Mechanisms of the Shape Memory Effect in Polymeric Materials
Abstract
:1. Introduction
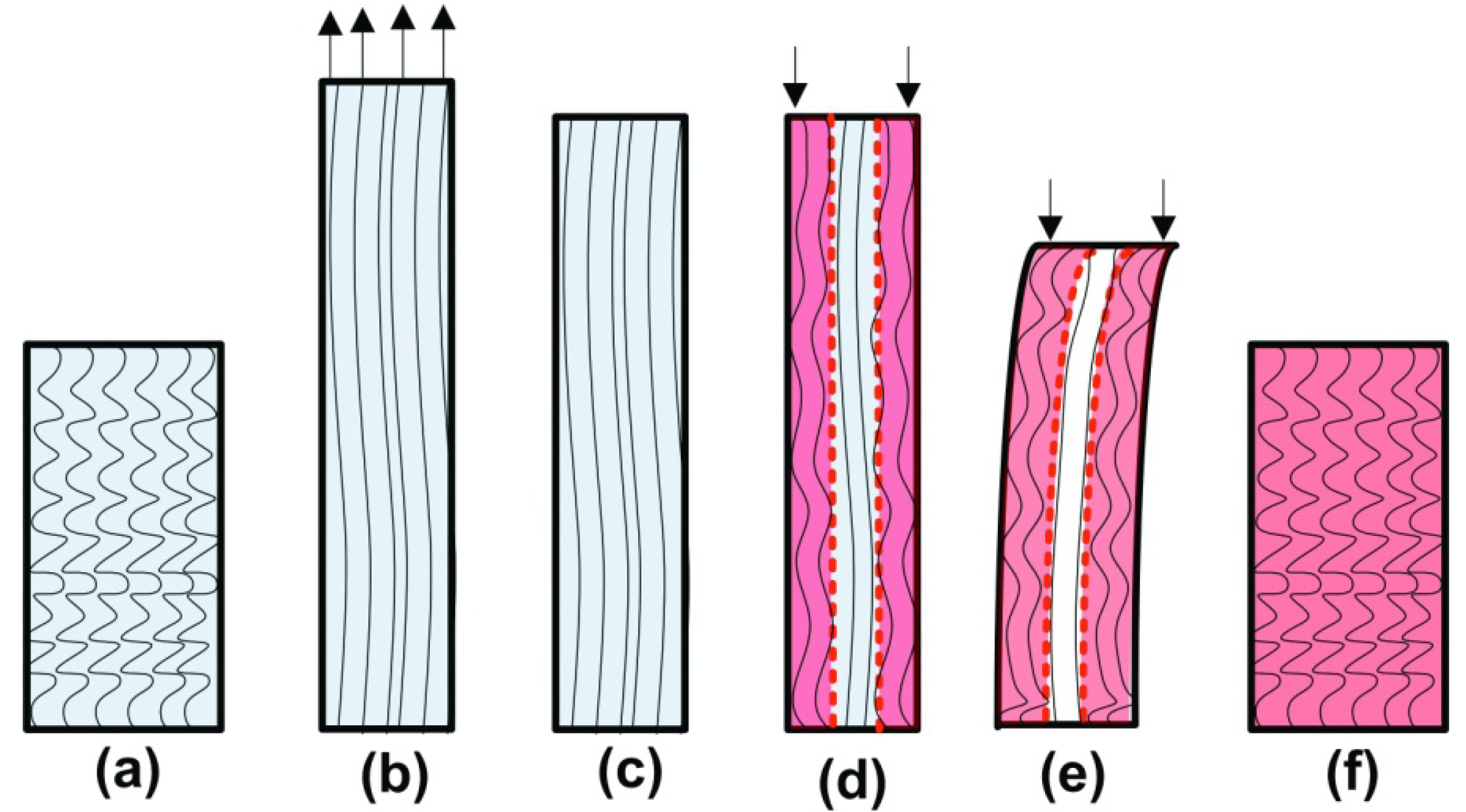
2. Working Mechanisms
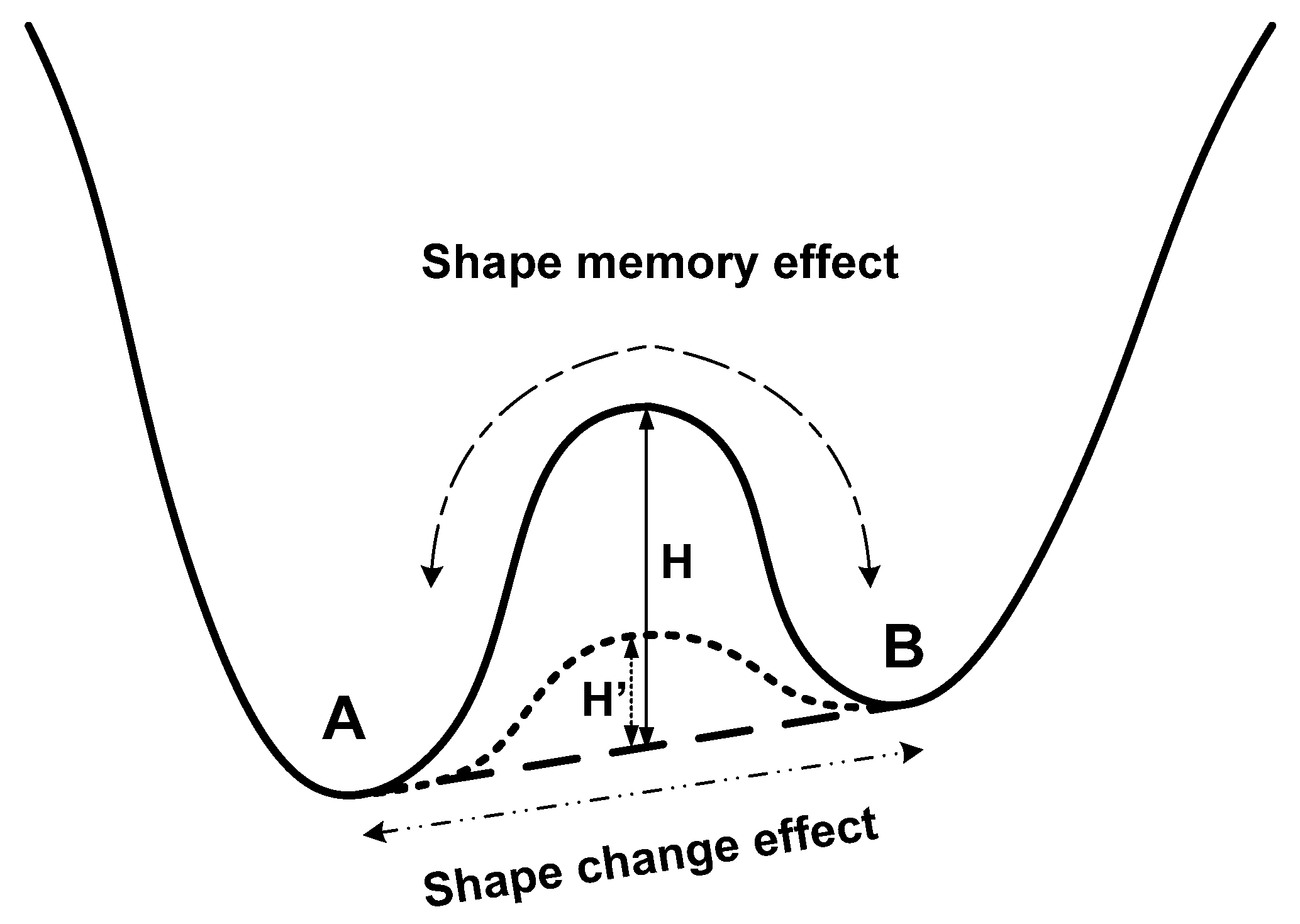
2.1. Basic Working Mechanisms

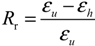
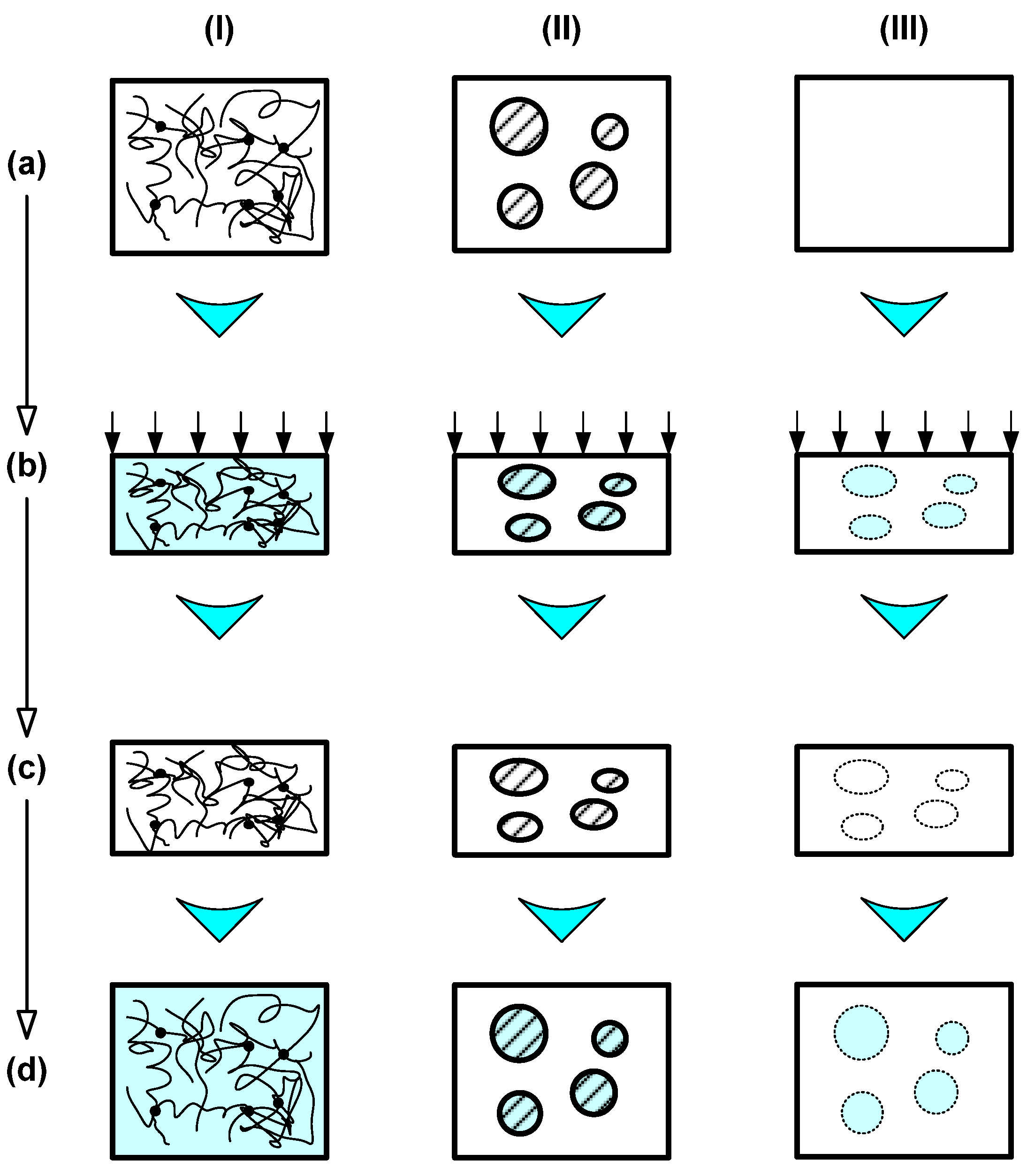
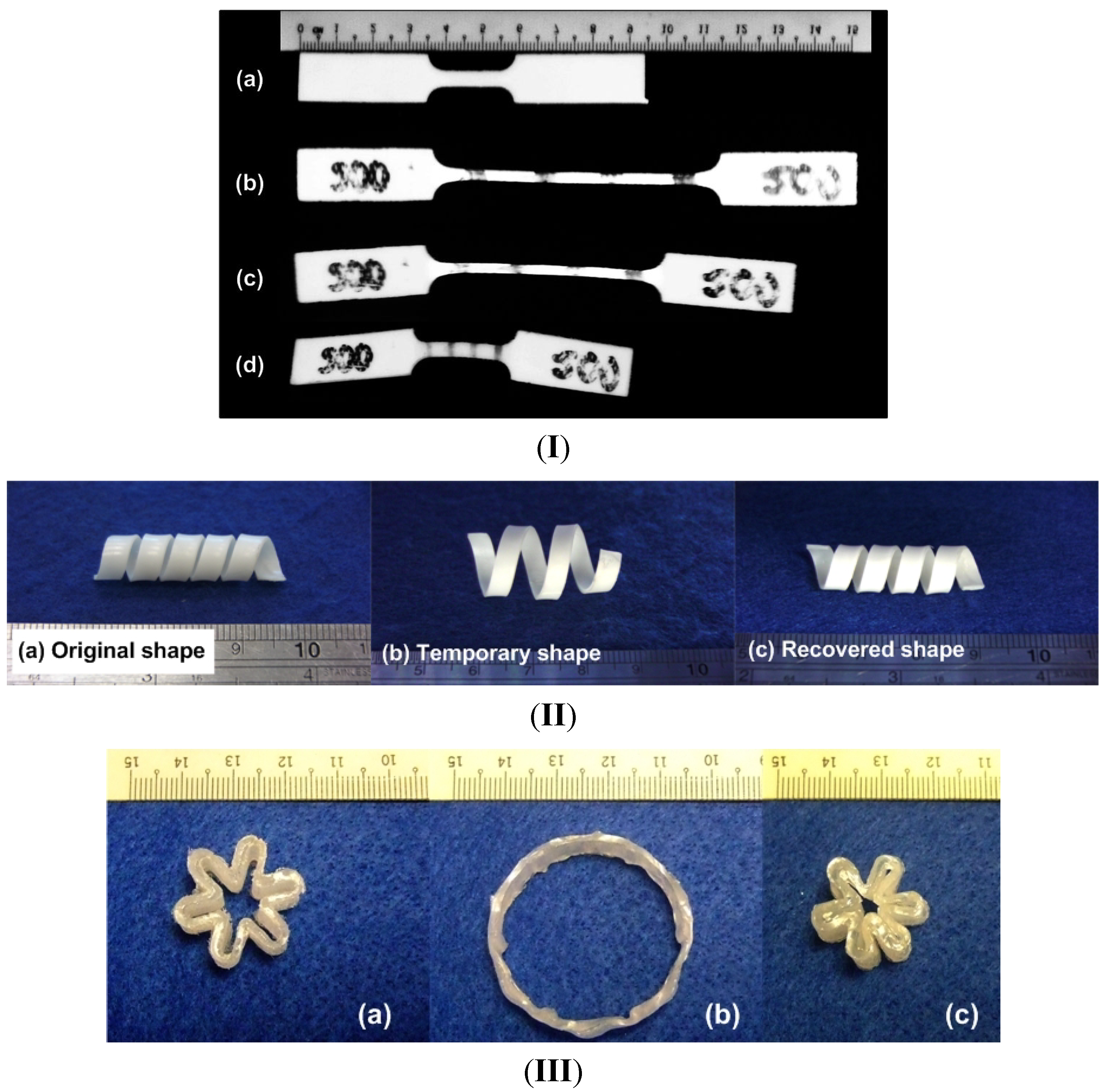
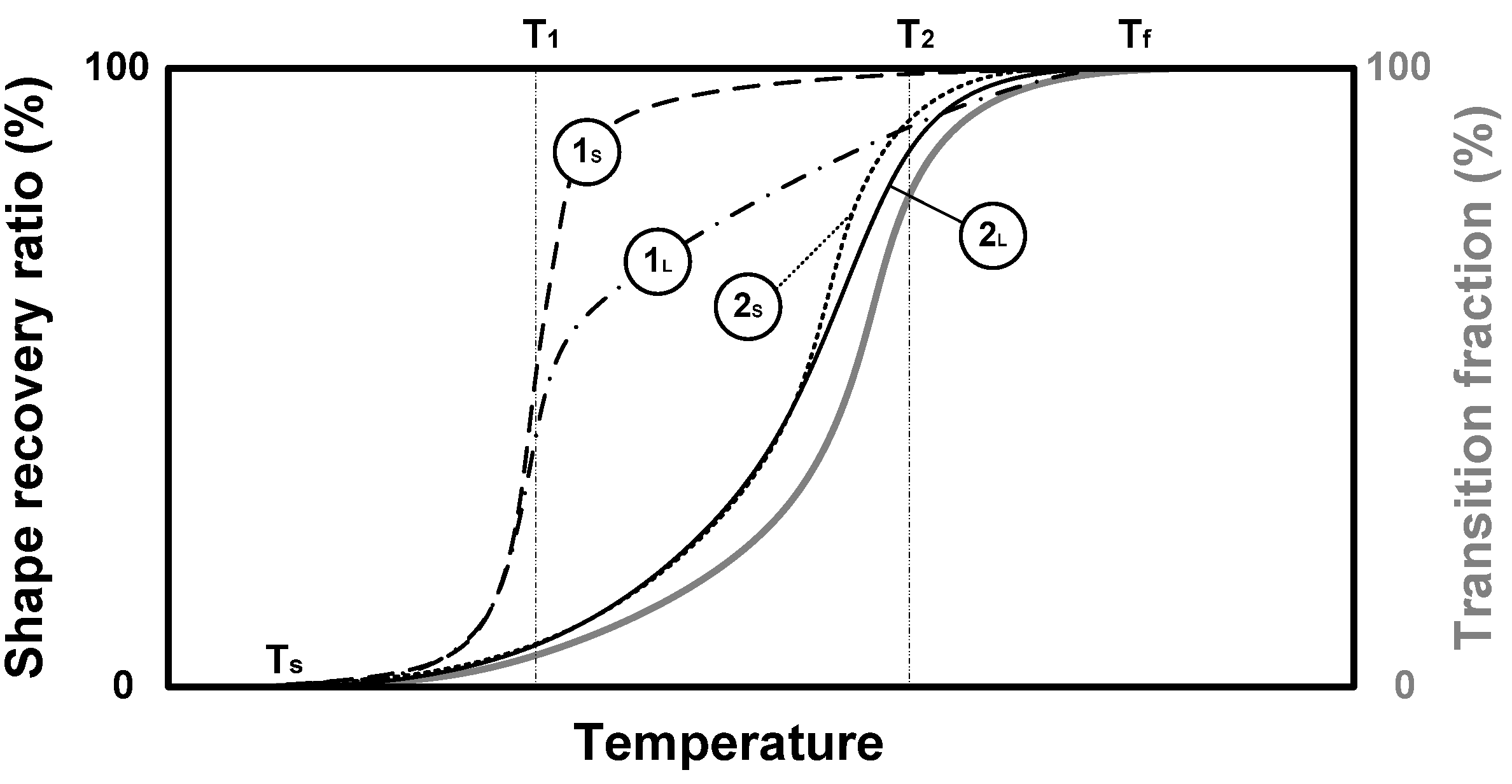
