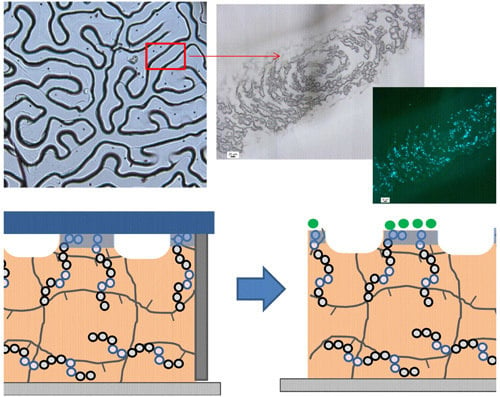
The wrinkled surfaces prepared by using the hydrophilic copolymer and a cleaned glass cover exhibit a different behavior (
Figure 6iv). In this case, bacterial attachment occurs as demonstrated by the presence of fluorescent dots in the fluorescent images. More interestingly, the bacterial adhesion did not occur homogeneously on the surface. This bacterial immobilization occurs on top of the wrinkles that correspond to those areas where the films are in contact with the glass cover. In these areas, the hydrophilic glass cover directs the surface enrichment in copolymer and forms rather hydrophilic areas. On the contrary, those areas forming the valley of the wrinkles did not exhibit any bacterial adhesion. In this case, the hydrophilic glass interface remains apart and the polymer is in contact to vacuum/air present and the hydrophilic functional groups of the copolymer remain embedded within the material. In summary, the presence of different functional groups at the wrinkle interface produces materials with either antifouling/adhesive properties towards bacteria.
3. Materials and Methods
Methyl methacrylate (MMA) (Aldrich, St. Louis, MO, USA, 99%) was washed three times with a basic aqueous solution (10% NaOH) and then washed three times with water. Then, the resulting monomer was dried on anhydrous magnesium sulfate, filtered and distilled. Ethylene glycol dimethacrylate (EGDMA) (Aldrich 99%), Irgacure 651 (IRG 651: 2,2-dimethoxy-1,2-diphenylethan-1-one) (Ciba, Basel, Switzerland), dichlorodimethylsilane (Aldrich 98.5%) tert-Butyl acrylate (tBuA), 2,2,2-trifluoroethyl methacrylate (TFMA) (Aldrich 99%), N,N,N',N'',N''-pentamethyldiethylenetriamine (PMDETA) (Aldrich, 99%), copper(I) bromide (CuBr) (Aldrich 98%), ethyl-2-bromoisobutyrate (EBrIB) and the rest of solvents were employed as received without further purification. Silicon wafers (Siegert Consulting e.k., Aachen, Germany) and 0.15 mm thickness glass covers (Menzel-Glaser, Braunschweig, Germany) were employed as supports.
Infrared spectra were obtained using a Spectrum One FT-IR (Fourier transform infrared) spectrometer (Perkin-Elmer, Waltham, MA, USA) fitted with an attenuated total reflectance (ATR) accessory under unforced conditions. The irradiated samples were placed in direct contact with the diamond crystal with no previous preparation. Measurements were collected at 8 cm−1 resolution and co-adding 6 scans per spectrum.
Water contact angles (CA) were measured using a KSV Theta goniometer from Attension (Stockholm, Sweden). The volume of the droplets was controlled to be approximately 2.0 μL and a charge coupled device camera was used to capture the images of the water droplets for the determination of the contact angles. In addition to the measured contact angles, the predicted contact angle values were calculated using the Wenzel model [
45]:
where
r is the roughness ratio calculated from the expression:
Sdr (area factor) corresponds to the ratio between the interfacial and the projected areas and was directly obtained from the 3D Optical profiler software.
Scanning electron microscopy (SEM) was used for surface structural and cross-sectional analysis. Micrographs were taken using a Philips XL30 (Amsterdam, The Netherlands) with an acceleration voltage of 25 kV. The samples were coated with gold-palladium (80/20) prior to scanning.
Cross sectional profiles and 3D images of the wrinkled surfaces were achieved by using a Zeta-20 True Color 3D Optical Profiler from Zeta Instruments (San Jose, CA, USA).
3.1. Synthesis of the Copolymers
3.1.1. Poly(methyl methacrylate-co-2,2,2-trifluoroethyl methacrylate)
A random copolymer of poly(methyl methacrylate-co-2,2,2-trifluoroethyl methacrylate) (P(MMA77-co-TFMA23) (i.e., 77 mol% of MMA and 23 mol% of TFMA) was synthesized by Atom Transfer Radical Polymerization (ATRP) in order to obtain low polydispersity and controlled chain length. The polymerization was performed in Schlenk flasks previously flamed and dried under vacuum. It was carried out using the following stoichiometry [M1]/[M2]/[I]/[CuBr]/[L] = 48:12:1:1:1, where M1 = MMA, M2 = TFMA, I = EBrIB, L = PMDETA in toluene. The reaction mixture was degassed by three-pump-thaw cycles and placed in a thermostatic oil bath at 90 °C; after the polymerization, the mixtures were cooled to room temperature; the contents were diluted with dichloromethane and passed through a neutral alumina column to remove the copper salt. After removing the solvent, the polymers were precipitated in hexane, washed and dried under vacuum. (Mn: 11,318 g/mol; Polydispersity (PD): 1.10).
