Embedding of Hollow Polymer Microspheres with Hydrophilic Shell in Nafion Matrix as Proton and Water Micro-Reservoir
Abstract
:1. Introduction
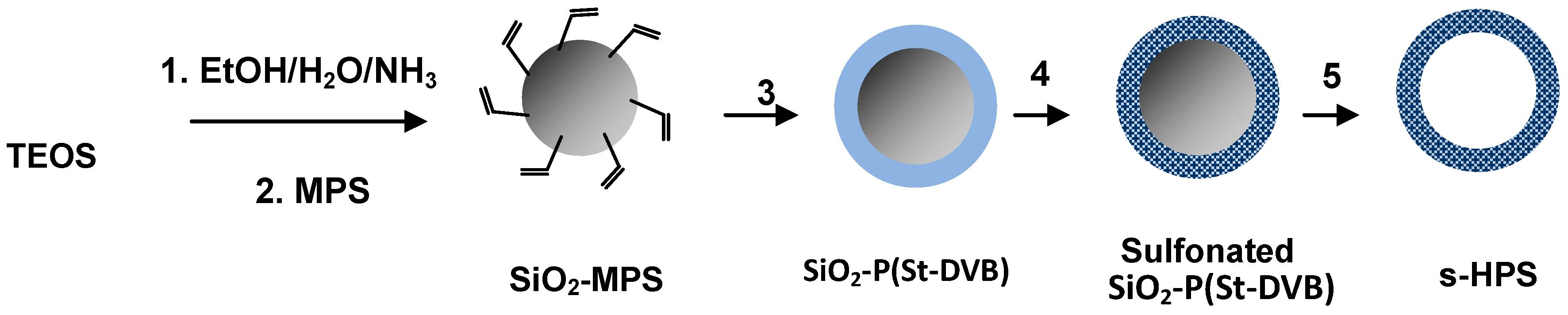
2. Experimental Section
2.1. Materials
2.2. Synthesis of SiO2-MPS Nanoparticles
2.3. Synthesis of SiO2/Polymer Core-Shell Nanoparticles
2.4. Synthesis of Hollow Polymer Micropheres (HPS)
2.5. Fabrication of the Composite Membranes
2.6. Characterizations
2.6.1. Structural Characterizations
2.6.2. Determination of Water Uptake and Ionic Exchange Capacity (IEC)
2.6.3. Thermal Analysis of the Cast Membranes

2.6.4. Electrochemical Properties of the Composite Membranes
3. Results and Discussion
3.1. Characteristics of the HPS
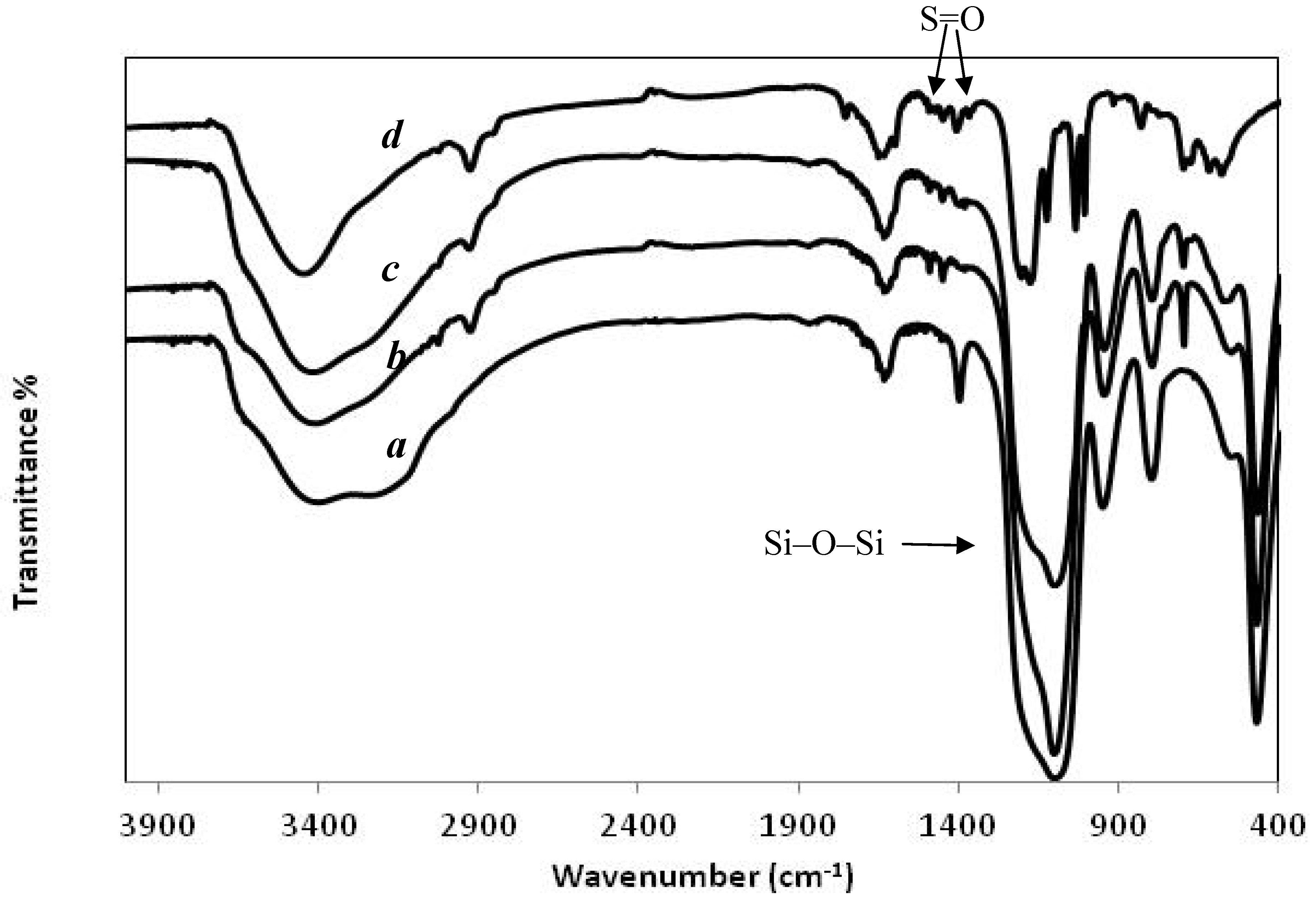

3.2. Broadening Hydrophilic Channel of Nafion by Hydrophilic HPS
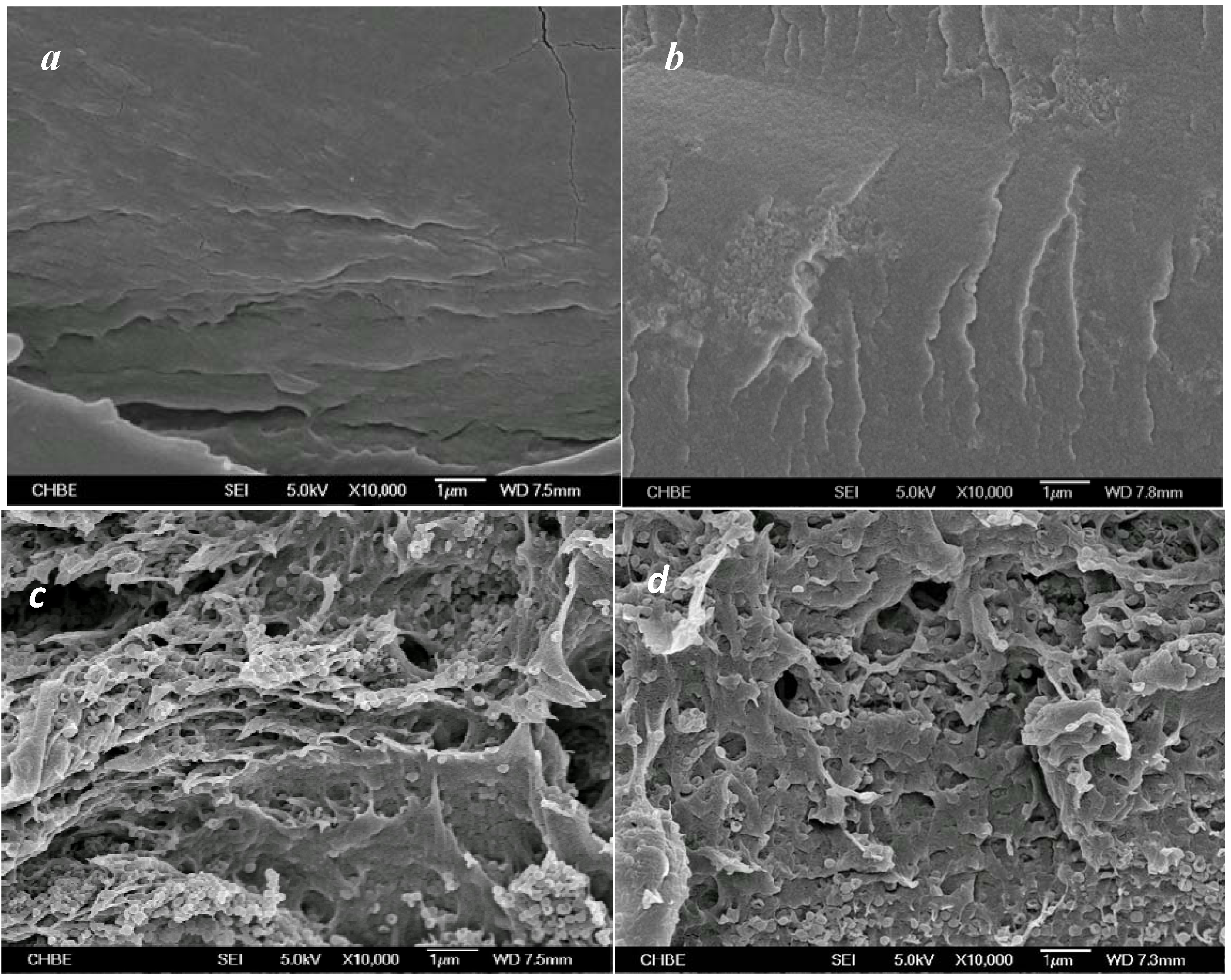
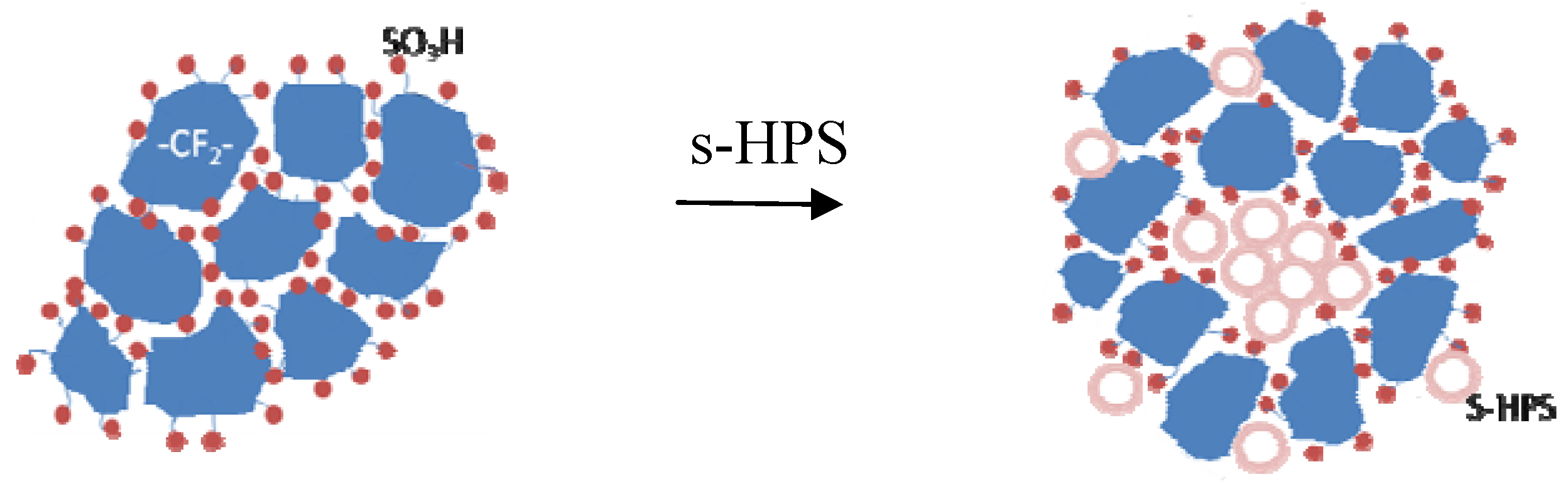
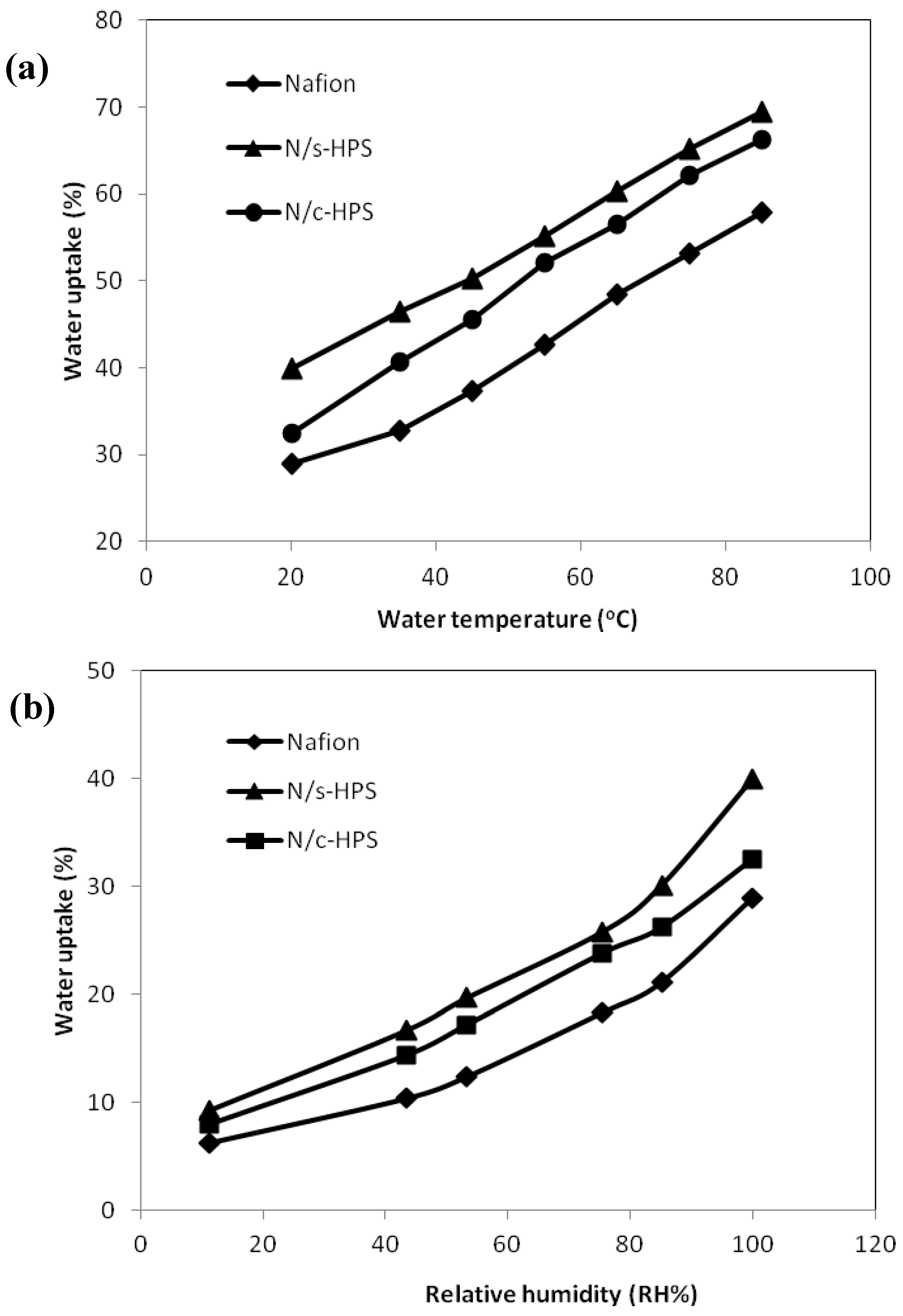
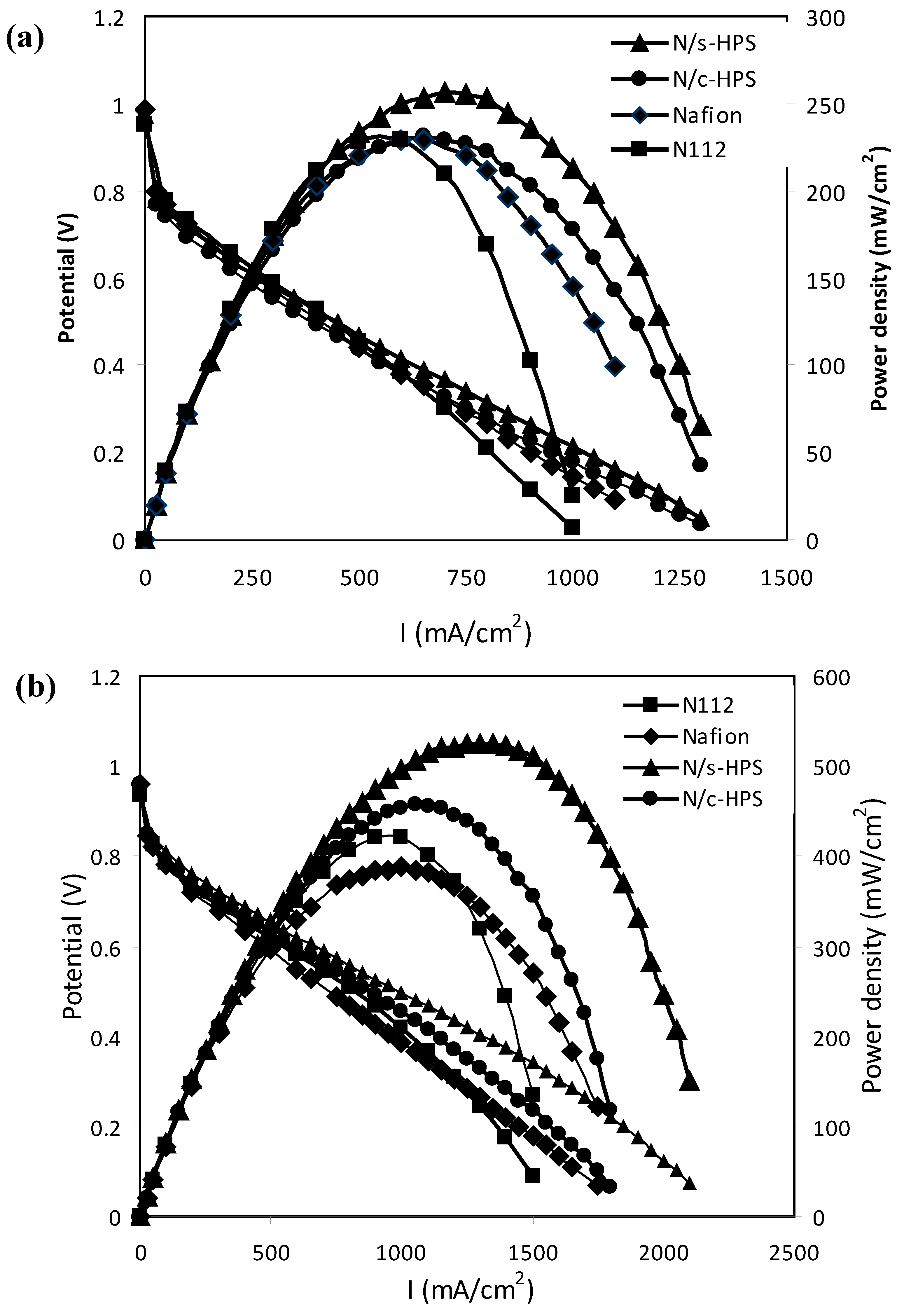
3.3. Effects of Water Micro-Reservoir in the Composite Membranes
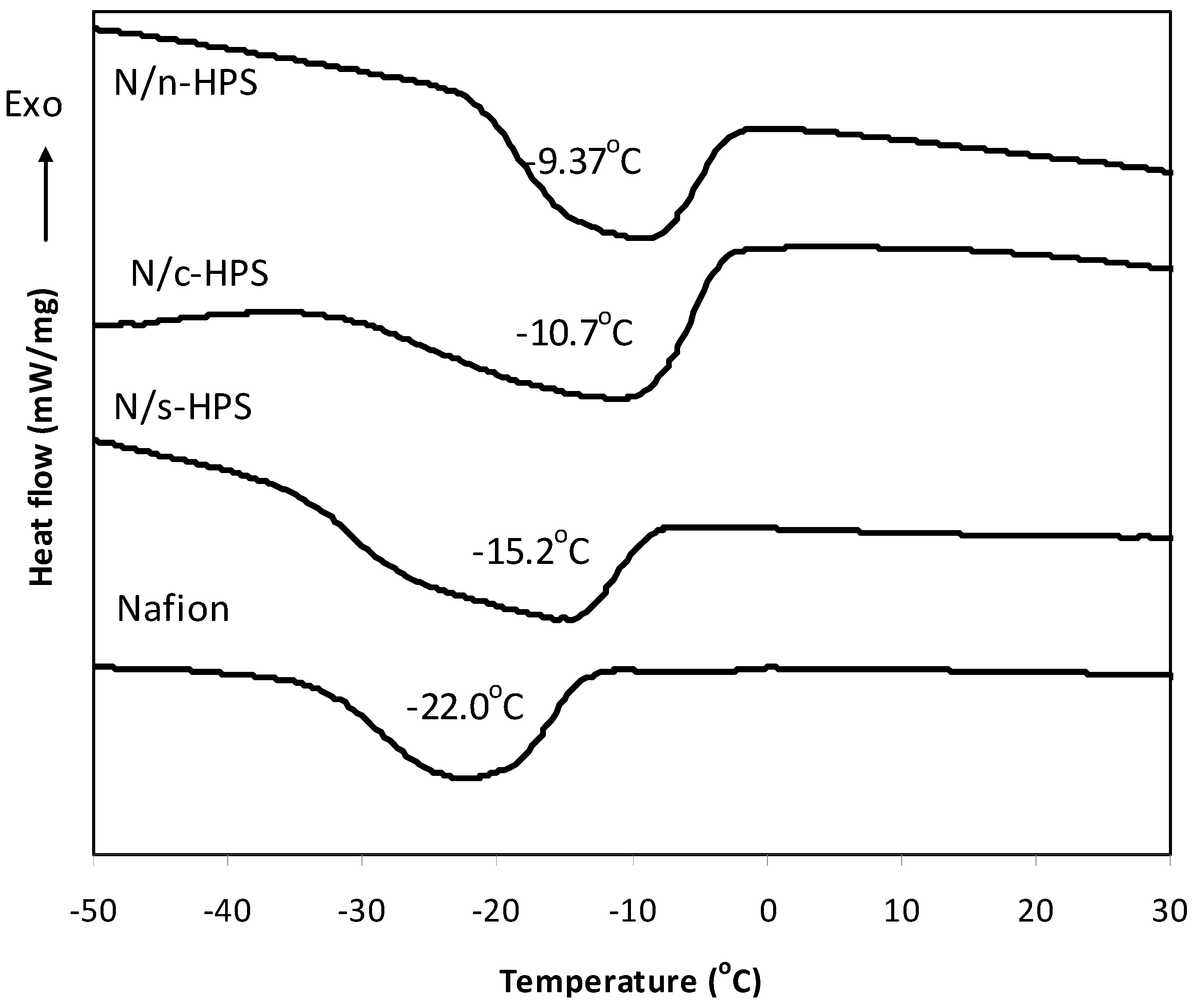
| Membrane | IEC (mmol/g) | Water uptake (%, 20 °C) | Melting peak (°C) | Freezable water (%) | Non Freezable water (%) |
|---|---|---|---|---|---|
| Nafion | 0.75 ± 0.01 | 28.9 ± 2.3 | −22.0 | 13.8 | 15.1 |
| N/n-HPS | 0.75 ± 0.03 | 30.1 ± 2.2 | −9.37 | 18.9 | 11.2 |
| N/c-HPS | 0.82 ± 0.02 | 32.5 ± 2.1 | −10.7 | 20.2 | 13.3 |