2.1.1. Dual-State Mechanism (DSM) (Figure 5I)
2.1.2. Dual-Component Mechanism (DCM) (Figure 5II)
2.1.3. Partial-Transition Mechanism (PTM) (Figure 5III)
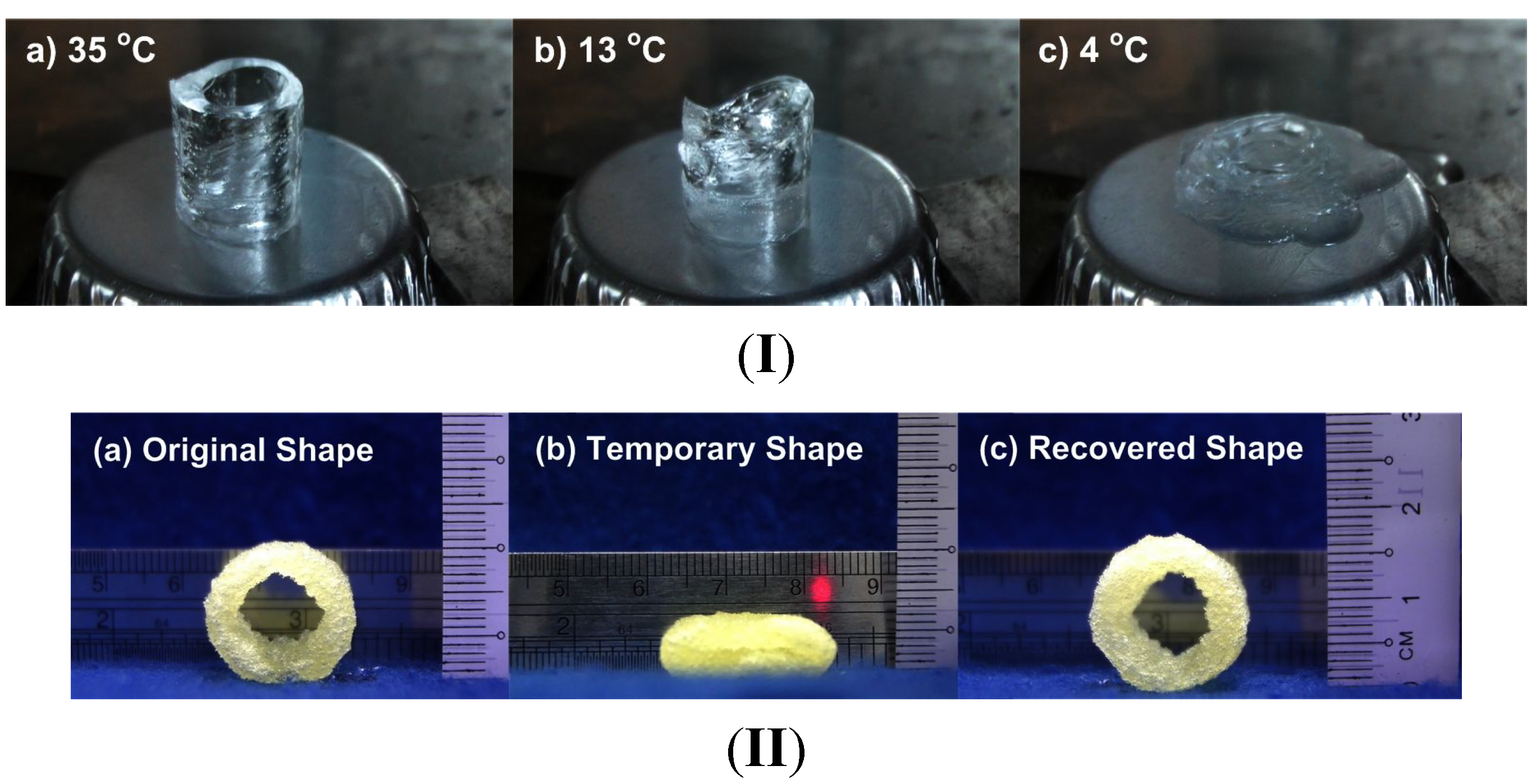
2.2. Other Types of Stimuli
2.2.1. Softening
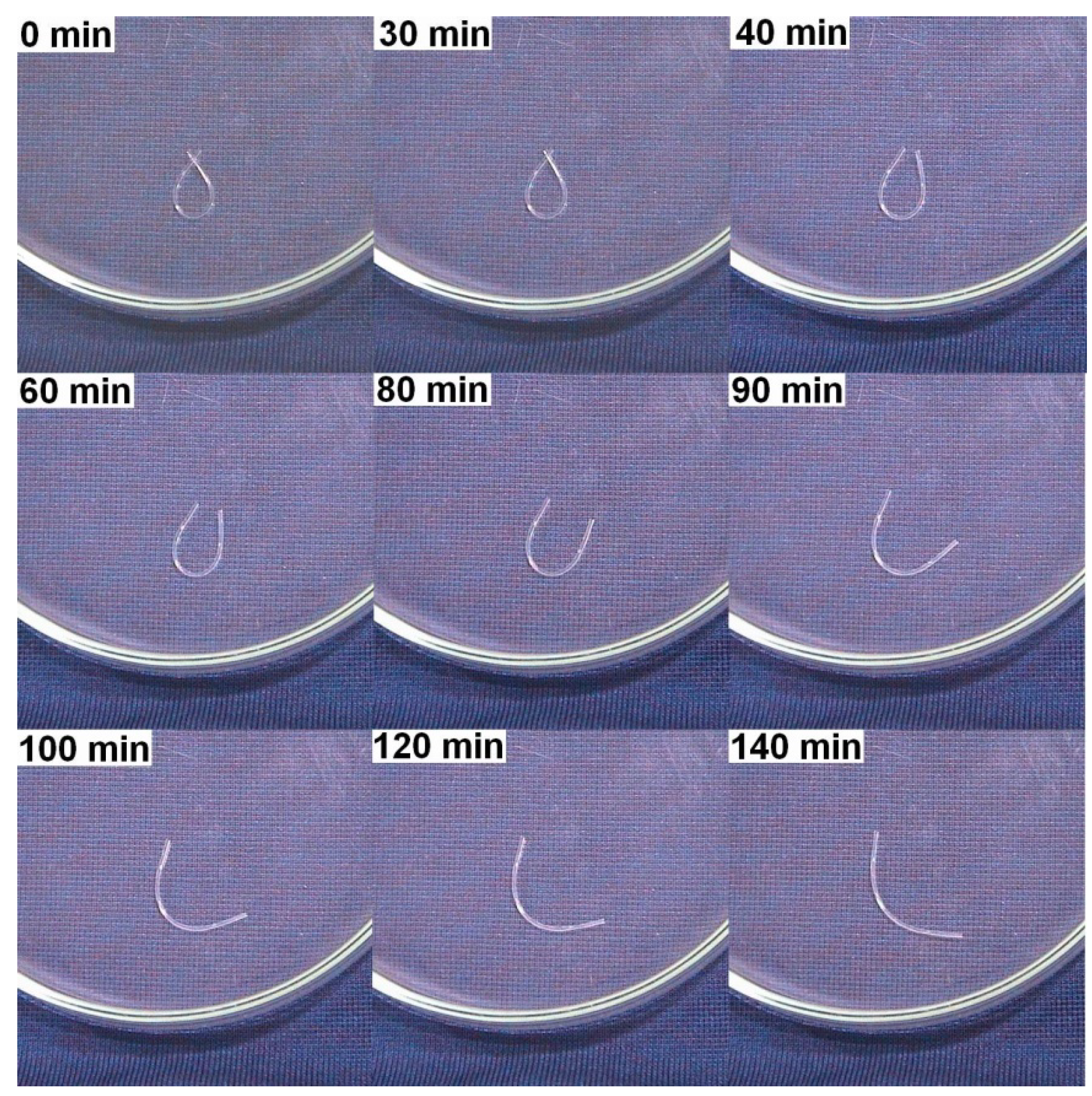
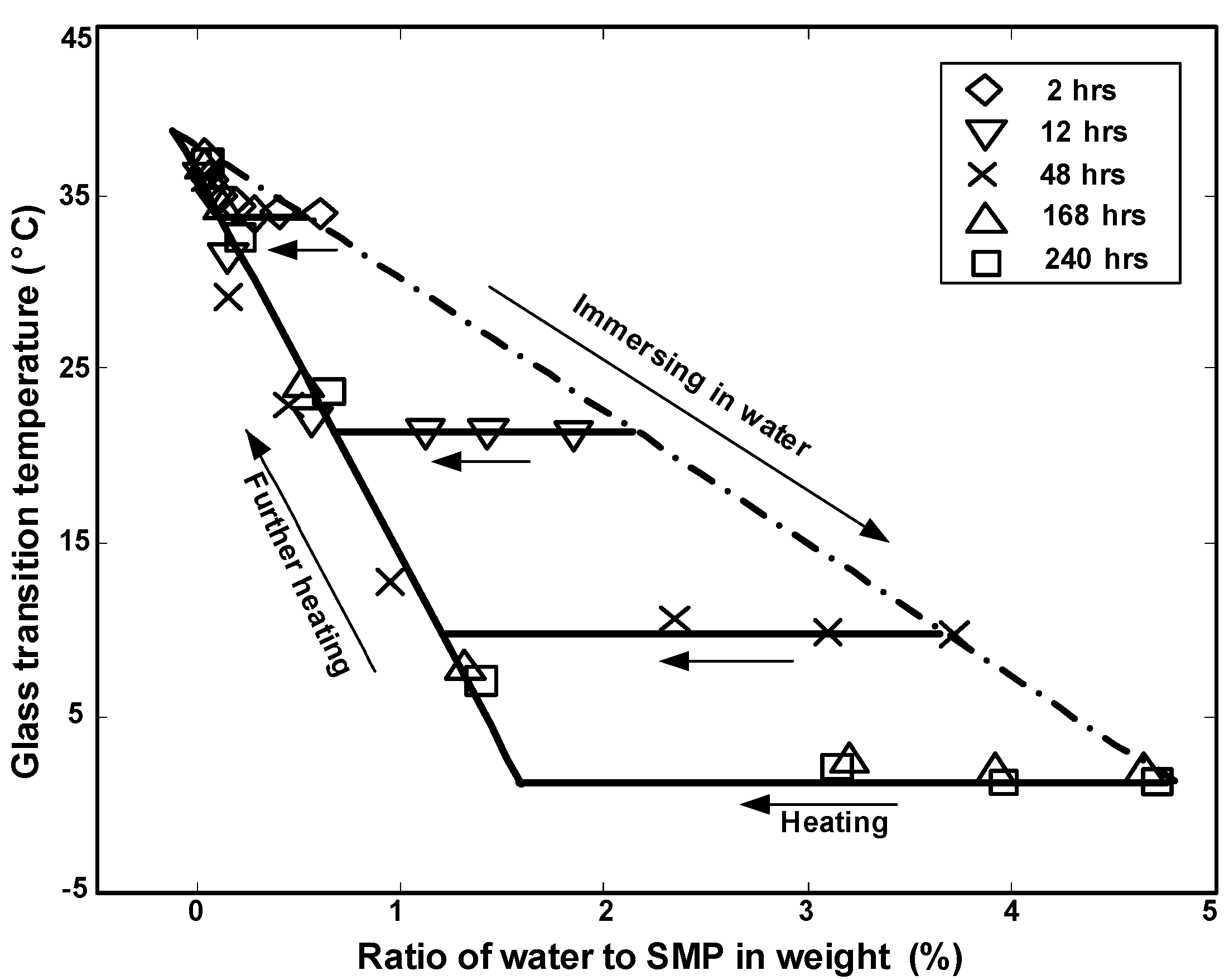
2.2.2. Swelling
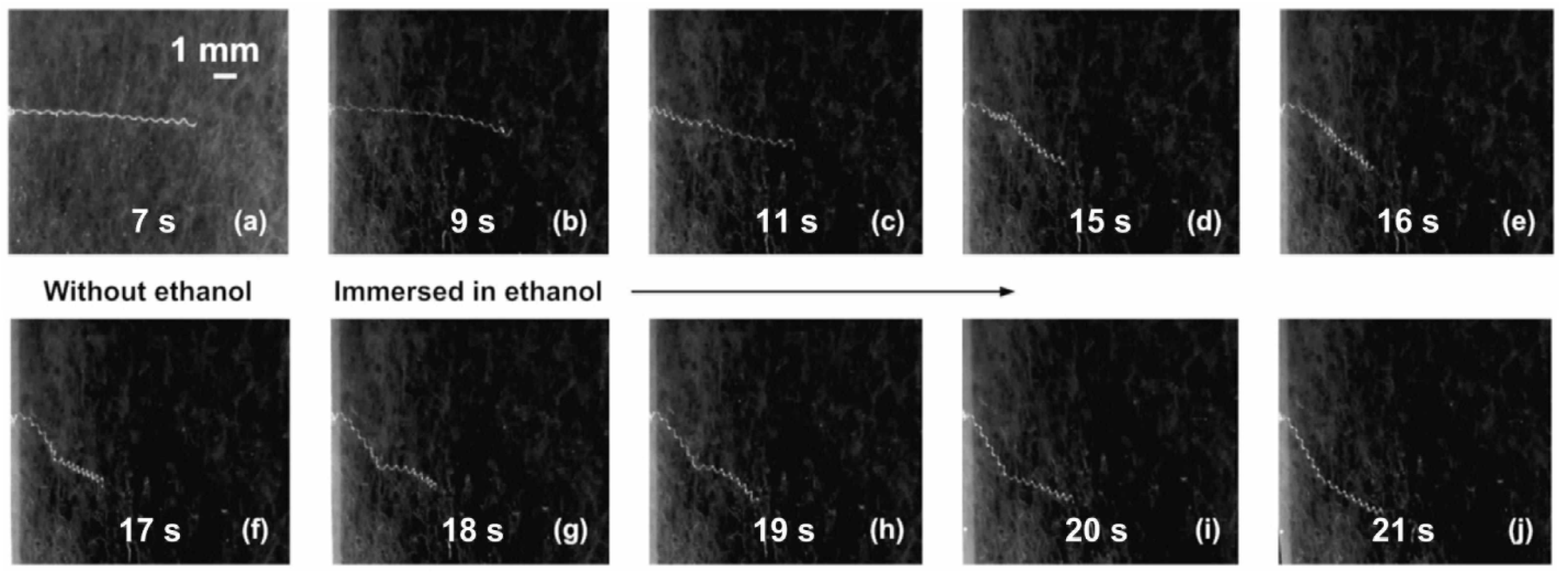
