Reverse Design of High Strength and High Modulus Epoxy Resin Systems Through Computational Modeling with Experimental Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Design
- (1)
- Formulation Prediction: The database of the AI polymer platform covers a large amount of structural property data of polymer materials and uses machine learning to construct a structure–performance quantitative prediction model, which realizes the simultaneous prediction of tensile strength and elastic modulus by capturing the constitutive relationship between the topological features of the functional groups and mechanical response. When the target strength (100 MPa) and target modulus (4 GPa) were entered, the platform reverse engineers the resin system to meet the target performance using a virtual design-high-throughput-screening method. The resulting resin formulation is shown in Table 1.
- (2)
- Regularity Analysis: According to Table 1, the essential design principles for the epoxy resin and curing agents are described as follows:
- (a)
- Multifunctional epoxy groups: Incorporating epoxy resins with multiple functional groups enhances the reactivity, facilitating the formation of a dense crosslinked network that simultaneously improves the tensile strength and tensile modulus.
- (b)
- Rigid organic structural motifs: Integrating rigid structural elements, such as aromatic rings and biphenyl frameworks, into the molecular structure of resins and curing agents increases their stiffness and resistance to deformation, thereby boosting both the tensile strength and tensile modulus.
- (c)
- Strong polar interactions: The inclusion of polar functional groups (e.g., hydroxyl and amino) enhances intermolecular hydrogen bonding and dipole–dipole interactions, thereby strengthening interfacial adhesion and enhancing modulus.
- (3)