| N/s-HPS | 0.88 ± 0.06 | 40.0 ± 3.3 | −15.2 | 26.7 | 13.3 |
3.4. Influence of Moisture Level on Proton Transport in the Composite Membranes
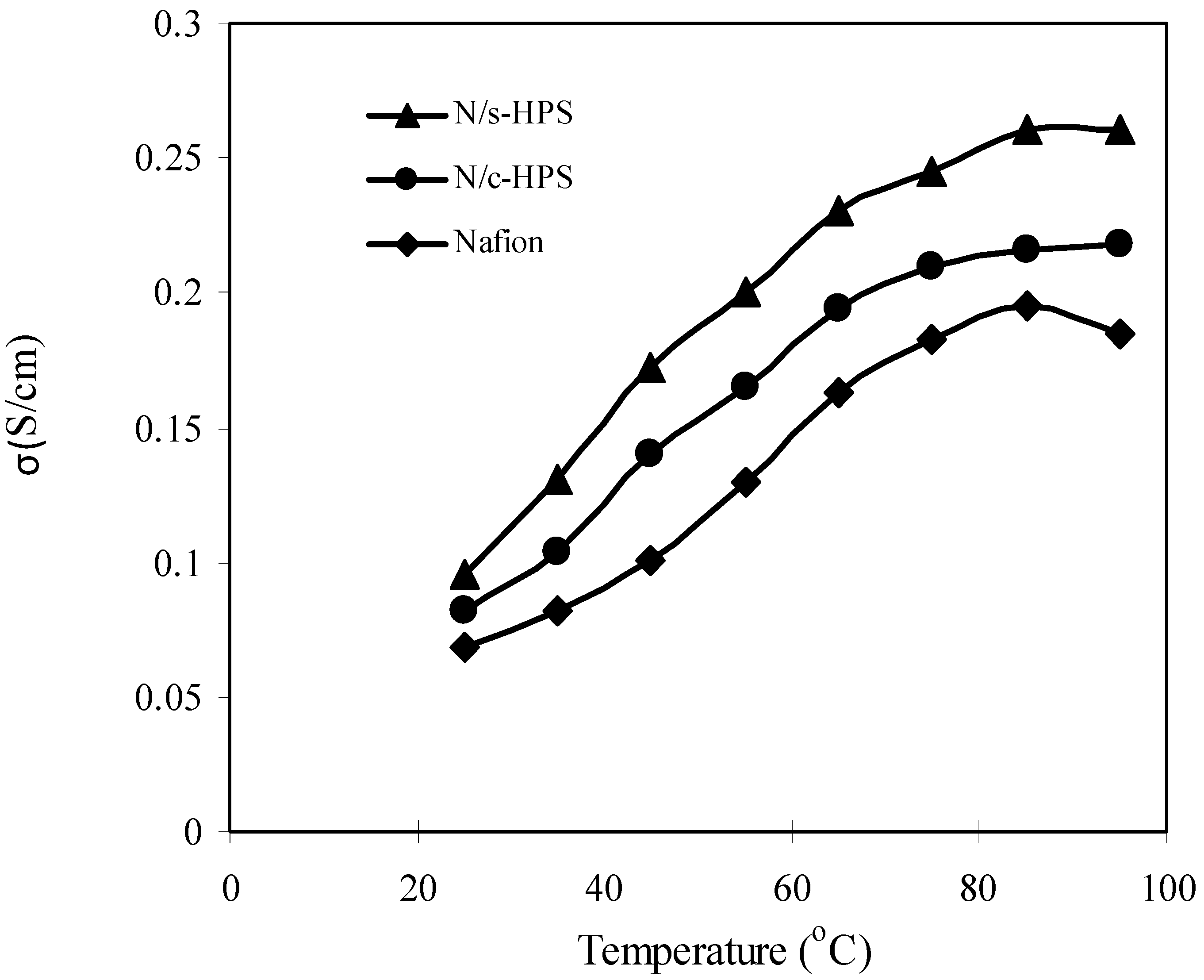
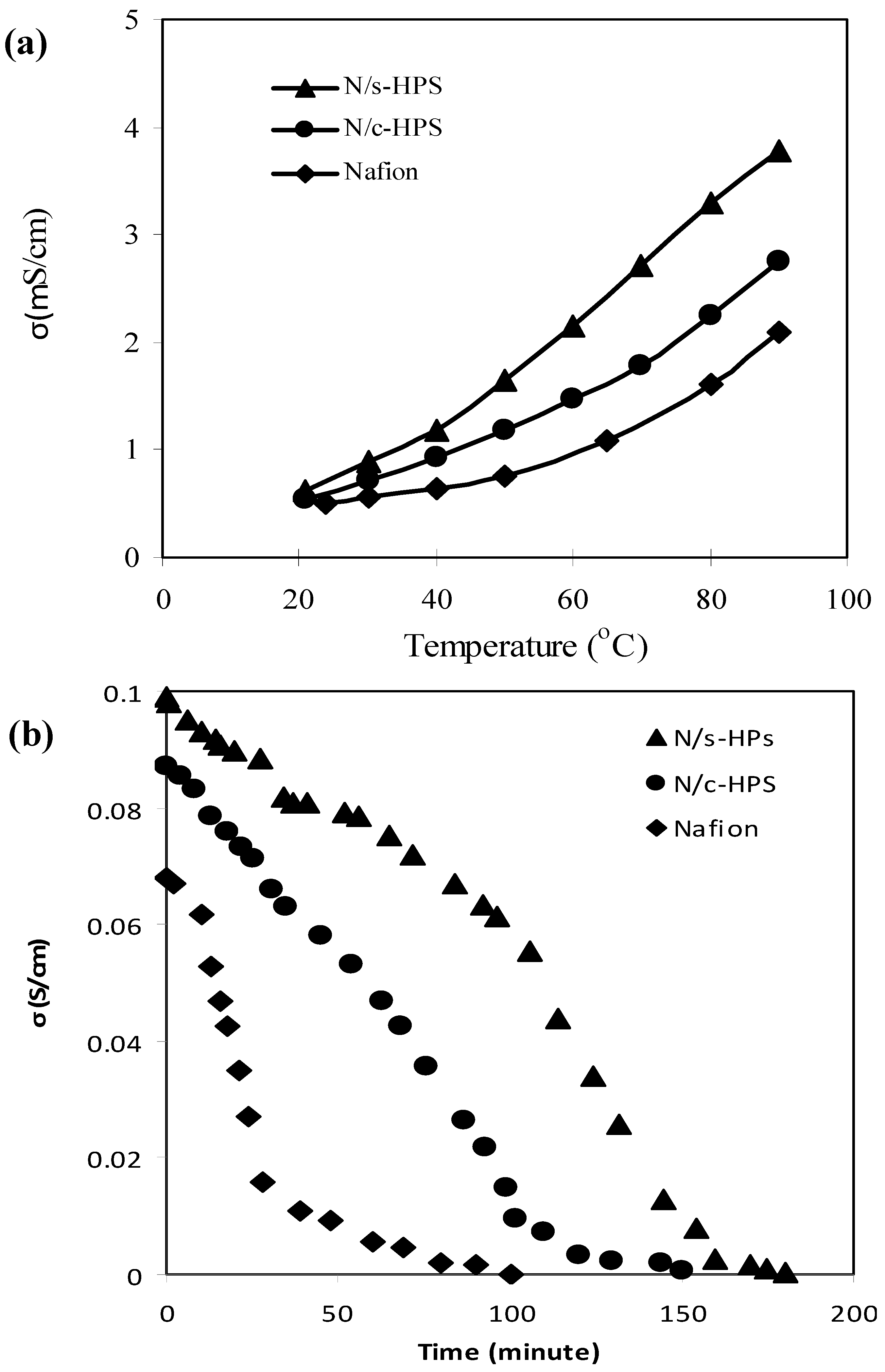
4. Conclusions
References
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energ. Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M. Composite membranes for medium-temperature PEM fuel cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Hickner, M.A. Ion-contaning polymers: New energy & clean water. Mater. Today 2010, 13, 34–41. [Google Scholar] [CrossRef]
- Lee, S.Y.; Scharfenberger, G.; Meyer, W.H.; Wegener, G. A novel water-free proton-conducting solid electrolyte based on an organic/inorganic hybrid. Adv. Mater. 2005, 17, 626–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.M.; Zhu, X.B.; Bi, C. Promotion of PEM self-humidifying effect by nanometer-sized sulfated zirconia-supported Pt catalyst hybrid with sulfonated poly(ether ether ketone). J. Phys. Chem.B 2007, 111, 6391–6399. [Google Scholar]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y.S. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Garrido, L.; Pozuelo, J.; López-González, M.; Fang, J.; Riande, E. Simulation and experimental studies on proton diffusion in polyelectrolytes based on sulfonated naphthalenic copolyimides. Macromolecules 2009, 42, 6572–6580. [Google Scholar]