2.2.3. Dissolving
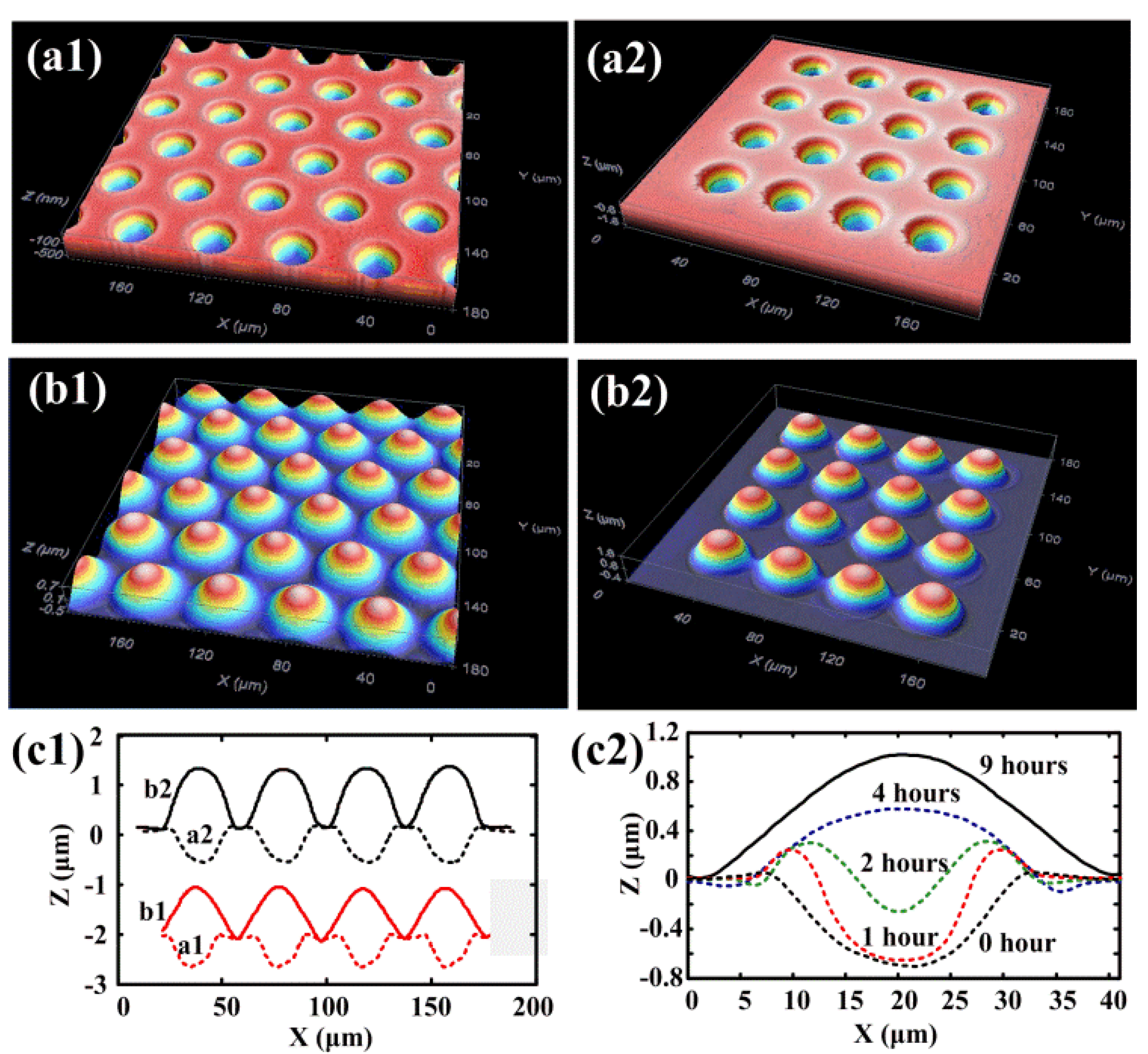
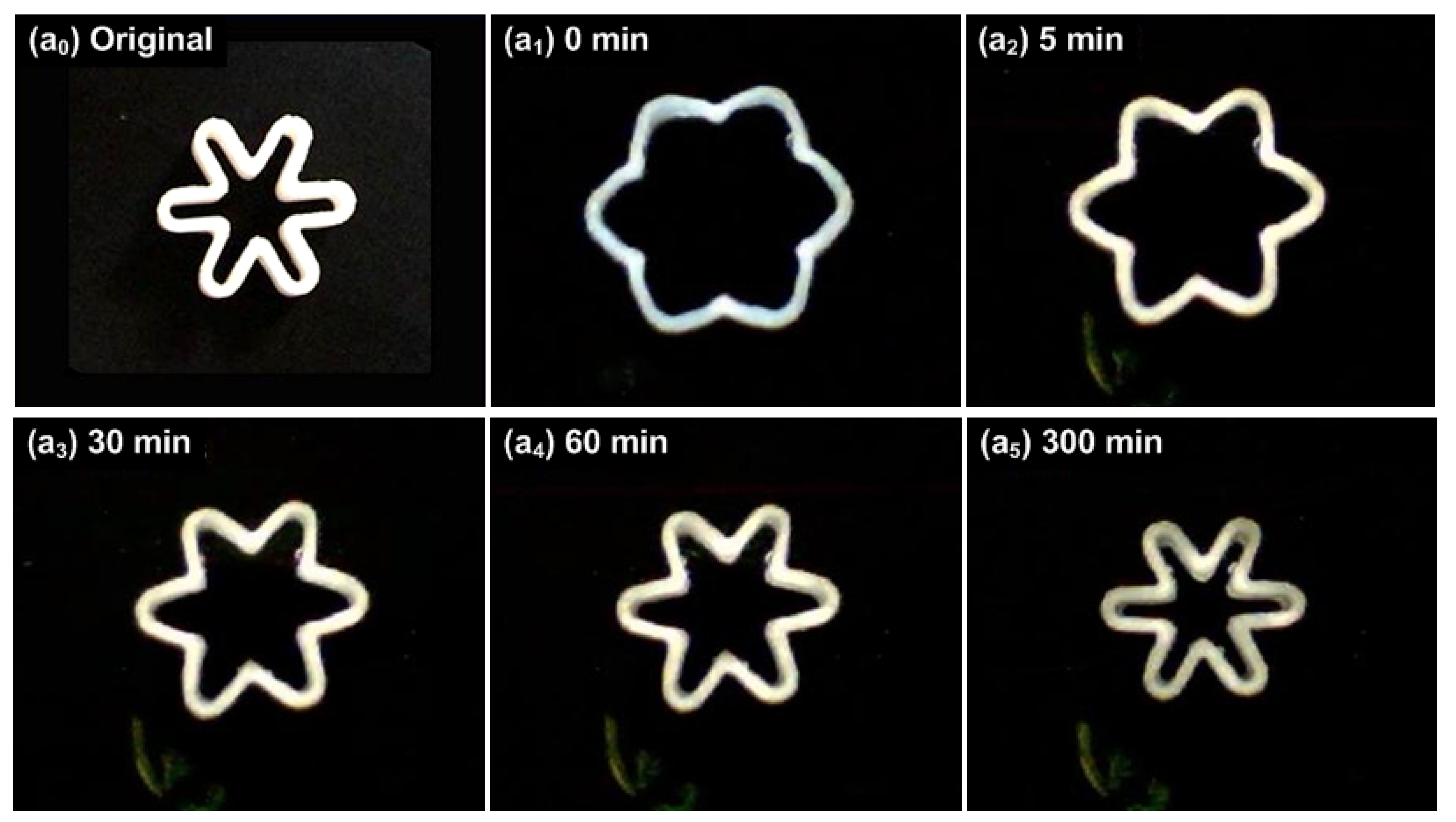
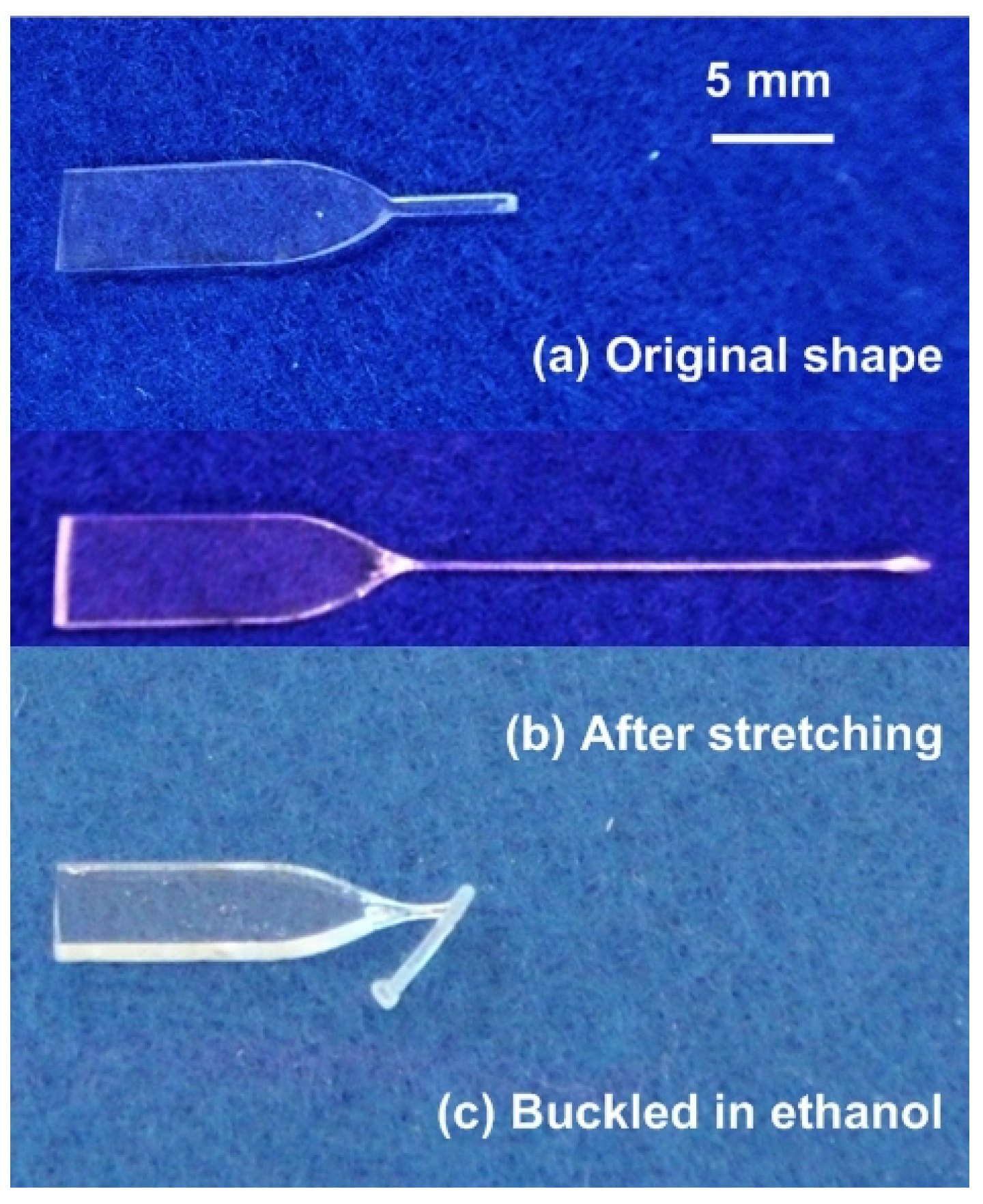

3. Advanced Shape Memory Phenomena
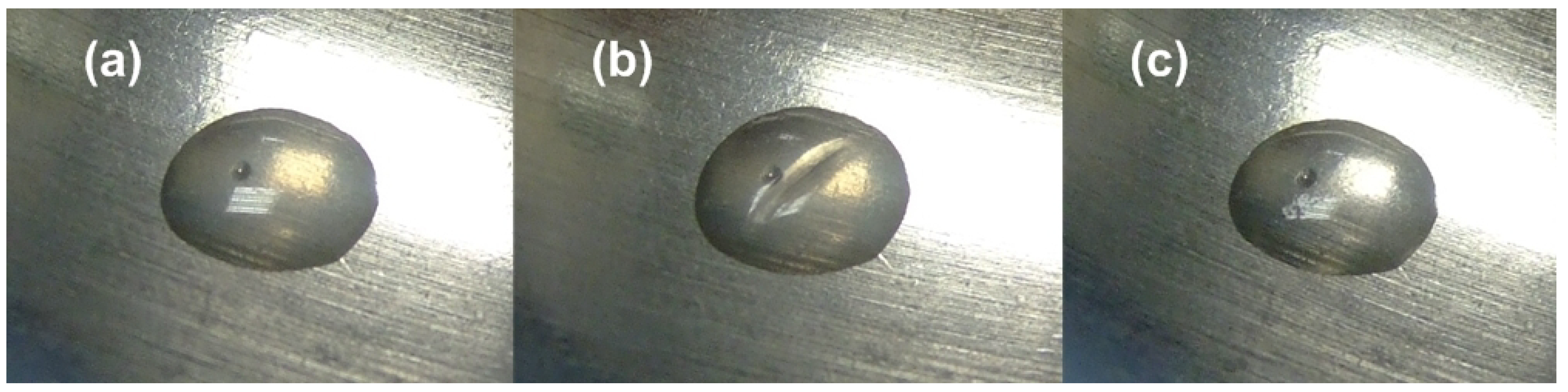
3.1. Multiple-SME (Shape Memory Effect)
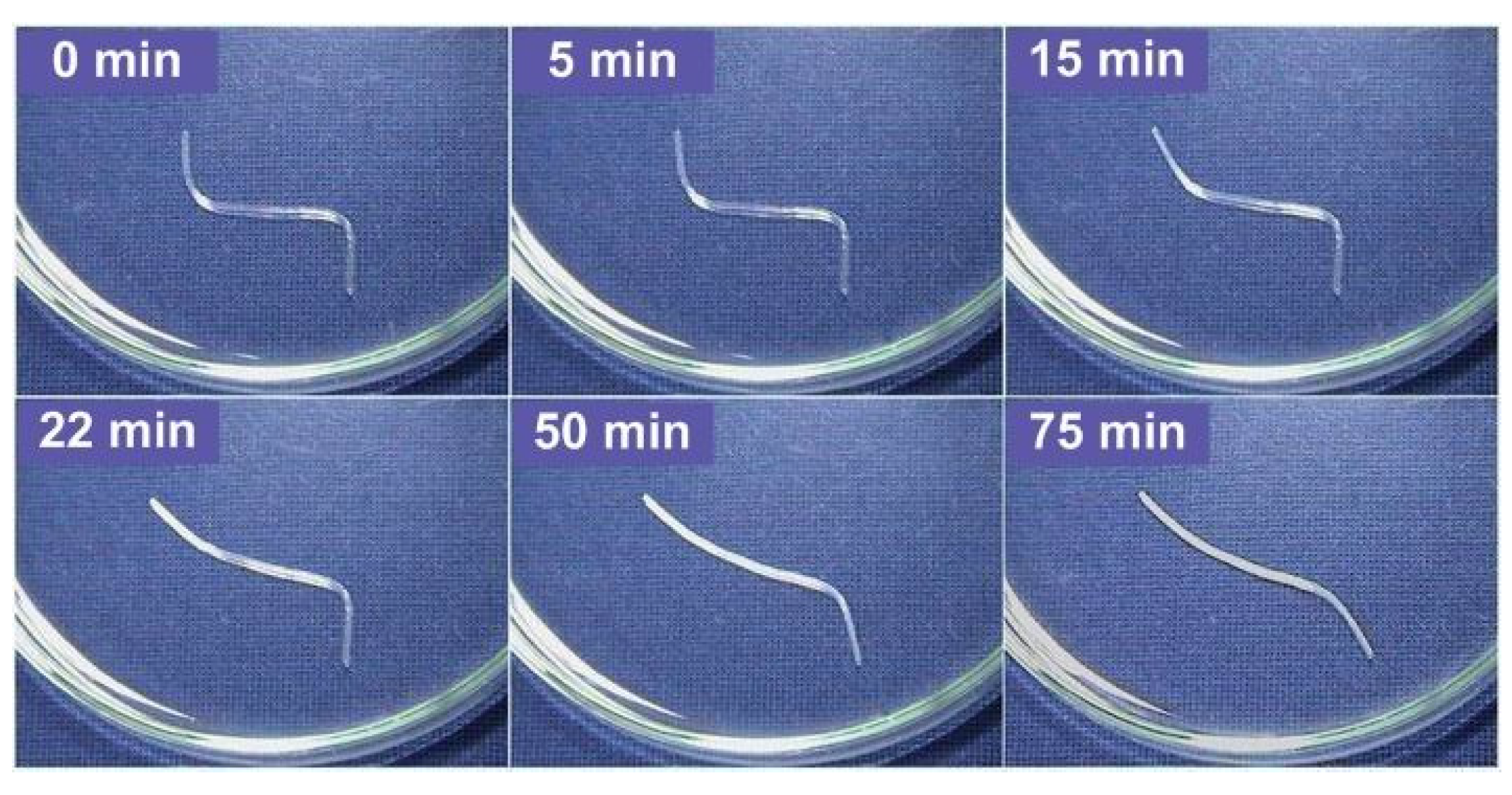
- -
- The hybrid is heated to 65 °C and then twisted.