- Preliminary Formulation: Based on the above screening criteria and principles, TDE-85 was selected as the epoxy matrix and MPD was used as the primary curing agent. TDE-85 was chosen for its trifunctional glycidyl ether structure, which enhances crosslinking density and chain rigidity, leading to improved mechanical properties. In addition, low viscosity favors effective impregnation during the processing of carbon-fiber-reinforced composites. MPD contains a rigid aromatic amine structure, which helps maintain high modulus while controlling curing reaction rates to prevent the formation of defects.
- (4)
- Formulation Optimization: However, a two-component resin system consisting of TDE-85 and MPD may face the following issues:
- (a)
- Uncontrolled exothermic reactions: The curing profile of a two-component system faces rapid curing and the concentration of internal stress, which likely leads to the formation of defects and the deterioration of mechanical properties.
- (b)
- High brittleness: The rigid structure of both TDE-85 and MPD leads to a brittle feature of the cured system with limited toughness, which is undesirable for applications in industrial sectors that require both enhanced durability and resistance to damage.
- (c)
- Poor processability: The viscosity of TDE-85 is suitable for the impregnation of prepregs, but it is still challenging due to the rigidity and high reactivity of the reaction system.
2.3. Preparation of Epoxy Resin
2.4. Characterizations
3. Results
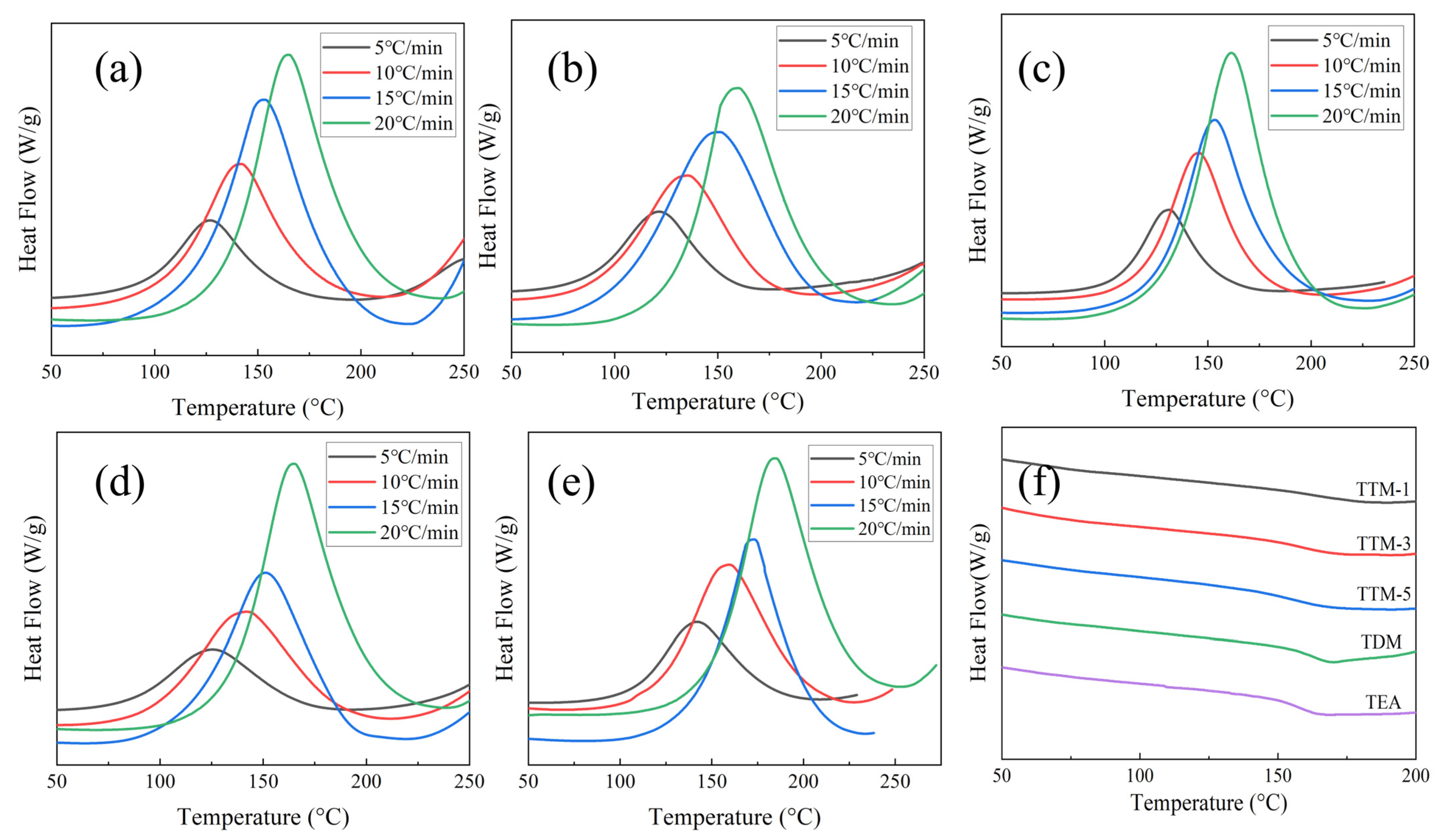
3.1. Curing Behavior of Resin Systems
3.2. FTIR Spectra
3.3. Viscosity Analysis
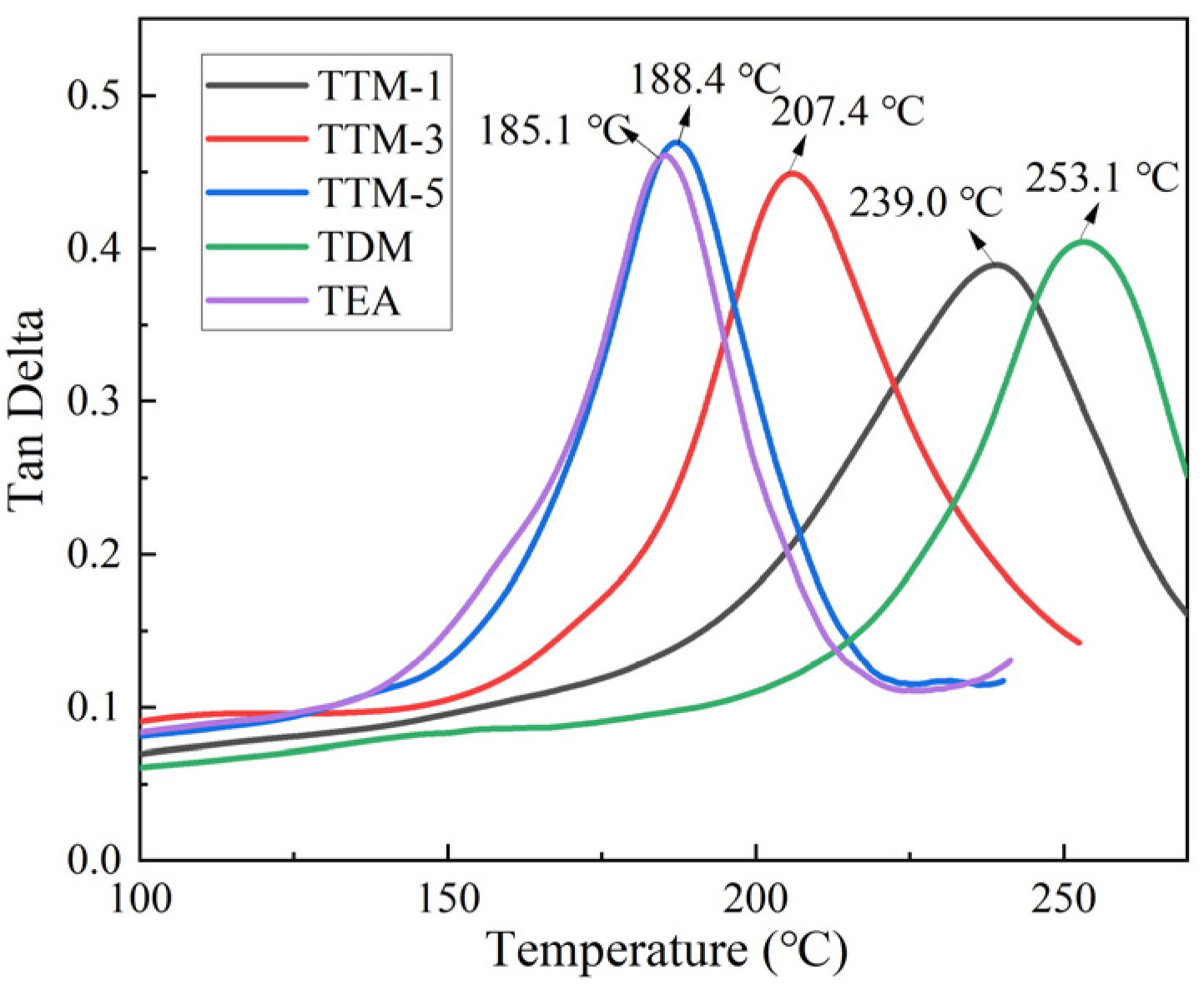
3.4. Thermal Properties
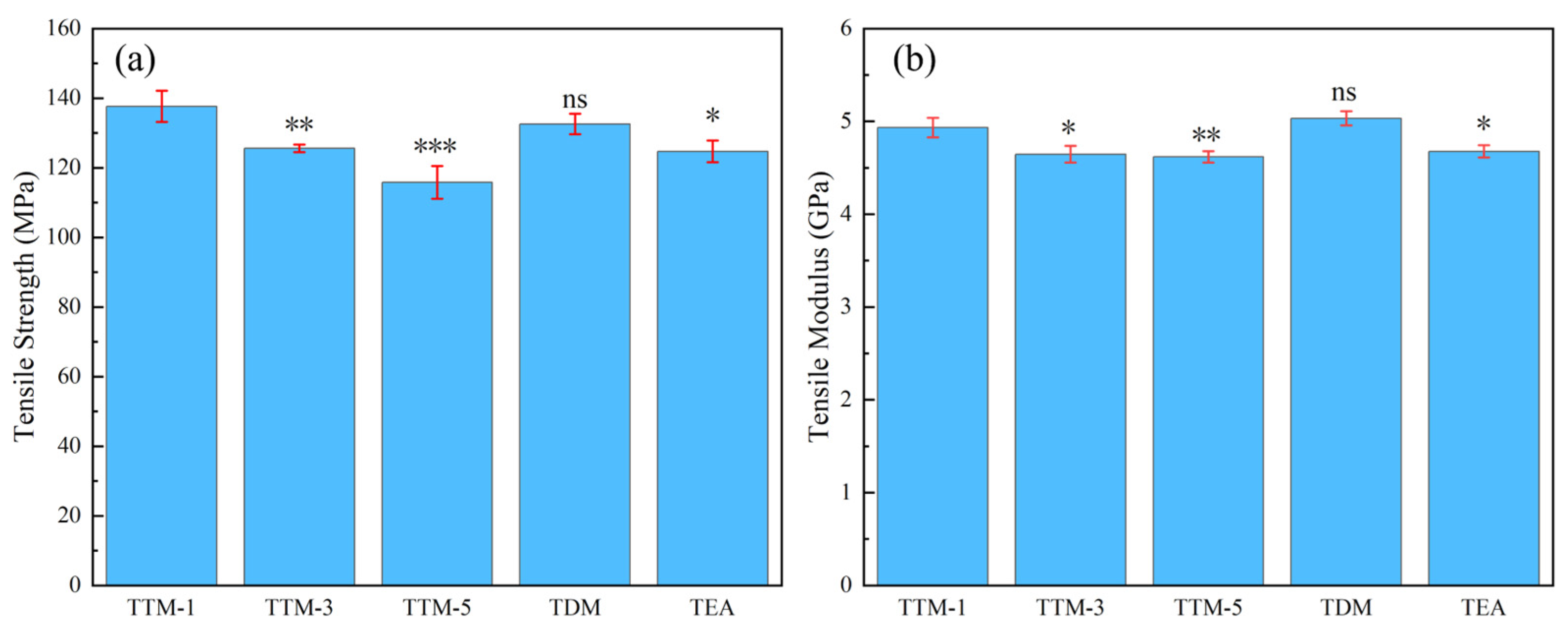
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Lu, Y.; Su, Z.; Meng, G. Functionalized composite structures for new generation airframes: A review. Compos. Sci. Technol. 2005, 65, 1436–1446. [Google Scholar] [CrossRef]