3.1.2. Poly(methyl methacrylate-co-acrylic acid)
Preparation of the copolymer poly(methyl methacrylate-co-acrylic acid) (PMMA80-co-PAA20). The polymerization was performed in Schlenk flasks previously flamed and dried under vacuum. ATRP was carried out using the following stoichiometry [Mt]/[I]/[CuBr]/[L] = 100:1:1:1 where Mt = M1 + M2; M1 = MMA, M2 = tBuA, I = Initiator (EBrIB), L = Ligand (PMDETA) in toluene. The reactants were added under N2. The reaction mixture was degassed by three-pump-thaw cycles and placed in a thermostatic oil bath at 65 °C. After the polymerization, the mixture were cooled to room temperature, diluted with dichloromethane and passed through a neutral alumina column to remove the copper salt. After removing the solvent, the polymers were precipitated in cold hexane, washed and dried under vacuum. Finally, the tert-butyl groups were hydrolyzed to afford the carboxylic acid functional groups. For this purpose, the copolymers were first dissolved in dichloromethane (CH2Cl2). Trifluoroacetic acid (TFA) was then added (20 equivalents to t-butyl ester units), and the mixture was stirred at room temperature for two days. The deprotected polymers precipitated in the reaction media and were filtered, washed with CH2Cl2, and finally dried under vacuum. (Mn: 8123 g/mol; PD: 1.25).
3.2. Methodology to Prepare the Wrinkles
Photosensitive mixtures were obtained from variable monomeric mixtures of a crosslinking agent, ethylene glycol dimethacrylate (EGDMA), and a linear monomer, methyl methacrylate (MMA). In addition, a radical photoinitiator, IRG 651 (2 wt% respect to the total amount of monomers) was employed to start the polymerization process. IRG 651 has two UV/Vis absorption peaks at 250 and 340 nm. A variable amount of copolymer comprised between 2 wt% and 20 wt% of either P(MMA-co-AA) or P(MMA-co-FTMA) was added to control de surface chemical composition. To prepare the films, 2 cm × 2 cm silicon wafers with variable spacers (50–150 μm) were employed. A few drops of the photosensitive mixture were placed in the center of the wafer and covered by a glass of 0.30 mm thickness. The samples were preheated and irradiated while the heating was kept constant. The photopolymerization of the films has been done under UV spot light irradiation source using a Hamamatsu model lightning cure L8868 (Hamamatsu, Japan) provided by Hg–Xe lamp with 200 W power. The irradiation intensity was kept constant throughout the experiments and focused on the samples with an optic fiber at a constant distance of 8 cm. The incident light intensity was maintained constant for all experiments at 6150 mW/m2, measured by a Luzchem radiometer SPR-01 (Ottawa, Ontario, Canada) (75% of the lamp capacity). Initial tests were carried out varying the ratio linear monomer versus crosslinking agent in order to optimize the wrinkle formation. The samples having additionally functional monomers were prepared using a constant ratio of linear monomers (i.e., both functional monomer and non-functional monomer) and crosslinking agent of 85/15 v/v%.
For the treatment of the glass covers to obtain hydrophobic surface a silanization process was carried out, where the glass is submerged in a dichlorodimethylsilane solution for one minute. After the treatment, the films were rinsed with a mixture ethanol/water and ethyl acetate. On the other hand, to obtain a clean hydrophilic surface, the glass covers were exposed to ozone by using a UV-cleaner lamp for 15 min.
3.3. Bacterial Immobilization Tests
S. aureus strain RN4220 carrying the plasmid pCN57 for green fluorescent protein (GFP) expression (generous gift from Iñigo Lasa’s Laboratory at Instituto de Agrobiotecnología, Universidad Politécnica de Navarra (UPNA-CSIC-Gobierno de Navarra) was grown overnight at 37 °C in Luria-Bertani (LB) media with erythromycin (10 μg/mL). The cells were centrifuged and washed three times in PBS (phosphate buffered saline) buffer (150 mM NaCl, 50 mM Na-phosphate pH 7.4). The solution was adjusted to a cell concentration that corresponds to an optical density (OD) at 600 nm of 1.0 using an UV-VIS Varian (Palo Alto, CA, USA) Cary 50 spectrophotometer.
The different patterned polymeric surfaces were incubated for 1 h with a bacterial suspension at OD = 1.0 in PBS buffer with 0.05% Tween 20. After incubation the surfaces were washed with PBS three times during 15 min.
Bacteria immobilization was monitored by fluorescence microscopy using a Leica (Wetzlar, Germany) DMI-3000-B fluorescence microscope. Images were acquired using 20× magnification and the corresponding set of filters for imaging green fluorescence and bright field.
4. Conclusions
In this contribution we described the preparation of wrinkled interfaces based on surface instabilities generated during a photocrosslinking process. In comparison with previously reported procedures, where wrinkles have been obtained upon surface treatment of a particular material (typically PDMS (Polydimethylsiloxane)) or by gradual crosslinking of polymer gels, wrinkled interfaces were obtained by confining a photosensitive monomeric mixture composed of monofunctional monomer and a crosslinking agent within a substrate and a cover. We described how, by using this approach, the wrinkle characteristics can be controlled by the monomer mixture, the spacer employed or the temperature of the photopolymerization.
More interestingly, the designed fabrication approach allowed, by blending, the incorporation within the material of a functional copolymer that permitted the variation of the surface chemical composition while maintaining the surface structure. For that purpose we incorporated either a fluorinated copolymer that enhanced the surface hydrophobicity of the wrinkled interface, or an acrylic acid containing copolymer that increased the hydrophilicity of the wrinkled surface.
In view of the application of such structured interfaces, we evaluated the role of the surface chemistry depending on the functional copolymer employed and the hydrophobicity of the interface on the bacterial adhesion using S. aureus. As a result, for the conditions employed the wrinkled surfaces prepared using fluorinated copolymer behave as anti-adhesive surfaces whereas the surfaces prepared with the hydrophilic copolymer promote the bacterial adhesion in those areas where the film is in contact with the glass cover. Moreover, as the surface chemistry depends on the environment of exposure, the proposed wrinkled surfaces are excellent candidates to design materials with tunable surface properties.