- -
- After cooling to 40 °C with the twisted shape maintained, it is bent.
- -
- Subsequently, it is cooled to 15 °C and the constraint is removed.
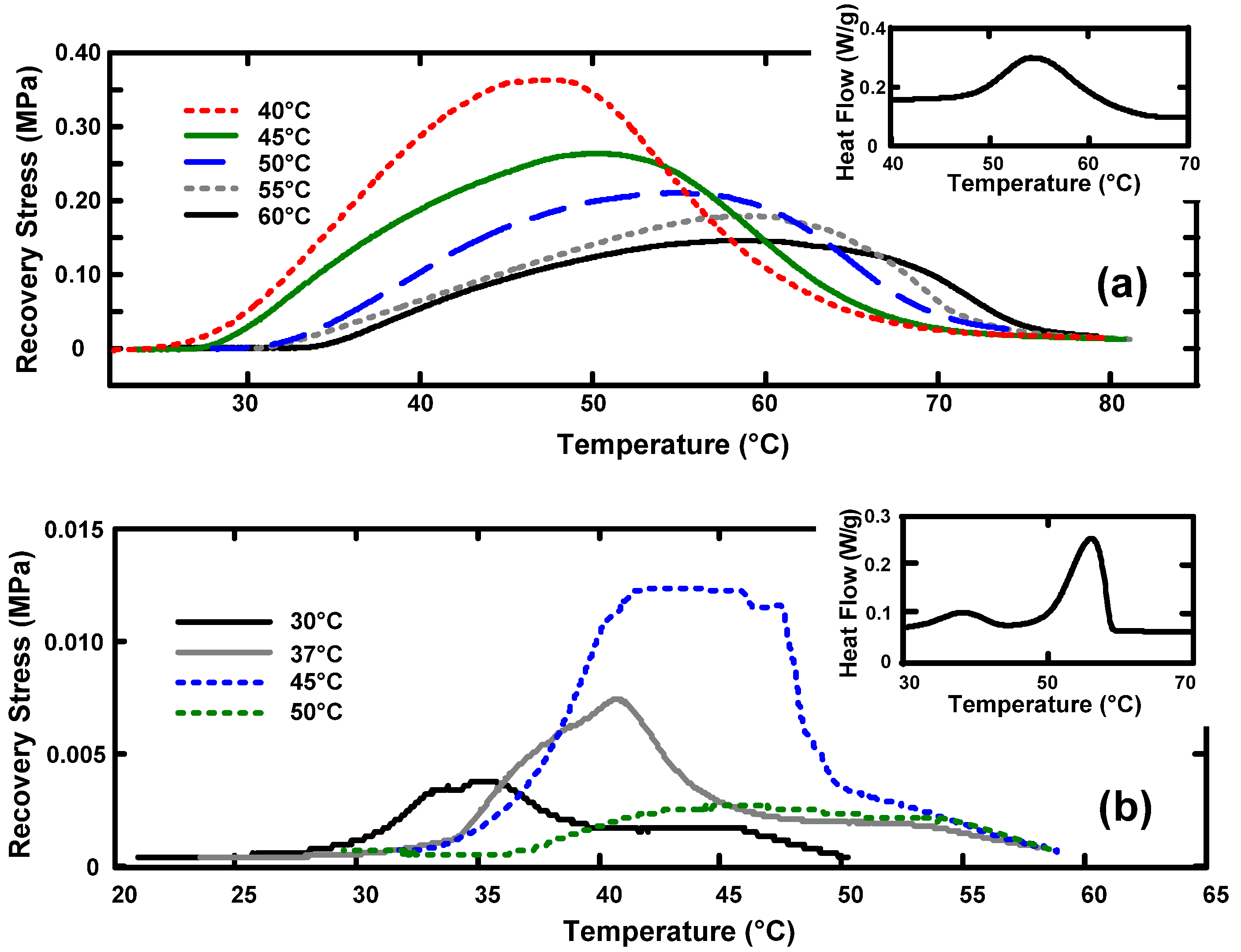
3.2. Temperature Memory Effect
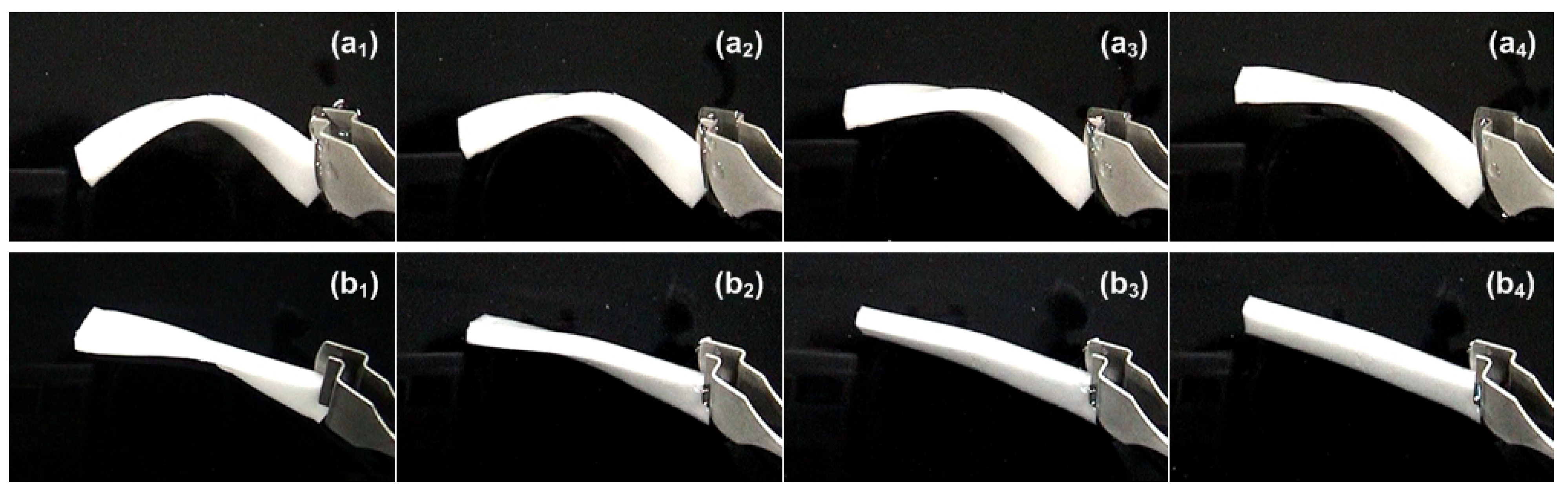
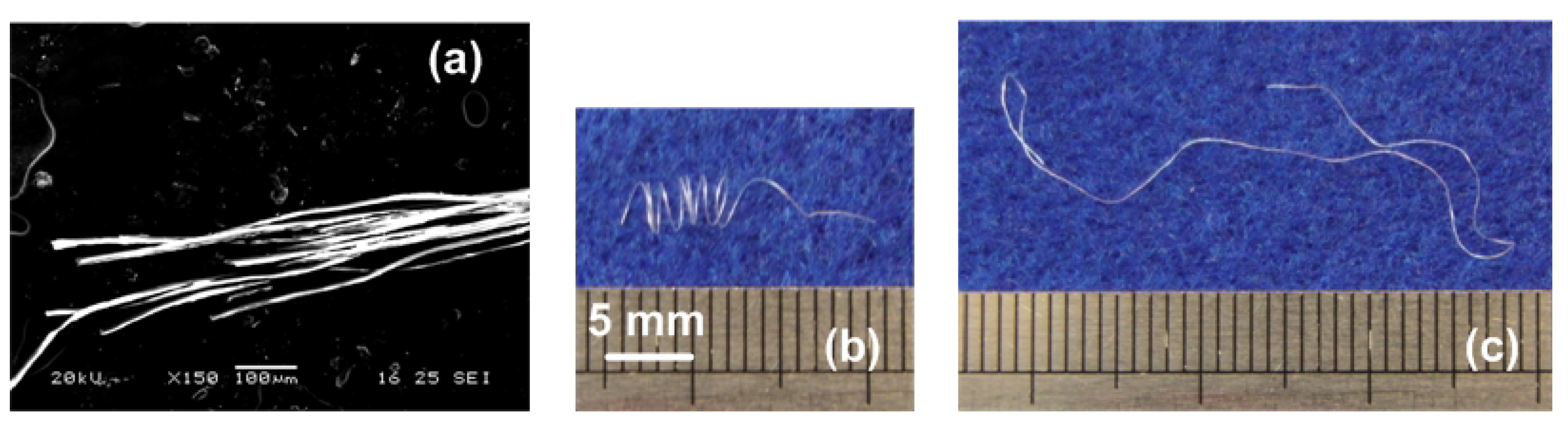
3.3. Reversible Motion
- -
- Strictly speaking, the reversible motion upon thermal cycling is actually the SCE.
- -
- A special mechanism to achieve softening during cooling upon loading is required. Right now, the fundamentals are still under construction [130]. As such, unlike the aforementioned multiple-SME and TME, reversible motion is not a generic shape memory phenomenon in polymers.