- Sun, Z.; Luo, Y.; Chen, C.; Dong, Z.; Jiang, G.; Chen, F.; Ma, P. Mechanical enhancement of carbon fiber-reinforced polymers: From interfacial regulating strategies to advanced processing technologies. Prog. Mater. Sci. 2024, 142, 101221. [Google Scholar] [CrossRef]
- Yuan, Y.; Yao, X.; Niu, K.; Liu, B.; Wuyun, Q. Compressive failure of fiber reinforced polymer composites by imperfection. Compos. Part A Appl. Sci. Manuf. 2019, 118, 106–116. [Google Scholar] [CrossRef]
- Nunna, S.; Ravindran, A.R.; Mroszczok, J.; Creighton, C.; Varley, R.J. A review of the structural factors which control compression in carbon fibres and their composites. Compos. Struct. 2023, 303, 116293. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Y.; Liu, D.; He, M.; Li, G.; Yang, X. Enhanced interfacial and mechanical properties of high-modulus carbon fiber composites: Establishing modulus intermediate layer between fiber and matrix based on tailored-modulus epoxy. Compos. Sci. Technol. 2018, 163, 26–33. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Lin, J.-Y.; Chen, W.-C.; Gamal Mohamed, M.; Huang, C.-F.; Chen, J.-H.; Kuo, S.W. High-Thermal Stable Epoxy Resin through Blending Nanoarchitectonics with Double-Decker-Shaped Polyhedral Silsesquioxane-Functionalized Benzoxazine Derivatives. Polymers 2024, 16, 112. [Google Scholar] [CrossRef]
- Pouladvand, A.R.; Mortezaei, M.; Fattahi, H.; Amraei, I.A. A novel custom-tailored epoxy prepreg formulation based on epoxy-amine dual-curable systems. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105852. [Google Scholar] [CrossRef]
- Qiao, F.; Ji, J.; Zhao, C.; Wang, F.; Deng, S. Synthesis of star-shaped epoxy resin with excellent mechanical properties using triazine as a skeleton. J. Appl. Polym. Sci. 2024, 141, 55763. [Google Scholar] [CrossRef]