- Park, M.J.; Downing, K.H.; Jackson, A. Increased water retention in polymer electrolyte membranes at elevated temperature assisted by capillary condensation. Nano Lett. 2007, 7, 3547–3552. [Google Scholar] [CrossRef]
- Shao, Z.G.; Joghee, P.; Hsing, M. Preparation and chacterization of hybrid Nafion-silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Membr. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Pereira, F.; Valle, K.; Belleville, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced mesostructured hybrid silica-Nafion membranes for high-performance PEM fuel cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; Leahy, J.; Srinivasan, S.; Benziger, J.B.; Bocarsly, A.B. Function and characterization of metak oxide-Nafion composite membranes for elevated-temperature H2/O2 PEM fuel cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar]
- Tay, S.W.; Zhang, X.H.; Liu, Z.L.; Hong, L. Composite Nafion® membrane embedded with hybrid nanofillers for promoting direct methanol fuel cell performance. J. Membr. Sci. 2008, 321, 139–145. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, L.; Liu, Z.; Lee, J.Y. Interfacial behavior of densely anchored hydrophilic oligomeric chains on silica microspheres. Colloid. Polym. Sci. 2008, 286, 1351–1360. [Google Scholar]
- Santiago, E.I.; Isidoro, R.A.; Dresch, M.A.; Matos, B.R.; Linardi, M.; Fonseca, F.C. Nafion-TiO2 hybrid electrolytes for stable operation of PEM fuel cells at high temperature. Electrochim. Acta. 2009, 54, 4111–4117. [Google Scholar]