- -
- Particular attention should be paid to distinguish it from the thermal mismatch-induced reversible motion.
4. Design of Polymeric Materials and Optimization
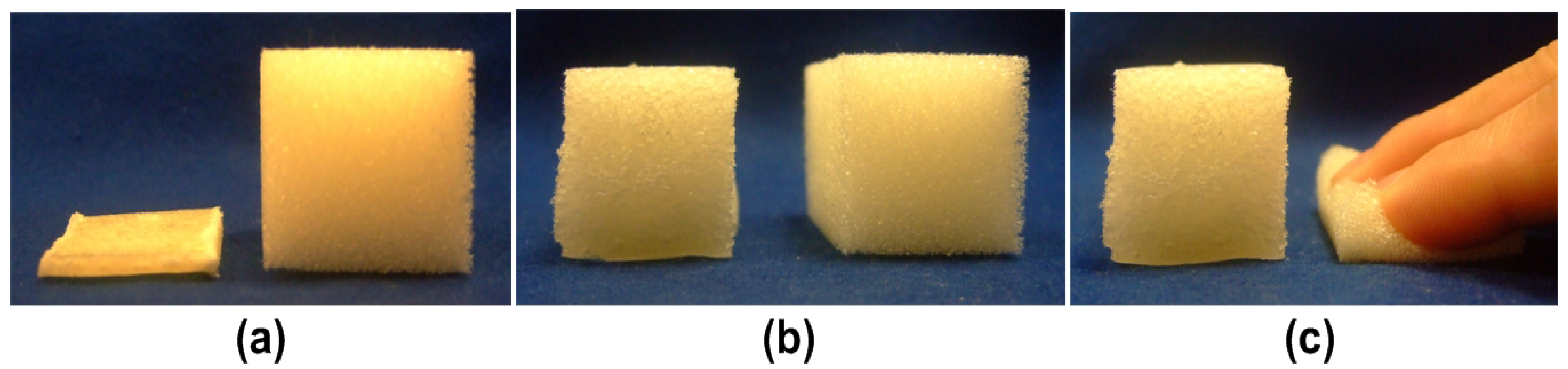
4.1. Design of Polymeric Materials
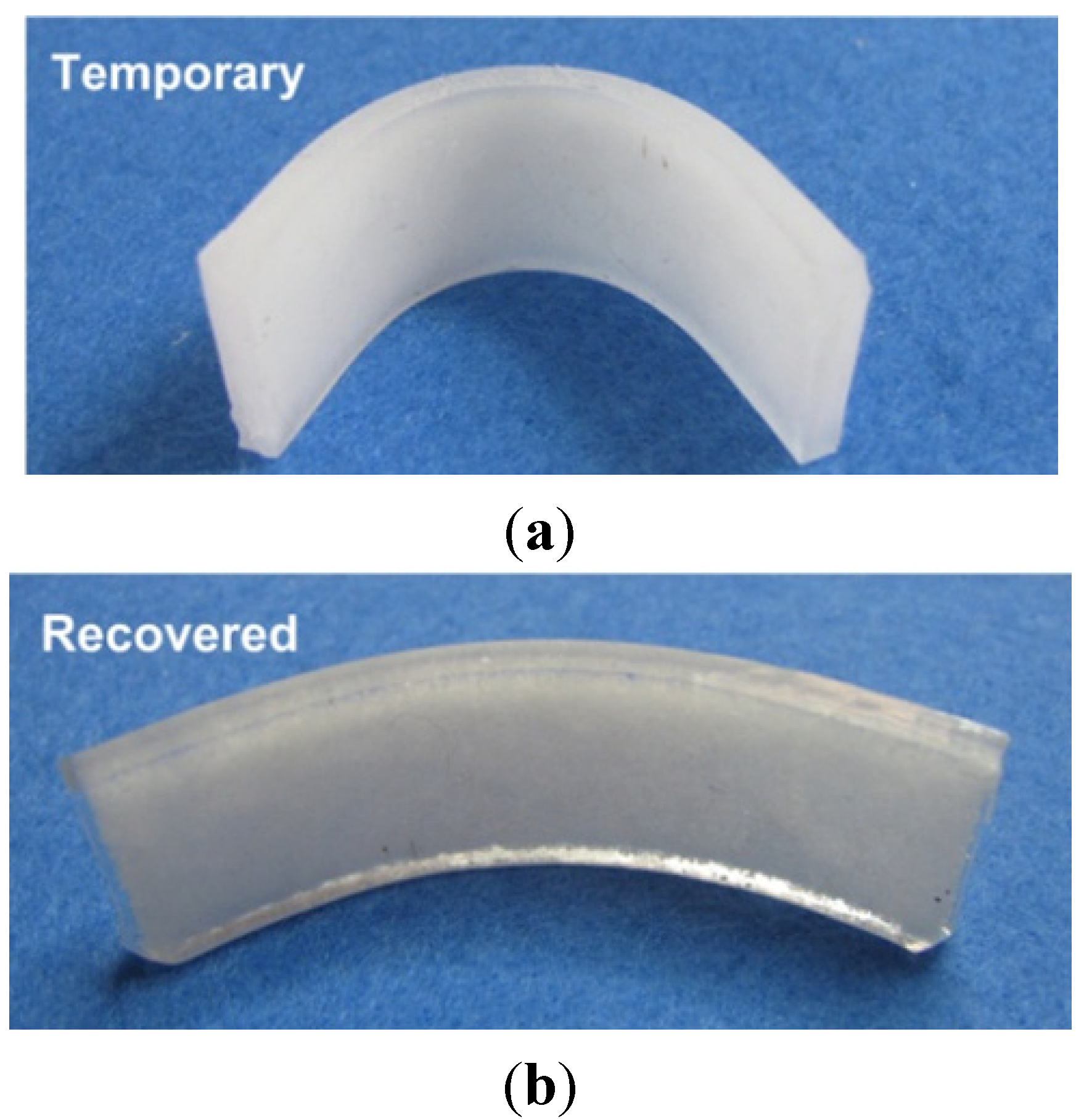

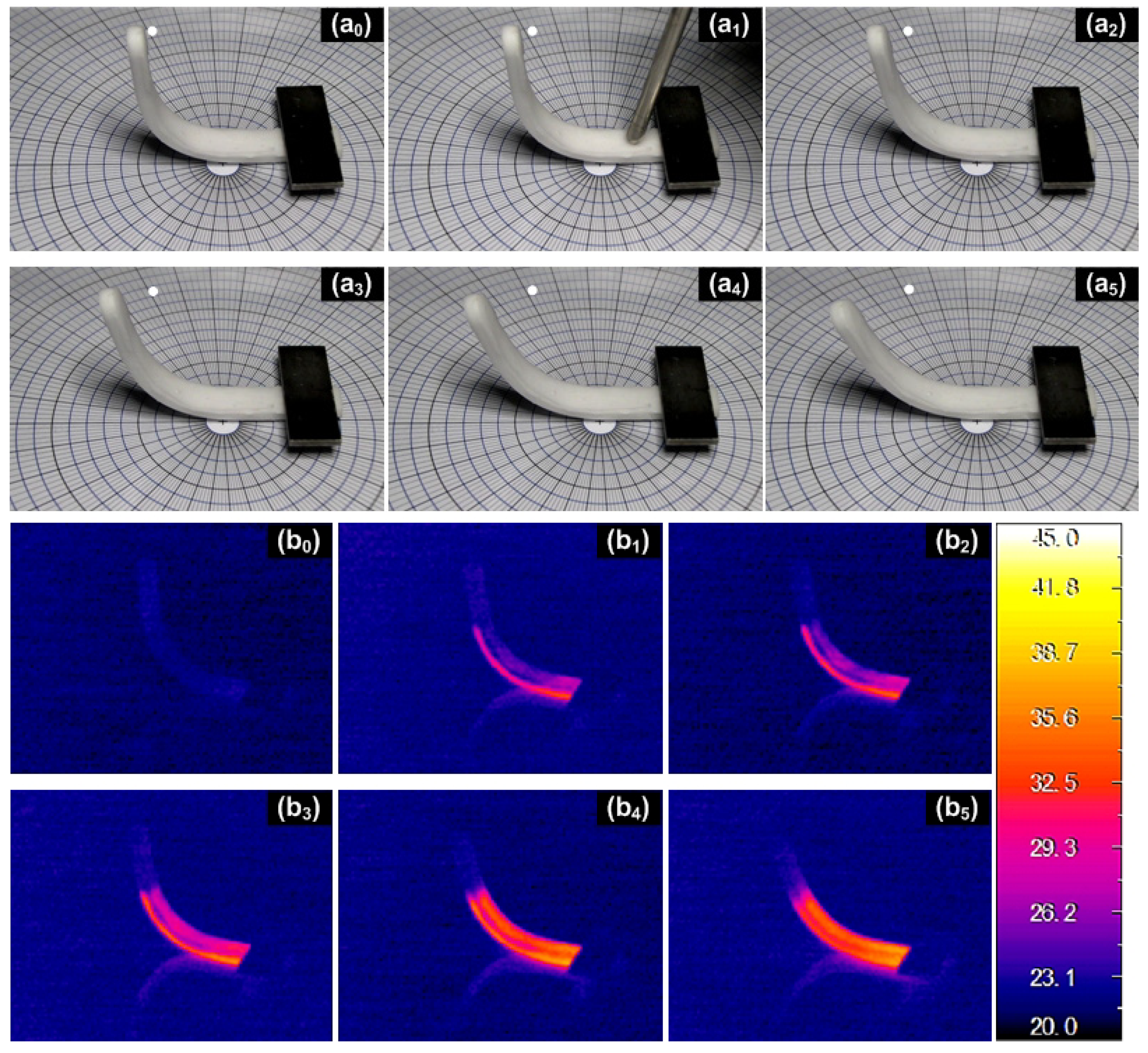
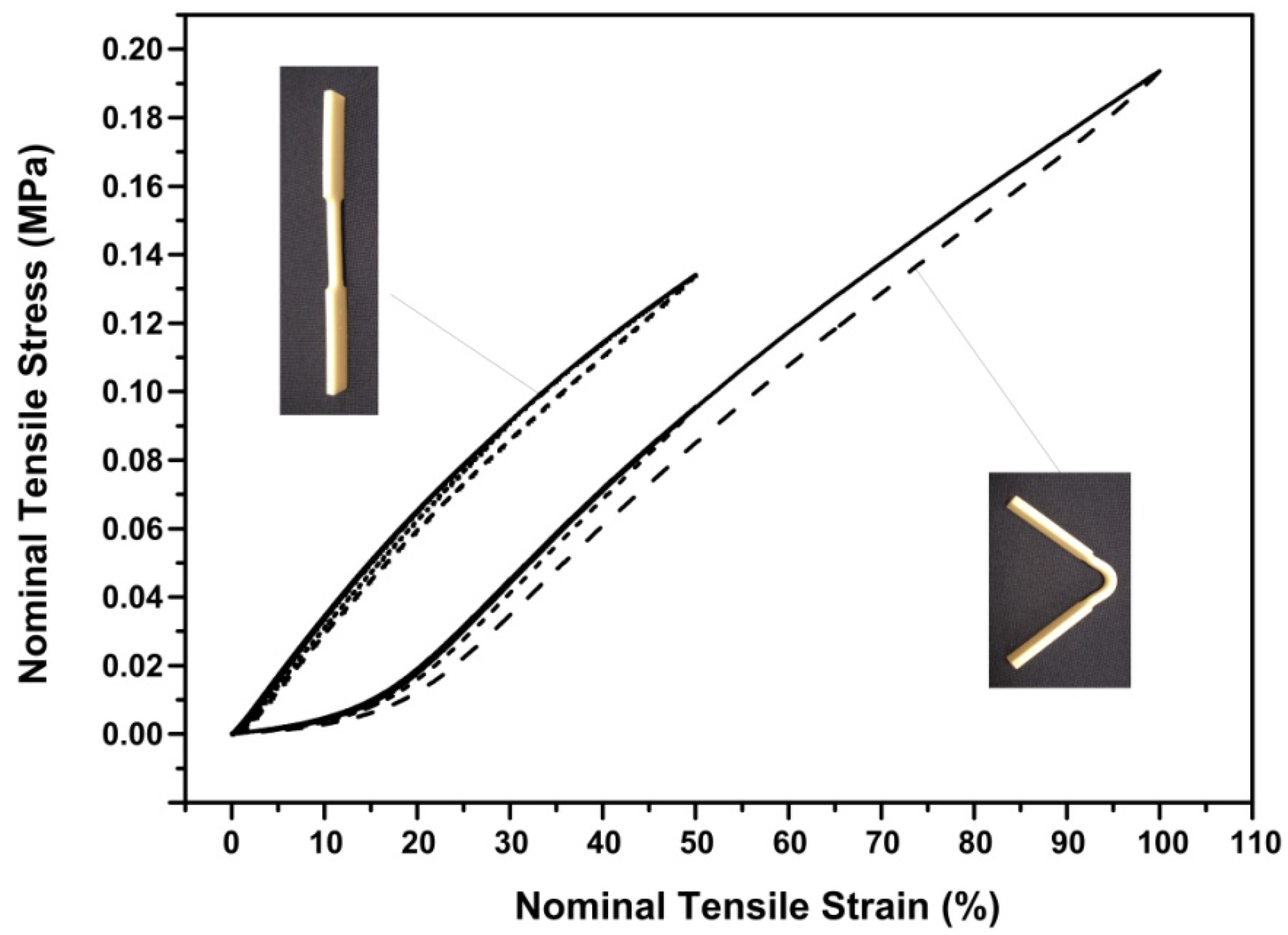

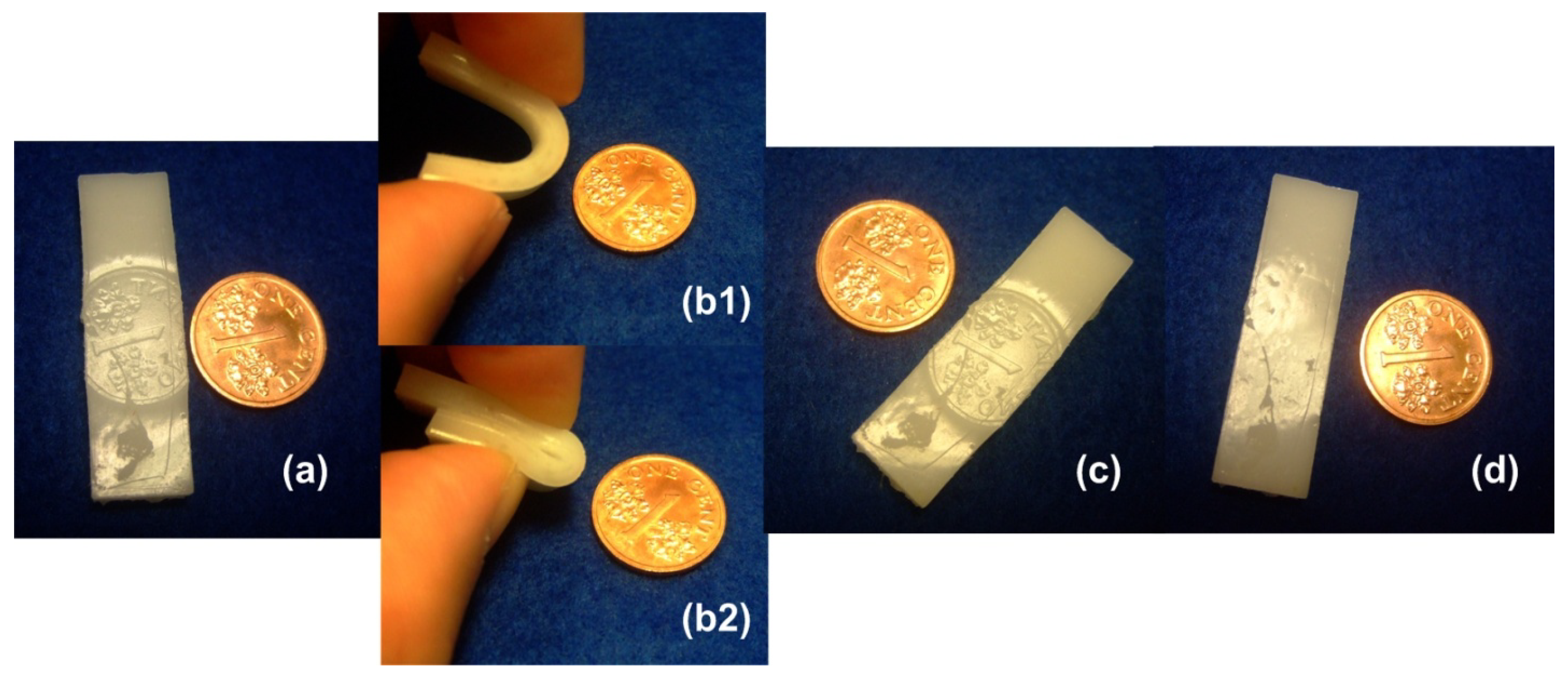
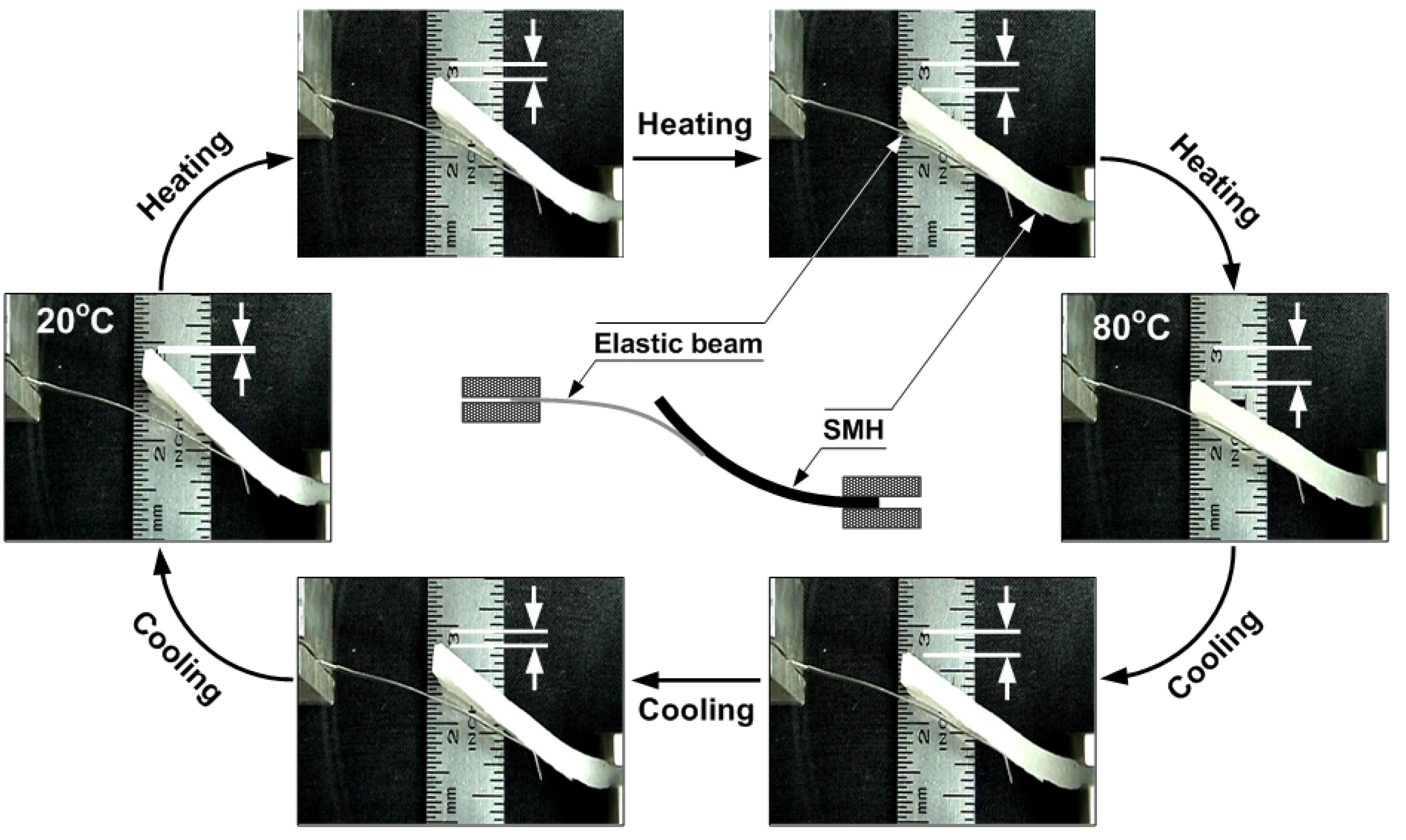

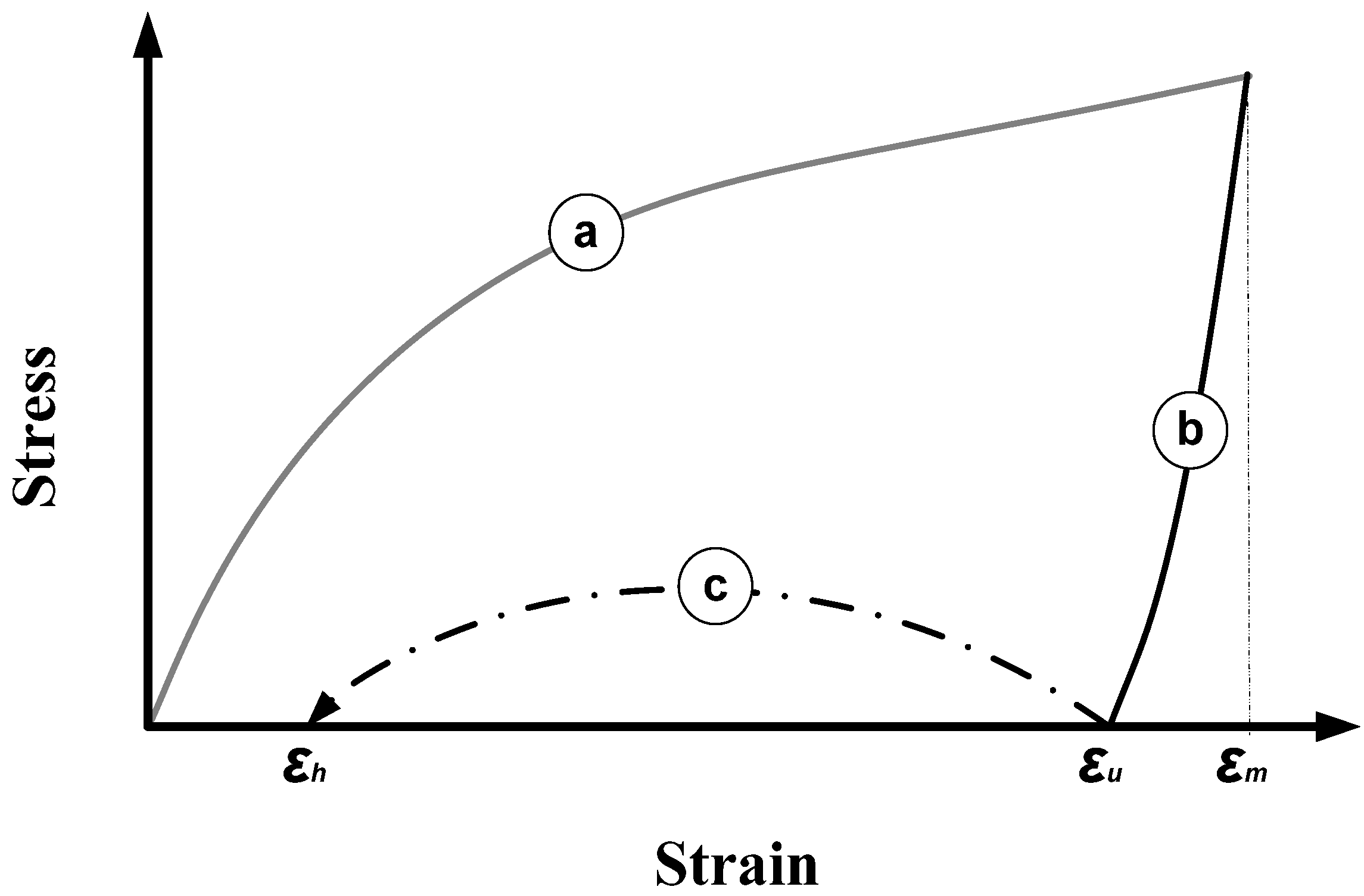
4.2. Optimization
- -
- The maximum programming strain is a constant;
- -
- Unloading in programming is always conducted after cooling the material to below Ts.