- Budelmann, D.; Schmidt, C.; Meiners, D. Tack of epoxy resin films for aerospace-grade prepregs: Influence of resin formulation, B-staging and toughening. Polym. Test. 2022, 114, 107709. [Google Scholar] [CrossRef]
- Liu, J.; Cui, X.; Qin, J.; Shi, M.; Wang, D.; Yang, L.; Lyu, M.; Liang, L. Ultra-high cross-linked active ester-cured epoxy resins: Side group cross-linking for performance enhancement. Polymer 2024, 301, 127063. [Google Scholar] [CrossRef]
- Shields, B.J.; Stevens, J.; Li, J.; Parasram, M.; Damani, F.; Alvarado, J.I.M.; Janey, J.M.; Adams, R.P.; Doyle, A.G. Bayesian reaction optimization as a tool for chemical synthesis. Nature 2021, 590, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Seisdedos, G.; Prisbrey, M.G.; Vakhlamov, P.; Fernandez, J.; De Freitas, R.; Rockward, T.; Davis, E.S. Data-Driven Tailoring Optimization of Thermoset Polymers Using Ultrasonics and Machine Learning. Polymers 2025, 17, 895. [Google Scholar] [CrossRef] [PubMed]
- Alimirzaei, S.; Ahmadi Najafabadi, M.; Nikbakht, A. Investigation of Mechanical Properties and Failure Behavior of CFRP Filament-Wound Composites Using an Acoustic Emission-Based Methodology and Numerical Simulation. Fibers Polym. 2023, 24, 693–707. [Google Scholar] [CrossRef]
- Pai, S.M.; Shah, K.A.; Sunder, S.; Albuquerque, R.Q.; Brütting, C.; Ruckdäschel, H. Machine learning applied to the design and optimization of polymeric materials: A review. Next Mater. 2025, 7, 100449. [Google Scholar] [CrossRef]
- Zhou, J.; He, J.; Wang, L.; Wang, Y.; Sun, T.; Zhang, H.; Heng, Z.; Chen, Y.; Zou, H.; Liang, M. Molecular mechanics-based design of high-modulus epoxy to enhance composite compressive properties. Compos. Sci. Technol. 2022, 229, 109678. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Lin, J.; Du, L. An Intelligent Manufacturing Platform of Polymers: Polymeric Material Genome Engineering. Engineering 2023, 27, 31–36. [Google Scholar] [CrossRef]