- Yuan, J.J.; Zhou, G.B.; Pu, H.T. Preparation and properties of Nafion®/hollow silica spheres composite membranes. J. Membr. Sci. 2008, 325, 742–748. [Google Scholar] [CrossRef]
- Pu, H.T.; Wang, D.; Yang, Z.L. Towards high water retention of proton exchange membranes at elevated temperature via hollow nanospheres. J. Membr. Sci. 2010, 360, 123–129. [Google Scholar] [CrossRef]
- Lou, L.D.; Pu, H.T. preparation and properties of proton exchange membranes based on Nafion and phosphonic aicd-functionalized hollow silica spheres. Int. J. Hydro. Energy. 2011, 36, 3123–3130. [Google Scholar]
- Wang, J.T.; Zhang, H.; Yang, X.L.; Jiang, S.; Lv, W.J.; Jiang, Z.Y.; Qiao, S.Z. Enhanced water retention by using polymeric microcapsules to confer high proton conductivity on membranes at low humidity. Adv. Funct. Mater. 2011, 21, 971–978. [Google Scholar] [CrossRef]
- Yuan, J.J.; Pu, H.T.; Yang, Z.L. Studies on sulfonic acid functionalized hollow silica spheres (SAFHSS)/Nafion®112 composite proton exchange membranes. J. Polym. Sci. A Polym. Chem. 2009, 47, 2647–2655. [Google Scholar]
- Pu, H.T.; Lou, L.D.; Guan, Y.S.; Chang, Z.H. Proton exchange membranes based on semi-interpenetrating polymer networks of polybenzimidazole and perfluorosulfonic acid polymer with hollow silica spheres as micro-reservoir. J. Membr. Sci. 