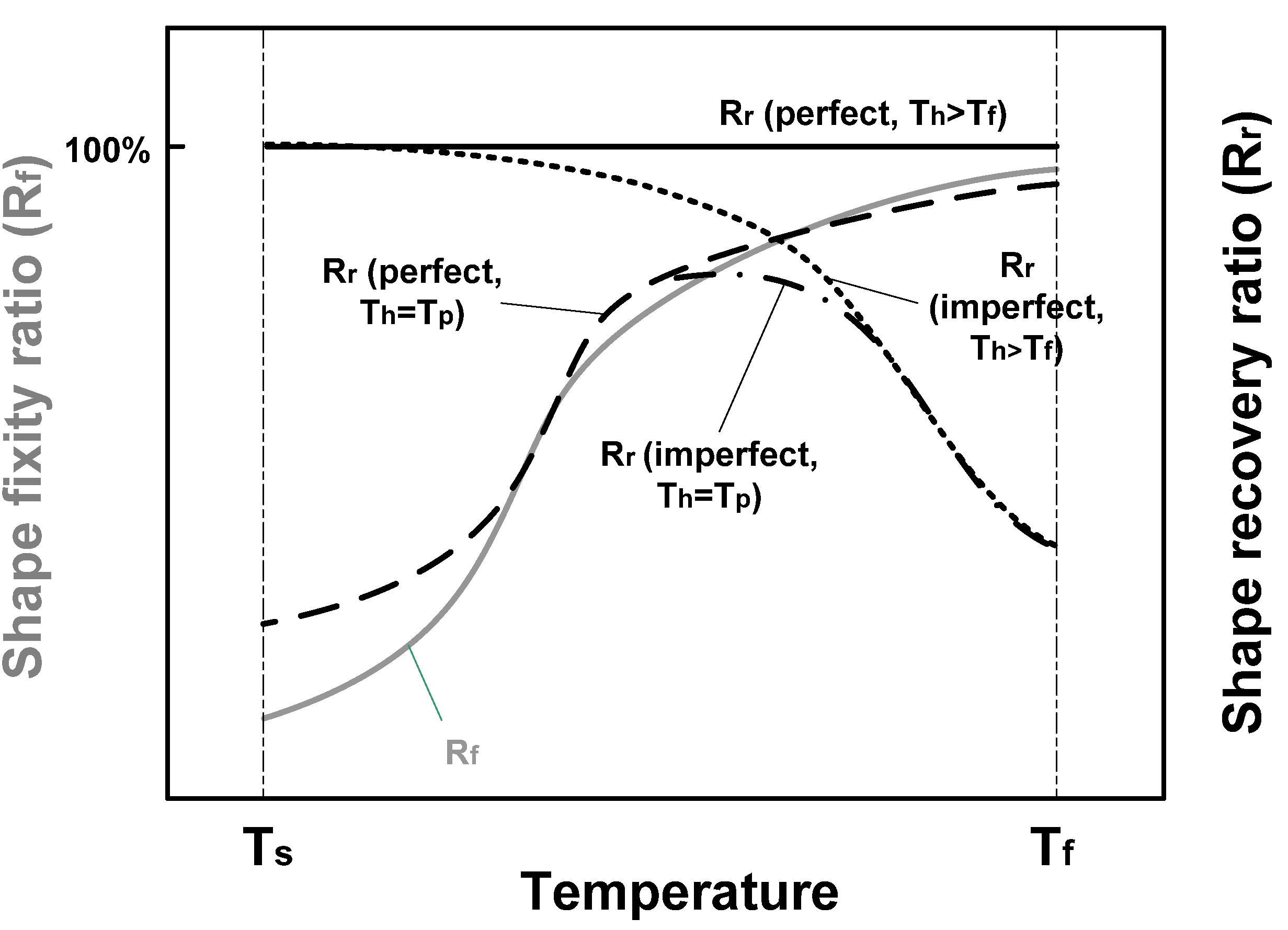
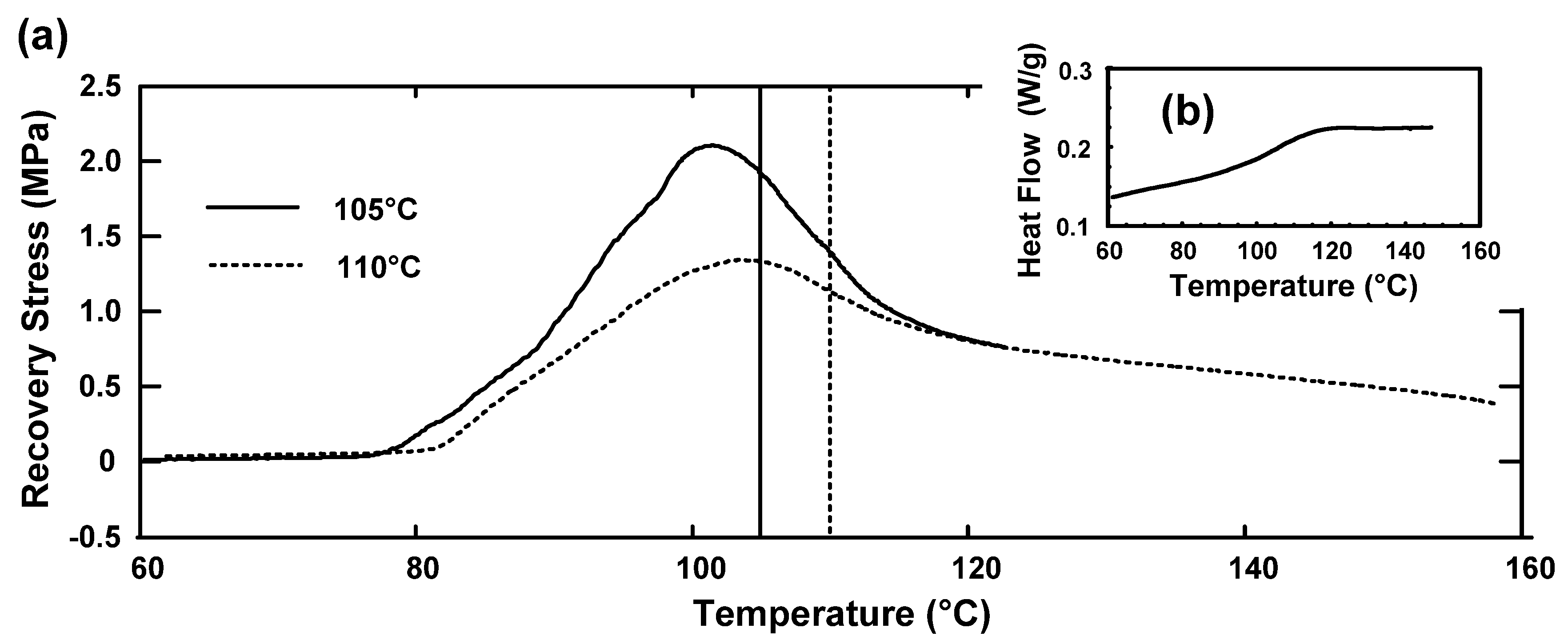
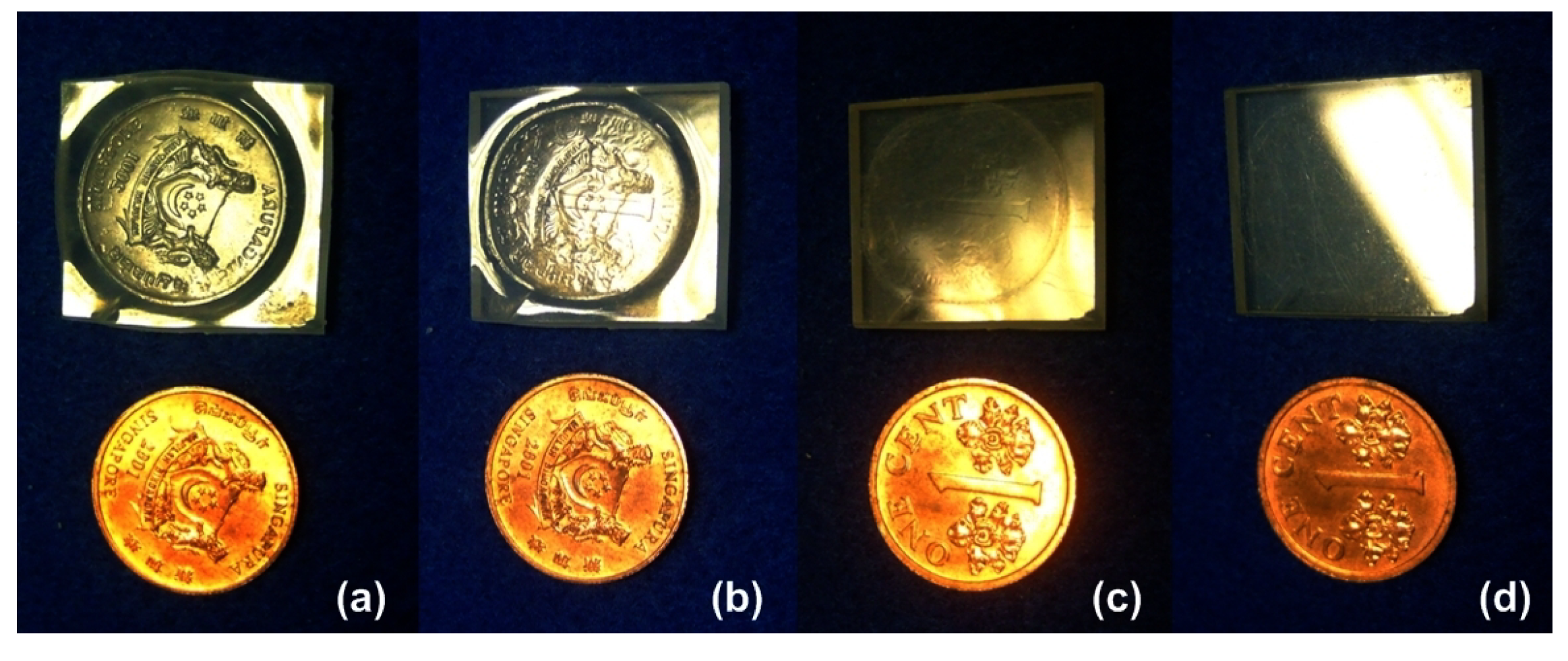
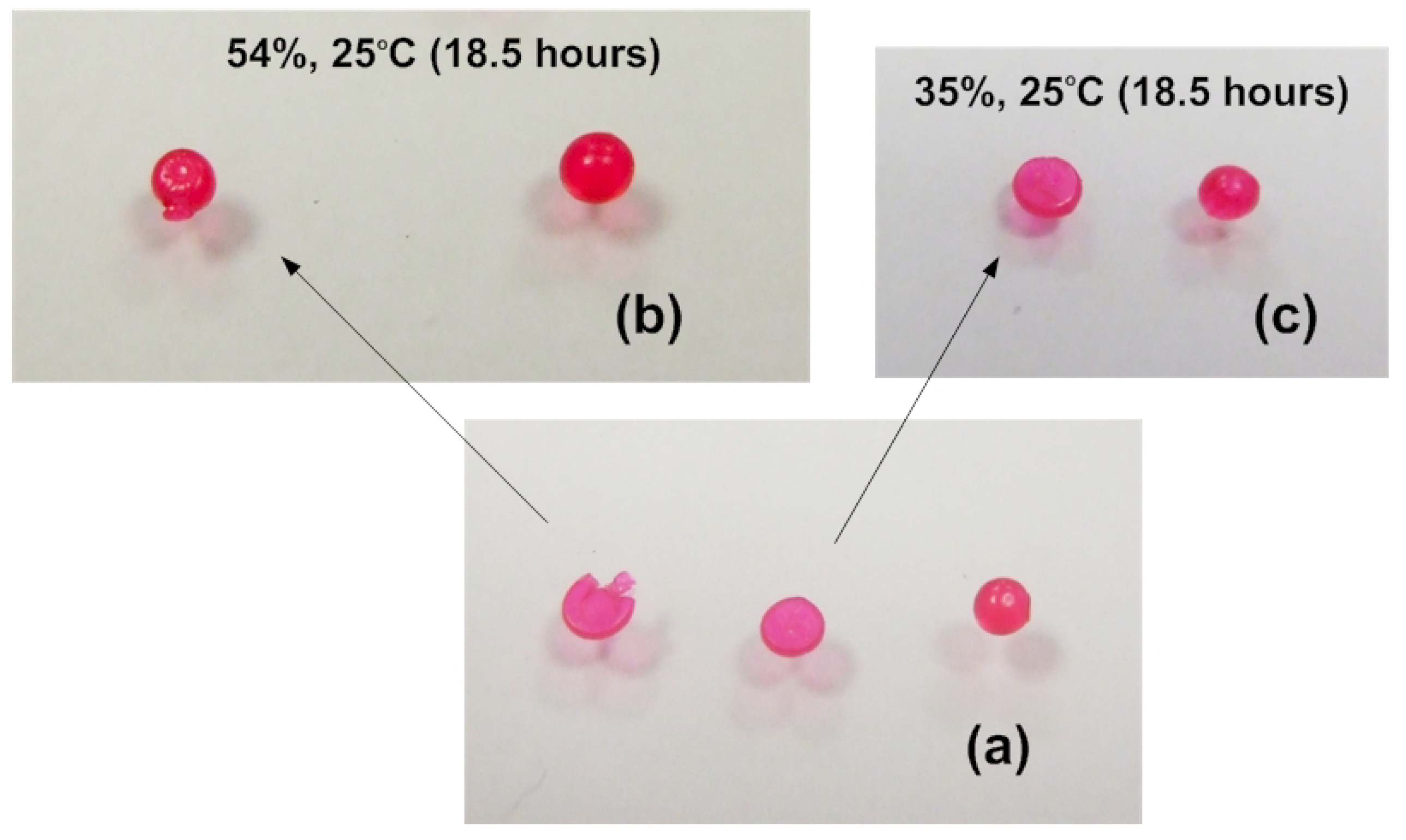
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Otsuka, K.; Wayman, C.M. Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998; pp. 36–38. [Google Scholar] [Green Version]
- Huang, W.M.; Ding, Z.; Wang, C.C.; Wei, J.; Zhao, Y.; Purnawali, H. Shape memory materials. Mater. Today 2010, 13, 54–61. [Google Scholar]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms, fundamentals and optimization. J. Polym. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Lendlein, A. Shape-Memory Polymers; Springer-Verlag: Heidelberg, Germany, 2010. [Google Scholar] [Green Version]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater.Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Funakubo, H. Shape Memory Alloys; Gordon and Breach Science Publishers: New York, NY, USA, 1987. [Google Scholar] [Green Version]
- Gunes, I.S.; Jana, S.C. Shape memory polymers and their nanocomposites: A review of science and technology of new multifunctional materials. J. Nanosci. Nanotechnol. 2008, 8, 1616–1637. [Google Scholar]
- Zhao, Q.; Kelch, S.; Lendlein, A. Shape-memory polymers with multiple transitions: Complex actively moving polymers. Soft Mater. 2013, 9, 1744–1755. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.F.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef]
- Wang, C.C.; Huang, W.M.; Ding, Z.; Zhao, Y.; Purnawali, H. Cooling-/water-responsive shape memory hybrids. Compos. Sci. Technol. 2012, 72, 1178–1182. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Satarkar, N.S.; Gall, K.; Likos, R.; Hilt, J.Z. Shape-memory polymer networks with Fe3O4 nanoparticles for remote activation. J. Appl. Polym. Sci. 2009, 112, 3166–3176. [Google Scholar] [CrossRef]
- Lu, H.B. A simulation method to analyze chemo-mechanical behavior of swelling-induced shape-memory polymer in response to solvent. J. Appl. Polym. Sci. 2012, 123, 1137–1146. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.L.; Luo, H.S.; Young, R.J.; Deng, L.B.; Zhang, S.; Fan, Y.; Ye, G.D. Rapidly switchable water-sensitive shape-memory cellulose/elastomer nano-composites. Soft Matter 2012, 8, 2509–2517. [Google Scholar]
- Beloshenko, V.A.; Varyukhin, V.N.; Voznyak, Y.V. The shape memory effect in polymers. Russ. Chem. Rev. 2005, 74, 265–283. [Google Scholar] [CrossRef]
- Razzaq, M.Y.; Behl, M.; Lendlein, A. Magnetic memory effect of nanocomposites. Adv. Funct. Mater. 2012, 22, 184–191. [Google Scholar] [CrossRef]
- Luo, X.F.; Mather, P.T. Conductive shape memory nanocomposites for high speed electrical actuation. Soft Matter 2010, 6, 2146–2149. [Google Scholar] [CrossRef]
- He, Z.W.; Satarkar, N.; Xie, T.; Cheng, Y.T.; Hilt, J.Z. Remote controlled multishape polymer nanocomposites with selective radiofrequency actuations. Adv. Mater. 2011, 23, 3192–3196. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; Fu, Y.Q. Polyurethane Shape Memory Polymers; CRC Press: Boca Raton, FL, USA, 2011; pp. 325–340. [Google Scholar] [Green Version]
- Huang, W.M.; Yang, B.; Zhao, Y.; Ding, Z. Thermo-moisture responsive polyurethane shape-memory polymer and composites: A review. J. Mater. Chem. 2010, 20, 3367–3381. [Google Scholar] [CrossRef]
- Fan, K.; Huang, W.M.; Wang, C.C.; Ding, Z.; Zhao, Y.; Purnawali, H.; Liew, K.C.; Zheng, L.X. Water-responsive shape memory hybrid: Design concept and demonstration. Express Polym. Lett. 2011, 5, 409–416. [Google Scholar] [CrossRef]
- Feng, Y.K.; Behl, M.; Kelch, S.; Lendlein, A. Biodegradable multiblock copolymers based on oligodepsipeptides with shape-memory properties. Macromol. Biosci. 2009, 9, 45–54. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Kelch, S.; Lendlein, A. Polymers move in response to light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Lendlein, A.; Behl, M. Shape-memory polymers for biomedical applications. Adv. Sci. Technol. 2009, 54, 96–102. [Google Scholar] [CrossRef]
- Lendlein, A.; Zotzmann, J.; Feng, Y.K.; Alteheld, A.; Kelch, S. Controlling the switching temperature of biodegradable, amorphous, shape-memory poly(rac-lactide)urethane networks by incorporation of different comonomers. Biomacromolecules 2009, 10, 975–982. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Capadona, J.R.; Rowan, S.J.; Weder, C. Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl. Mater. Interface 2010, 2, 165–174. [Google Scholar] [CrossRef]
- Kim, Y.B.; Chung, C.W.; Kim, H.W.; Rhee, Y.H. Shape memory effect of bacterial poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)]. Macromol. Rapid Comm. 2005, 26, 1070–1074. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar]
- Zotzmann, J.; Behl, M.; Hofmann, D.; Lendlein, A. Reversible triple-shape effect of polymer networks containing polypentadecalactone- and poly(ε-caprolactone)-segments. Adv. Mater. 2010, 22, 3424–3429. [Google Scholar] [CrossRef]
- Luo, H.S.; Hu, J.L.; Zhu, Y.; Zhang, S.; Fan, Y.; Ye, G.D. Achieving shape memory: Reversible behaviors of cellulose-pu blends in wet-dry cycles. J. Appl. Polym. Sci. 2012, 125, 657–665. [Google Scholar] [CrossRef]
- Bellin, I.; Kelch, S.; Lendlein, A. Dual-shape properties of triple-shape polymer networks with crystallizable network segments and grafted side chains. J. Mater. Chem. 2007, 17, 2885–2891. [Google Scholar] [CrossRef]
- Xie, T.; Xiao, X.C.; Cheng, Y.T. Revealing triple-shape memory effect by polymer bilayers. Macromol. Rapid Comm. 2009, 30, 1823–1827. [Google Scholar] [CrossRef]
- Qin, H.; Mather, P.T. Combined one-way and two-way shape memory in a glass-forming nematic network. Macromolecules 2008, 42, 273–280. [Google Scholar]
- Chung, T.; Romo-Uribe, A.; Mather, P.T. Two-way reversible shape memory in a semicrystalline network. Macromolecules 2008, 41, 184–192. [Google Scholar] [CrossRef]
- Jung, D.H.; Jeong, H.M.; Kim, B.K. Organic-inorganic chemical hybrids having shape memory effect. J. Mater. Chem. 2010, 20, 3458–3466. [Google Scholar] [CrossRef]
- Luo, X.; Mather, P.T. Triple-shape polymeric composites (TSPCS). Adv. Funct. Mater. 2010, 20, 2649–2656. [Google Scholar] [CrossRef]
- Luo, H.S.; Hu, J.L.; Zhu, Y. Tunable shape recovery of polymeric nano-composites. Mater. Lett. 2011, 65, 3583–3585. [Google Scholar] [CrossRef]
- Pretsch, T. Triple-shape properties of a thermoresponsive poly(ester urethane). Smart Mater. Struct. 2010, 19. [Google Scholar] [CrossRef]
- Small, W.; Buckley, P.R.; Wilson, T.S.; Benett, W.J.; Hartman, J.; Saloner, D.; Maitland, D.J. Shape memory polymer stent with expandable foam: A new concept for endovascular embolization of fusiform aneurysms. IEEE Trans. Bio. Med. Eng. 2007, 54, 1157–1160. [Google Scholar] [CrossRef]
- Sokolowski, W.; Metcalfe, A.; Hayashi, S.; Yahia, L.; Raymond, J. Medical applications of shape memory polymers. Biomed. Mater. 2007, 2, S23–S27. [Google Scholar] [CrossRef]
- Sortino, S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar] [CrossRef]
- Wischke, C.; Neffe, A.T.; Steuer, S.; Lendlein, A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J. Control. Release 2009, 138, 243–250. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Safranski, D.; Ortega, A.M.; Sassaman, K.; Gall, K. Strong, tailored, biocompatible shape-memory polymer networks. Adv. Funct. Mater. 2008, 18, 2428–2435. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Li, L.; Chor, J.H. Qualitative separation of the effects of carbon nano-powder and moisture on the glass transition temperature of polyurethane shape memory polymer. Scr. Mater. 2005, 53, 105–107. [Google Scholar] [CrossRef]
- Tobushi, H.; Hayashi, S.; Ikai, A.; Hara, H. Thermomechanical properties of shape memory polymers of polyurethane series and their applications. J. Phys. 1996, 6, 377–384. [Google Scholar]
- Tobushi, H.; Hayashi, S.; Pieczyska, E.; Date, K.; Nishimura, Y. Three-way actuation of shape memory composite. Arch. Mech. 2011, 63, 443–457. [Google Scholar]
- Westbrook, K.K.; Mather, P.T.; Parakh, V.; Dunn, M.L.; Ge, Q.; Lee, B.M.; Qi, H.J. Two-way reversible shape memory effects in a free-standing polymer composite. Smart Mater. Struct. 2011, 20. [Google Scholar] [CrossRef]
- Dietsch, B.; Tong, T. A review—Features and benefits of shape memory polymers (SMPS). J. Adv. Mater. 2007, 39, 3–12. [Google Scholar]
- Hu, J.L.; Meng, H.P.; Li, G.Q.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21. [Google Scholar] [CrossRef]
- Havens, E.; Snyder, E.A.; Tong, T.H. Light-activated shape memory polymers and associated applications. Smart Struct. Mater. 2005 Ind. Commerc. Appl. Smart Struct. Technol. 2005, 5762, 48–55. [Google Scholar]
- Sokolowski, W.M.; Tan, S.C. Advanced self-deployable structures for space applications. J. Spacecr. Rocket. 2007, 44, 750–754. [Google Scholar] [CrossRef]
- Pretsch, T.; Ecker, M.; Schildhauer, M.; Maskos, M. Switchable information carriers based on shape memory polymer. J. Mater. Chem. 2012, 22, 7757–7766. [Google Scholar] [CrossRef]
- Small, W.; Singhal, P.; Wilson, T.S.; Maitland, D.J. Biomedical applications of thermally activated shape memory polymers. J. Mater. Chem. 2010, 20, 3356–3366. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Triple-shape polymers. J. Mater. Chem. 2010, 20, 3335–3345. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Lanning, C.; Rech, B.; Eckstein, A.; Gall, K. Unconstrained recovery characterization of shape-memory polymer networks for cardiovascular applications. Biomaterials 2007, 28, 2255–2263. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers as stimuli-sensitive implant materials. Clin. Hemorheol. Microcirc. 2005, 32, 105–116. [Google Scholar]
- Alarcon, C.D.H.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef]