- Zhang, S.; Du, S.; Wang, L.; Lin, J.; Du, L.; Xu, X.; Gao, L. Design of silicon-containing arylacetylene resins aided by machine learning enhanced materials genome approach. Chem. Eng. J. 2022, 448, 137643. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, X.; Lan, H.; Wang, L.; Lin, J.; Du, L.; Zhang, C.; Tian, X. Designing Multicomponent Thermosetting Resins through Machine Learning and High-Throughput Screening. Macromolecules 2025, 58, 744–753. [Google Scholar] [CrossRef]
- Dispenza, C.; Carter, J.T.; McGrail, P.T.; Spadaro, G. Cure behaviour of epoxy resin matrices for carbon fibre composites. Polym. Int. 1999, 48, 1229–1236. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Kaelble, D.H.; Smith, T. Analysis of curing kinetics in polymer composites. J. Polym. Sci. Polym. Lett. Ed. 1974, 12, 473–475. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Ilyin, S.O.; Kostyuk, A.V.; Bondarenko, G.N.; Antonov, S.V. Acceleration of Epoxy Resin Curing by Using a Combination of Aliphatic and Aromatic Amines. Polym. Bull. 2020, 77, 1519–1540. [Google Scholar] [CrossRef]
- Tanks, J.; Arao, Y.; Kubouchi, M. Network-level analysis of damage in amine-crosslinked diglycidyl ether resins degraded by acid. Express Polym. Lett. 2022, 16, 488–499. [Google Scholar] [CrossRef]
- Bratasyuk, N.A.; Zuev, V.V. The study of the curing mechanism, kinetic and mechanical performance of polyurethane/epoxy composites using aliphatic and aromatic amines as curing agents. Thermochim. Acta 2020, 687, 178598. [Google Scholar] [CrossRef]
- Demir, B.; Henderson, L.C.; Walsh, T.R. Design Rules for Enhanced Interfacial Shear Response in Functionalized Carbon Fiber Epoxy Composites. ACS Appl. Mater. Interfaces 2017, 9, 11846–11857. [Google Scholar] [CrossRef]
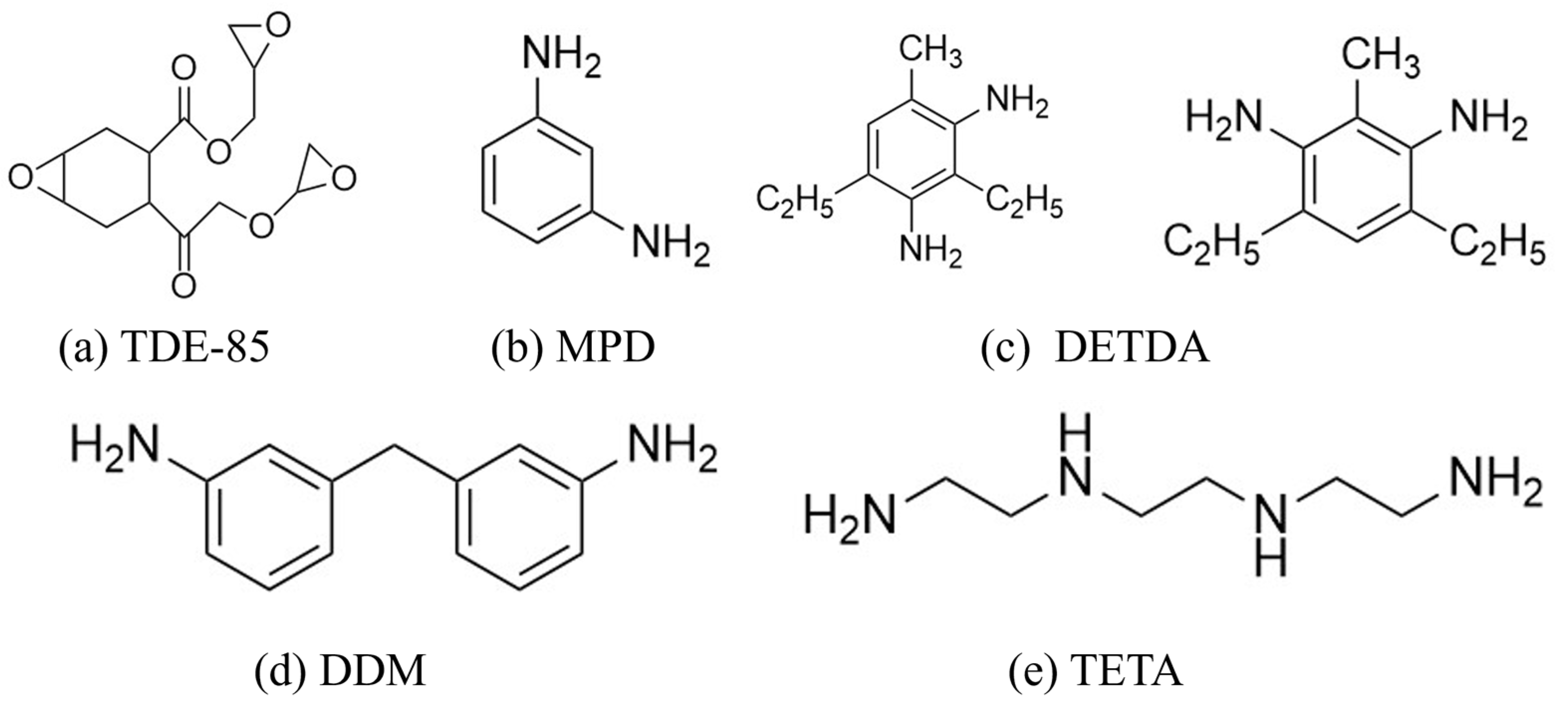