2012, in press. [Google Scholar]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Controlled Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Gemeinhart, R.A.; Chen, J.; Park, H.; Park, K. pH-sensitivity of fast responsive superporous hydrogels. J. Biomater. Sci. Polym. Ed. 2000, 11, 1371–1380. [Google Scholar] [CrossRef]
- Kannan, R.; Kakade, B.A.; Pillai, V.K. Polymer electrolyte fuel cells using Nafion-based composite membanes with functionalized carbon nanotubes. Angew. Chem. Int. Ed. 2008, 47, 2653–2656. [Google Scholar]
- Li, G.L.; Liu, G.; Kang, E.T.; Neoh, K.G.; Yang, X.L. pH-responsive hollow polymeric microspheres and concentric hollow silica microspheres from silica-polymer core-shell microspheres. Langmuir 2008, 24, 9050–9055. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodispersed silica spheres in the micron size. J. Colloid. Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lang, J. Encapsulation of inorganic particles by dispersion polymerization in polar media: 1. silica nanoparticles encapsulated by polystyrene. J. Colloid. Interface Sci. 1998, 197, 293–308. [Google Scholar] [CrossRef]
- lvarez-Gallego, Y.; de Heer, M.P. Sub-freezing conductivity of PFSA membranes. Fuel Cells 2009, 9, 421–431. [Google Scholar] [CrossRef]
- Saito, M.; Hayamizu, K.; Okada, T. Temperature dependence of ion and water transport in perfluorinated ionomer membranes for fuel cells. J. Phys. Chem. B 2005, 109, 3112–3119. [Google Scholar] [CrossRef]