- Buckley, P.R.; McKinley, G.H.; Wilson, T.S.; Small, W.; Benett, W.J.; Bearinger, J.P.; McElfresh, M.W.; Maitland, D.J. Inductively heated shape memory polymer for the magnetic actuation of medical devices. IEEE Trans. Bio. Med. Eng. 2006, 53, 2075–2083. [Google Scholar] [CrossRef]
- Davis, K.A.; Burke, K.A.; Mather, P.T.; Henderson, J.H. Dynamic cell behavior on shape memory polymer substrates. Biomaterials 2011, 32, 2285–2293. [Google Scholar] [CrossRef]
- Feninat, E.F.; Laroche, G.; Fiset, M.; Mantovani, D. Shape memory materials for biomedical applications. Adv. Eng. Mater. 2002, 4, 91–104. [Google Scholar] [CrossRef]
- Huang, W.M. Thermo-moisture responsive polyurethane shape memory polymer for biomedical devices. Open Med. Dev. J. 2010, 2, 11–19. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; Liu, N.; Phee, S.J. Water-responsive programmable shape memory polymer devices. Int. Conf. Smart Mater. Nanotechnol. Eng. 2007, 6423. [Google Scholar] [CrossRef]
- Metcalfe, A.; Desfaits, A.C.; Salazkin, I.; Yahia, L.; Sokolowski, W.M.; Raymond, J. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials 2003, 24, 491–497. [Google Scholar] [CrossRef]
- Serrano, M.C.; Carbajal, L.; Ameer, G.A. Novel biodegradable shape-memory elastomers with drug-releasing capabilities. Adv. Mater. 2011, 23, 2211–2215. [Google Scholar] [CrossRef]
- Feng, Y.K.; Zhang, S.F.; Wang, H.Y.; Zhao, H.Y.; Lu, J.; Guo, J.T.; Behl, M.; Lendlein, A. Biodegradable polyesterurethanes with shape-memory properties for dexamethasone and aspirin controlled release. J. Control. Release 2011, 152, E21–E23. [Google Scholar] [CrossRef]
- Feng, Y.K.; Zhang, S.F.; Wang, H.Y.; Zhao, H.Y.; Lu, J.; Guo, J.T.; Behl, M.; Lendlein, A. Drug release from biodegradable polyesterurethanes with shape-memory effect. J. Control. Release 2011, 152, E20–E21. [Google Scholar] [CrossRef]
- Wischke, C.; Tripodo, G.; Choi, N.Y.; Lendlein, A. Hydrolytic degradation behavior of poly(rac-lactide)-block-poly(propylene glycol)-block-poly(rac-lactide) dimethacrylate derived networks designed for biomedical applications. Macromol. Biosci. 2011, 11, 1637–1646. [Google Scholar] [CrossRef]
- Xue, L.; Dai, S.Y.; Li, Z. Synthesis and characterization of elastic star shape-memory polymers as self-expandable drug-eluting stents. J. Mater. Chem. 2012, 22, 7403–7411. [Google Scholar] [CrossRef]
- Li, G.; Fei, G.X.; Xia, H.S.; Han, J.J.; Zhao, Y. Spatial and temporal control of shape memory polymers and simultaneous drug release using high intensity focused ultrasound. J. Mater. Chem. 2012, 22, 7692–7696. [Google Scholar] [CrossRef]
- Miyazaki, S.; Fu, Y.Q.; Huang, W.M. Thin Film Shape Memory Alloys: Fundamentals and Device Applications; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Ge, Y.L.; Heczko, O.; Soderberg, O.; Hannula, S.P. Magnetic domain evolution with applied field in a Ni-Mn-Ga magnetic shape memory alloy. Scr. Mater. 2006, 54, 2155–2160. [Google Scholar] [CrossRef]
- Huang, W. On the selection of shape memory alloys for actuators. Mater. Des. 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Yang, B.; Huang, W.M.; Li, C.; Li, L. Effects of moisture on the thermomechanical properties of a polyurethane shape memory polymer. Polymer 2006, 47, 1348–1356. [Google Scholar] [CrossRef]
- Tobushi, H.; Hara, H.; Yamada, E.; Hayashi, S. Thermomechanical properties in a thin film of shape memory polymer of polyurethane series. Smart Mater. Struct. 1996, 5, 483–491. [Google Scholar] [CrossRef]
- Tey, S.J.; Huang, W.M.; Sokolowski, W.M. Influence of long-term storage in cold hibernation on strain recovery and recovery stress of polyurethane shape memory polymer foam. Smart Mater. Struct. 2001, 10, 321–325. [Google Scholar] [CrossRef]
- Tobushi, H.; Matsui, R.; Hayashi, S.; Shimada, D. The influence of shape-holding conditions on shape recovery of polyurethane-shape memory polymer foams. Smart Mater. Struct. 2004, 13, 881–887. [Google Scholar] [CrossRef]
- Tobushi, H.; Hayashi, S.; Hoshio, K.; Ejiri, Y. Shape recovery and irrecoverable strain control in polyurethane shape-memory polymer. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Muller, W.W.; Pretsch, T. Hydrolytic aging of crystallizable shape memory poly(ester urethane): Effects on the thermo-mechanical properties and visco-elastic modeling. Eur. Polym. J. 2010, 46, 1745–1758. [Google Scholar] [CrossRef]
- Pretsch, T.; Muller, W.W. Shape memory poly(ester urethane) with improved hydrolytic stability. Polym. Degrad. Stab. 2010, 95, 880–888. [Google Scholar] [CrossRef]
- Castro, F.; Westbrook, K.K.; Hermiller, J.; Ahn, D.U.; Ding, Y.F.; Qi, H.J. Time and temperature dependent recovery of epoxy-based shape memory polymers. J. Eng. Mater. Technol. 2011, 133, 021025:1–021025:9. [Google Scholar]
- Bhattacharyya, A.; Tobushi, H. Analysis of the isothermal mechanical response of a shape memory polymer rheological model. Polym. Eng. Sci. 2000, 40, 2498–2510. [Google Scholar] [CrossRef]
- Ge, Q.; Luo, X.F.; Rodriguez, E.D.; Zhang, X.; Mather, P.T.; Dunn, M.L.; Qi, H.J. Thermomechanical behavior of shape memory elastomeric composites. J. Mech. Phys. Solids 2012, 60, 67–83. [Google Scholar] [CrossRef]
- Azra, C.; Plummer, C.J.G.; Manson, J.A.E. Isothermal recovery rates in shape memory polyurethanes. Smart Mater. Struct. 2011, 20. [Google Scholar] [CrossRef]
- Choi, J.; Ortega, A.M.; Xiao, R.; Yakacki, C.M.; Nguyen, T.D. Effect of physical aging on the shape-memory behavior of amorphous networks. Polymer 2012, 53, 2453–2464. [Google Scholar] [CrossRef]
- Ortega, A.M.; Yakacki, C.M.; Dixon, S.A.; Likos, R.; Greenberg, A.R.; Gall, K. Effect of crosslinking and long-term storage on the shape-memory behavior of (meth)acrylate-based shape-memory polymers. Soft Matter 2012, 8, 7381–7392. [Google Scholar] [CrossRef]
- Gunes, I.S.; Jimenez, G.A.; Jana, S.C. Carbonaceous fillers for shape memory actuation of polyurethane composites by resistive heating. Carbon 2009, 47, 981–997. [Google Scholar] [CrossRef]
- Ding, Z. Shape Memory Hybrids: Mechanism and Design for Tailored Properties. Ph.D. Thesis, Nanyang Technological University , Singapore, 2011. [Google Scholar]
- Wang, C.C.; Huang, W.M.; Ding, Z.; Zhao, Y.; Purnawali, H.; Zheng, L.X.; Fan, H.; He, C.B. Rubber-like shape memory polymeric materials with repeatable thermal-assisted healing function. Smart Mater. Struct. 2012, 21. [Google Scholar] [CrossRef]
- Weiss, R.A.; Izzo, E.; Mandelbaum, S. New design of shape memory polymers: Mixtures of an elastomeric ionomer and low molar mass fatty acids and their salts. Macromolecules 2008, 41, 2978–2980. [Google Scholar] [CrossRef]
- Shi, Y.; Yoonessi, M.; Weiss, R.A. High temperature shape memory polymers. Macromolecules 2013, 46, 4160–4167. [Google Scholar] [CrossRef]
- Dong, J.; Weiss, R.A. Effect of crosslinking on shape-memory behavior of zinc stearate/ionomer compounds. Macromol. Chem. Phys. 2013, 214, 1238–1246. [Google Scholar] [CrossRef]
- Koerner, H.; Strong, R.J.; Smith, M.L.; Wang, D.H.; Tan, L.S.; Lee, K.M.; White, T.J.; Vaia, R.A. Polymer design for high temperature shape memory: Low crosslink density polyimides. Polymer 2013, 54, 391–402. [Google Scholar] [CrossRef]
- Lee, K.M.; Wang, D.H.; Koerner, H.; Vaia, R.A.; Tan, L.S.; White, T.J. Photomechanical response of pre-strained azobenzene-functionalized polyimide materials. Macromol. Chem. Phys. 2013, 214, 1189–1194. [Google Scholar] [CrossRef]
- Ahn, S.; Kasi, R.M. Exploiting microphase-separated morphologies of side-chain liquid crystalline polymer networks for triple shape memory properties. Adv. Funct. Mater. 2013, 21, 4543–4549. [Google Scholar]
- Zhang, D.W.; Petersen, K.M.; Grunlan, M.A. Inorganic-organic shape memory polymer (SMP) foams with highly tunable properties. ACS Appl. Mater. Interfaces 2013, 5, 186–191. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; Li, C.; Chan, Y.S.; Li, A. Response to comment on water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2010, 97, 056102. [Google Scholar] [CrossRef]
- Jung, Y.C.; So, H.H.; Cho, J.W. Water-responsive shape memory polyurethane block copolymer modified with polyhedral oligometric silsesquioxane. J. Macromol. Sci. B 2006, 45, 1189. [Google Scholar] [CrossRef]
- Yang, B. Influence of Moisture in Polyurethane Shape Memory Polymers and their Electrical Conductive Composites. Ph.D. Thesis, Nanyang Technological University, Singapore, 2007. [Google Scholar]
- Yang, B.; Huang, W.M.; Li, C.; Lee, C.M.; Li, L. On the enects of moisture in a polyurethane shape memory polymer. Smart Mater. Struct. 2004, 13, 191–195. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; Li, A.; Li, C.; Chan, Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105:1–114105:3. [Google Scholar]
- Wang, C.C.; Zhao, Y.; Purnawali, H.; Huang, W.M.; Sun, L. Chemically induced morphing in polyurethane shape memory polymer micro fibers/springs. React. Funct. Polym. 2012, 72, 757–764. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.C.; Huang, W.M.; Purnawali, H.; An, L. Formation of micro protrusive lens arrays atop poly(methyl methacrylate). Opt. Express 2011, 19, 26000–26005. [Google Scholar]
- Huang, W.M.; Song, C.L.; Fu, Y.Q.; Wang, C.C.; Zhao, Y.; Purnawali, H.; Lu, H.B.; Tang, C. Shaping tissue with shape memory materials. Adv. Drug Deliv. Rev. 2013, 65, 515–535. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.C.; Huang, W.M.; Purnawali, H. Buckling of poly(methyl methacrylate) in stimulus-responsive shape recovery. Appl. Phys. Lett. 2011, 99, 131911:1–131911:3. [Google Scholar]
- DiOrio, A.M.; Luo, X.F.; Lee, K.M.; Mather, P.T. A functionally graded shape memory polymer. Soft Matter 2011, 7, 68–74. [Google Scholar] [CrossRef]