| No. | Epoxy Resin | Curing Agent | Tensile Strength (MPa) | Tensile Modulus (GPa) |
|---|---|---|---|---|
| 1 | 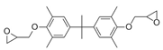 |  | 118.2 ± 40.7 | 4.3 ± 1.5 |
| 2 | 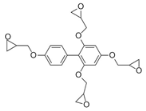 | 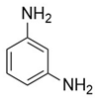 | 101.5 ± 44.8 | 4.3 ± 1.6 |
| 3 | 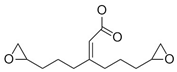 |  | 105.3 ± 42.3 | 4.0 ± 2.1 |
| 4 |  |  | 100.5 ± 37.8 | 4.1 ± 1.4 |
| 5 | 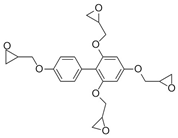 |  | 120.2 ± 41.4 | 4.4 ± 1.5 |
| Sample ID | TDE-85 (g) | MPD (g) | The Secondary Curing Agent | Content of the Secondary Curing Agent (mol%) | ||
|---|---|---|---|---|---|---|
| DETDA/g | DDM/g | TETA/g | ||||
| TTM-1 | 100 | 19.41 | 4.6 | 10 | ||
| TTM-3 | 100 | 13.5 | 9.5 | 30 | ||
| TTM-5 | 100 | 8.7 | 14.3 | 50 | ||
| TDM | 100 | 19.1 | 3.9 | 10 | ||
| TEA | 100 | 20.0 | 3.0 | 10 | ||
| Method | TTM-1 | TTM-3 | TTM-5 | TDM | TEA |
|---|---|---|---|---|---|
| Kissinger Ea (kJ/mol) | 49.33 | 68.97 | 60.40 | 55.47 | 43.96 |
| Ozawa Ea (kJ/mol) | 53.63 | 49.35 | 64.02 | 59.94 | 48.81 |
| Average Ea (kJ/mol) | 51.48 | 59.16 | 62.21 | 57.71 | 46.39 |
| n | 0.912 | 1.140 | 0.924 | 0.921 | 0.903 |
| System | β (°C·min−1) | Tp (K) | ln(β/Tp2) | lnβ | 103/Tp/K−1 |
|---|---|---|---|---|---|
| TTM-1 | 5 | 399.00 | −10.36 | 1.60 | 2.50 |
| 10 | 415.87 | −9.76 | 2.30 | 2.40 | |
| 15 | 428.29 | −9.41 | 2.70 | 2.33 | |
| 20 | 435.09 | −9.16 | 3.00 | 2.30 | |
| TTM-3 | 5 | 394.74 | −10.34 | 1.60 | 2.53 |
| 10 | 410.33 | −9.73 | 2.30 | 2.43 | |
| 15 | 420.15 | −9.37 | 2.70 | 2.38 | |
| 20 | 432.63 | −9.14 | 3.00 | 2.31 | |
| TTM-5 | 5 | 403.69 | −10.39 | 1.60 | 2.48 |
| 10 | 417.92 | −9.76 | 2.30 | 2.39 | |
| 15 | 426.07 | −9.40 | 2.71 | 2.34 | |
| 20 | 434.06 | −9.15 | 3.00 | 2.30 | |
| TDM | 5 | 404.84 | −10.40 | 1.61 | 2.47 |
| 10 | 421.51 | −9.79 | 2.30 | 2.37 | |
| 15 | 428.18 | −9.41 | 2.71 | 2.34 | |