- Hou, J.; Yu, H.; Wang, L.; Zhou, D.X.; Ming, P.; Shao, Z.; Yi, B. Conductivity of aromatic-based proton exchange membranes at subzero temperatures. J. Power Sources 2008, 180, 232–237. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Z.L.; Hong, L. Doping Nafion® matrix by p-aramide flakes for a proton transport less reliance on moisture. J. Mater. Chem. 2011, 21, 12414–12421. [Google Scholar]
- Guo, B.; Liu, Z.L.; Hong, L. Substituted poly(p-phenylene) oligomer as a physical crosslinker in Nafion® membrane. J. Membr. Sci. 2011, 379, 279–286. [Google Scholar] [CrossRef]
- Tsang, E.M.W.; Zhang, Z.; Shi, Z.; Soboleva, T.; Holdcroft, S. Considerations of macromolecular structure in the design of proton conducting polymer membranes: Graft versus diblock polyelectrolytes. J. Am. Chem. Soc. 2007, 129, 15106–15107. [Google Scholar]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of proton conductance in polymer electrolyte membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef]
- Kornshev, A.A.; Kuznetsov, A.M.; Spohr, E.; Ulstrup, J. Kinetics of proton transport in water. J. Phys. Chem. B 2003, 107, 3351–3366. [Google Scholar]
- Spohr, E.; Commer, P.; Kornyshev, A.A. Enhancing proton mobility in polymer electrolyte membranes: lessopns from molecular dynamics simulations. J. Phys. Chem. B 2002, 106, 10560–10569. [Google Scholar] [CrossRef]