- Bellin, I.; Kelch, S.; Langer, R.; Lendlein, A. Polymeric triple-shape materials. Proc. Natl. Acad. Sci. USA 2006, 103, 18043–18047. [Google Scholar]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M. Mechanisms of the multi-shape memory effect and temperature memory effect in shape memory polymers. Soft Matter 2010, 6, 4403–4406. [Google Scholar] [CrossRef]
- Bae, C.Y.; Park, J.H.; Kim, E.Y.; Kang, Y.S.; Kim, B.K. Organic–inorganic nanocomposite bilayers with triple shape memory effect. J. Mater. Chem. 2011, 21, 11288–11295. [Google Scholar] [CrossRef]
- Bothe, M.; Mya, K.Y.; Lin, E.M.J.; Yeo, C.C.; Lu, X.H.; He, C.B.; Pretsch, T. Triple-shape properties of star-shaped poss-polycaprolactone polyurethane networks. Soft Matter 2012, 8, 965–972. [Google Scholar] [CrossRef]
- Kumar, U.N.; Kratz, K.; Behl, M.; Lendlein, A. Shape-memory properites of magnetically active triple-shape nanocomposities based on a grafted polymer network with two crystallizable switching segments. Express Polym. Lett. 2012, 6, 26–40. [Google Scholar]
- Zotzmann, J.; Behl, M.; Lendlein, A. The influence of programming conditions on the triple-shape effect of copolymer networks with poly(ω-pentadecalactone) and poly(ε-caprolactone) as switching segments. Macromol. Symp. 2011, 309/310, 147–153. [Google Scholar] [CrossRef]
- Li, J.J.; Xie, T. Significant impact of thermo-mechanical conditions on polymer triple-shape memory effect. Macromolecules 2011, 44, 175–180. [Google Scholar] [CrossRef]
- Chen, S.J.; Hu, J.L.; Yuen, C.W.M.; Chan, L.K.; Zhuo, H.T. Triple shape memory effect in multiple crystalline polyurethanes. Polym. Adv. Technol. 2010, 21, 377–380. [Google Scholar]
- Kumar, U.N.; Kratz, K.; Wagermaier, W.; Behl, M.; Lendlein, A. Non-contact actuation of triple-shape effect in multiphase polymer network nanocomposites in alternating magnetic field. J. Mater. Chem. 2010, 20, 3404–3415. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Rubio, R.; German, L.; Laza, J.M.; Vilas, J.L.; Rodriguez, M.; Leon, L.M. Triple-shape memory effect of covalently crosslinked polyalkenamer based semicrystalline polymer blends. Soft Matter 2012, 8, 4928–4935. [Google Scholar] [CrossRef]
- Li, J.; Liu, T.; Xia, S.; Pan, Y.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. A versatile approach to achieve quintuple-shape memory effect by semi-interpenetrating polymer networks containing broadened glass transition and crystalline segments. J. Mater. Chem. 2011, 21, 12213–12217. [Google Scholar] [CrossRef]
- Behl, M.; Bellin, I.; Kelch, S.; Wagermaier, W.; Lendlein, A. One-step process for creating triple-shape capability of ab polymer networks. Adv. Funct. Mater. 2009, 19, 102–108. [Google Scholar] [CrossRef]
- Airoldi, G.; Besseghini, S.; Riva, G. Micromemory effects in shape memory alloys. Il Nuovo Cimento D 1993, 15, 365–374. [Google Scholar] [CrossRef]
- Riva, G.; Airoldi, G.; Besseghini, S. The step-wise martensite to austenite reversible transformation. Meccanica 1995, 30, 495–503. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Cheah, J.Y. The temperature memory effect and the influence of thermo-mechanical cycling in shape memory alloys. Smart Mater. Struct. 2010, 19. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.M. Comments on incomplete transformation induced multiple-step transformation in tini shape memory alloys. Scr. Mater. 2006, 55, 493–495. [Google Scholar] [CrossRef]
- Miaudet, P.; Derré, A.; Maugey, M.; Zakri, C.; Piccione, P.M.; Inoubli, R.; Poulin, P. Shape and temperature memory of nanocomposites with broadened glass transition. Science 2007, 318, 1294–1296. [Google Scholar] [CrossRef]
- Kratz, K.; Voigt, U.; Lendlein, A. Temperature-memory effect of copolyesterurethanes and their application potential in minimally invasive medical technologies. Adv. Funct. Mater. 2012, 22, 3057–3065. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Wang, C.C.; Zhao, Y.; Ding, Z.; Purnawali, H. Optimization of the shape memory effect in shape memory polymers. J. Polym. Sci. A Polym. Chem. 2011, 49, 3574–3581. [Google Scholar] [CrossRef]
- Lee, K.M.; Bunning, T.J.; White, T.J. Autonomous, hands-free shape memory in glassy, liquid crystalline polymer networks. Adv. Mater. 2012, 24, 2839–2843. [Google Scholar] [CrossRef]
- Hong, S.J.; Yu, W.R.; Youk, J.H. Two-way shape memory behavior of shape memory polyurethanes with a bias load. Smart Mater. Struct. 2010, 19. [Google Scholar] [CrossRef]
- Pandini, S.; Passera, S.; Messori, M.; Paderni, K.; Toselli, M.; Gianoncelli, A.; Bontempi, E.; Ricco, T. Two-way reversible shape memory behaviour of crosslinked poly(ε-caprolactone). Polymer 2012, 53, 1915–1924. [Google Scholar] [CrossRef]
- Martin, B.; Thorsten, P. Two-way shape changes of a shape-memory poly(ester urethane). Macromol. Chem. Phys. 2012, 213, 2378–2385. [Google Scholar] [CrossRef]
- Du, H.Y.; Zhang, J.H. Solvent induced shape recovery of shape memory polymer based on chemically cross-linked poly(vinyl alcohol). Soft Matter 2010, 6, 3370–3376. [Google Scholar] [CrossRef]
- Lu, H.B.; Liu, Y.J.; Gou, J.H.; Leng, J.S.; Du, S.Y. Surface coating of multi-walled carbon nanotube nanopaper on shape-memory polymer for multifunctionalization. Compos. Sci. Technol. 2011, 71, 1427–1434. [Google Scholar] [CrossRef]
- Sauter, T.; Lutzow, K.; Schossig, M.; Kosmella, H.; Weigel, T.; Kratz, K.; Lendlein, A. Shape-memory properties of polyetherurethane foams prepared by thermally induced phase separation. Adv. Eng. Mater. 2012, 14, 818–824. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.K.; Purnawali, H.; Huang, W.M.; King, P.J.; Chalker, P.R.; Mireftab, M.; Geng, J. Making shape memory polymers reprocessable and reusable by a simple chemical method. J. Mater. Chem. 2012, 22, 8192–8194. [Google Scholar]
- Zhang, Y.M.; Wang, Q.H.; Wang, C.; Wang, T.M. High-strain shape memory polymer networks crosslinked by SiO2. J. Mater. Chem. 2011, 21, 9073–9078. [Google Scholar] [CrossRef]
- Schoener, C.A.; Weyand, C.B.; Murthy, R.; Grunlan, M.A. Shape memory polymers with silicon-containing segments. J. Mater. Chem. 2010, 20, 1787–1793. [Google Scholar] [CrossRef]
- Gunes, I.S.; Perez-Bolivar, C.; Cao, F.N.; Jimenez, G.A.; Anzenbacher, P.; Jana, S.C. Analysis of non-covalent interactions between the nanoparticulate fillers and the matrix polymer as applied to shape memory performance. J. Mater. Chem. 2010, 20, 3467–3474. [Google Scholar]
- Rousseau, I.A.; Xie, T. Shape memory epoxy: Composition, structure, properties and shape memory performances. J. Mater. Chem. 2010, 20, 3431–3441. [Google Scholar] [CrossRef]
- Xu, B.; Fu, Y.Q.; Ahmad, M.; Luo, J.K.; Huang, W.M.; Kraft, A.; Reuben, R.; Pei, Y.T.; Chen, Z.G.; de Hosson, J.T.M. Thermo-mechanical properties of polystyrene-based shape memory nanocomposites. J. Mater. Chem. 2010, 20, 3442–3448. [Google Scholar] [CrossRef]
- Jang, M.K.; Hartwig, A.; Kim, B.K. Shape memory polyurethanes cross-linked by surface modified silica particles. J. Mater. Chem. 2009, 19, 1166–1172. [Google Scholar] [CrossRef]
- Kunzelman, J.; Chung, T.; Mather, P.T.; Weder, C. Shape memory polymers with built-in threshold temperature sensors. J. Mater. Chem. 2008, 18, 1082–1086. [Google Scholar] [CrossRef]
- Ionov, L. Soft microorigami: Self-folding polymer films. Soft Matter 2011, 7, 6786–6791. [Google Scholar] [CrossRef]
- Xie, T.; Xiao, X.C.; Li, J.J.; Wang, R.M. Encoding localized strain history through wrinkle based structural colors. Adv. Mater. 2010, 22, 4390–4394. [Google Scholar]
- Li, J.J.; An, Y.H.; Huang, R.; Jiang, H.Q.; Xie, T. Unique aspects of a shape memory polymer as the substrate for surface wrinkling. ACS Appl. Mater. Interfaces 2012, 4, 598–603. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, W.M.; Fu, Y.Q. Formation of micro/nano-scale wrinkling patterns atop shape memory polymers. J. Micromech. Microeng. 2011, 21. [Google Scholar] [CrossRef]
- Chaunier, L.; Lourdin, D. The shape memory of starch. Starch-Starke 2009, 61, 116–118. [Google Scholar] [CrossRef]
- Luo, X.F.; Mather, P.T. Preparation and characterization of shape memory elastomeric composites. Macromolecules 2009, 42, 7251–7253. [Google Scholar] [CrossRef]
- Pan, G.H.; Huang, W.M.; Ng, Z.C.; Liu, N.; Phee, S.J. The glass transition temperature of polyurethane shape memory polymer reinforced with treated/non-treated attapulgite (playgorskite) clay in dry and wet conditions. Smart Mater.Struct. 2008, 17. [Google Scholar] [CrossRef]
- Xu, B.; Huang, W.M.; Pei, Y.T.; Chen, Z.G.; Kraft, A.; Reuben, R.; de Hosson, J.T.M.; Fu, Y.Q. Mechanical properties of attapulgite clay reinforced polyurethane shape-memory nanocomposites. Eur. Polym. J. 2009, 45, 1904–1911. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Nguyen, T.D.; Likos, R.; Lamell, R.; Guigou, D.; Gall, K. Impact of shape-memory programming on mechanically-driven recovery in polymers. Polymer 2011, 52, 4947–4954. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, X.; Huang, W.M.; Zhao, Y.; Ding, Z.; Tang, C.; Zhang, J. Mechanisms of the Shape Memory Effect in Polymeric Materials. Polymers 2013, 5, 1169-1202. https://doi.org/10.3390/polym5041169
Wu X, Huang WM, Zhao Y, Ding Z, Tang C, Zhang J. Mechanisms of the Shape Memory Effect in Polymeric Materials. Polymers. 2013; 5(4):1169-1202. https://doi.org/10.3390/polym5041169
Chicago/Turabian StyleWu, Xuelian, Wei Min Huang, Yong Zhao, Zheng Ding, Cheng Tang, and Jiliang Zhang. 2013. "Mechanisms of the Shape Memory Effect in Polymeric Materials" Polymers 5, no. 4: 1169-1202. https://doi.org/10.3390/polym5041169
APA StyleWu, X., Huang, W. M., Zhao, Y., Ding, Z., Tang, C., & Zhang, J. (2013). Mechanisms of the Shape Memory Effect in Polymeric Materials. Polymers, 5(4), 1169-1202. https://doi.org/10.3390/polym5041169