| 20 | 438.40 | −9.17 | 3.00 | 2.28 | |
| TEA | 5 | 414.95 | −10.45 | 1.61 | 2.41 |
| 10 | 431.02 | −9.83 | 2.30 | 2.32 | |
| 15 | 445.29 | −9.49 | 2.71 | 2.25 | |
| 20 | 457.62 | −9.26 | 3.00 | 2.19 |
| Blended System/Reference | Tensile Strength (MPa) | Tensile Modulus (GPa) | Tg (°C) | Volume Density (g/cm3) |
|---|---|---|---|---|
| TTM-1 | 137.7 ± 4.5 | 4.96 ± 0.06 | 239.0 | 1.30 |
| TTM-3 | 125.6 ± 1.1 | 4.67 ± 0.05 | 207.4 | 1.29 |
| TTM-5 | 115.8 ± 4.7 | 4.61 ± 0.06 | 188.4 | 1.28 |
| TDM | 132.6 ± 3.2 | 5.06 ± 0.07 | 253.1 | 1.32 |
| TEA | 124.7 ± 3.1 | 4.71 ± 0.08 | 185.1 | 1.28 |
| [8] | 134.72 | 5.04 | 155.5 | - |
| [15] | 86.4 | 6.46 | 218.0 | - |
| [18] | 132.79 | 4.49 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zhu, S.; Zhang, B.; Lv, H.; Wu, J.; Yang, Y.; Zhang, B.; Gao, J. Reverse Design of High Strength and High Modulus Epoxy Resin Systems Through Computational Modeling with Experimental Validation. Polymers 2025, 17, 1214. https://doi.org/10.3390/polym17091214
Tang Y, Zhu S, Zhang B, Lv H, Wu J, Yang Y, Zhang B, Gao J. Reverse Design of High Strength and High Modulus Epoxy Resin Systems Through Computational Modeling with Experimental Validation. Polymers. 2025; 17(9):1214. https://doi.org/10.3390/polym17091214
Chicago/Turabian StyleTang, Yilin, Shipeng Zhu, Boya Zhang, Haozhong Lv, Jingshu Wu, Yunhua Yang, Ben Zhang, and Jianli Gao. 2025. "Reverse Design of High Strength and High Modulus Epoxy Resin Systems Through Computational Modeling with Experimental Validation" Polymers 17, no. 9: 1214. https://doi.org/10.3390/polym17091214
APA StyleTang, Y., Zhu, S., Zhang, B., Lv, H., Wu, J., Yang, Y., Zhang, B., & Gao, J. (2025). Reverse Design of High Strength and High Modulus Epoxy Resin Systems Through Computational Modeling with Experimental Validation. Polymers, 17(9), 1214. https://doi.org/10.3390/polym17091214