- Kalapos, T.L.; Decker, B.; Every, H.A.; Ghassemi, H.; Zawodzinski, T.A., Jr. Thermal studies of the state of water in proton conducting fuel cell membranes. J. Power Sources 2007, 172, 14–19. [Google Scholar] [CrossRef]
- Lue, S.J.; Shieh, S.J. Water states in perflurosulfonic acid membranes using differential scanning calorimetry. J. Macromol. Sci. B Phys. 2009, 48, 114–127. [Google Scholar] [CrossRef]
- Chen, W.F.; Kuo, P.L. Covalently cross-linked perfluorosulfonated membranes with polysiloxane framework. Macromolecules 2007, 40, 1987–1994. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Satoh, M.; Komiyama, J. A differential scanning calorimetry study of the states of water in swollen poly(vinyl alcohol) membranes containing nonvolatile additives. J. Membr. Sci. 1989, 42, 303–314. [Google Scholar] [CrossRef]
- Gupta, B.; Büchi, F.N.; Staub, M.; Grman, D.; Scherer, G.G. Cation exchange membranes by pre-irradiation grafting of styrene into FEP films. II. Properties of copolymer membranes. J. Polym. Sci. A Polym. Chem. 1996, 34, 1873–1880. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.B.; Chung, Y.S.; Lee, Y.M.; Freeman, B.D. Water sorption, proton conduction, and methanol permeation properties of sulfonated polyimide membranes cross-linked with N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES). Macromolecules 2006, 39, 755–764. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.L.; Sun, Y.M.; Lai, J.Y.; Wang, D.M.; Gao, Y.; Liu, B.; Guiver, M.D. Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Membr. Sci. 2007, 296, 21–28. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. Proton-conducting aromatic polymers carrying hypersulfonated side chains for fuel cell applications. Adv. Funct. Mater. 2007, 17, 2823–2834. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenolog. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, B.; Tay, S.W.; Liu, Z.; Hong, L. Embedding of Hollow Polymer Microspheres with Hydrophilic Shell in Nafion Matrix as Proton and Water Micro-Reservoir. Polymers 2012, 4, 1499-1516. https://doi.org/10.3390/polym4031499
Guo B, Tay SW, Liu Z, Hong L. Embedding of Hollow Polymer Microspheres with Hydrophilic Shell in Nafion Matrix as Proton and Water Micro-Reservoir. Polymers. 2012; 4(3):1499-1516. https://doi.org/10.3390/polym4031499
Chicago/Turabian StyleGuo, Bing, Siok Wei Tay, Zhaolin Liu, and Liang Hong. 2012. "Embedding of Hollow Polymer Microspheres with Hydrophilic Shell in Nafion Matrix as Proton and Water Micro-Reservoir" Polymers 4, no. 3: 1499-1516. https://doi.org/10.3390/polym4031499
APA StyleGuo, B., Tay, S. W., Liu, Z., & Hong, L. (2012). Embedding of Hollow Polymer Microspheres with Hydrophilic Shell in Nafion Matrix as Proton and Water Micro-Reservoir. Polymers, 4(3), 1499-1516. https://doi.org/10.3390/polym4031499




