Preparation and Characterization of Fenofibrate-Loaded Fibers Based on 2-Hydroxylpropyl-β-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fiber Preparation by Electrospinning
2.3. Scanning Electron Microscopy (SEM)
2.4. Viscosity Measurement
2.5. Conductivity Measurements
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Drug Content Determination of the FEN-Loaded Fibers
2.8. Differential Scanning Calorimetry (DSC)
2.9. In Vitro Dissolution Tests
3. Results
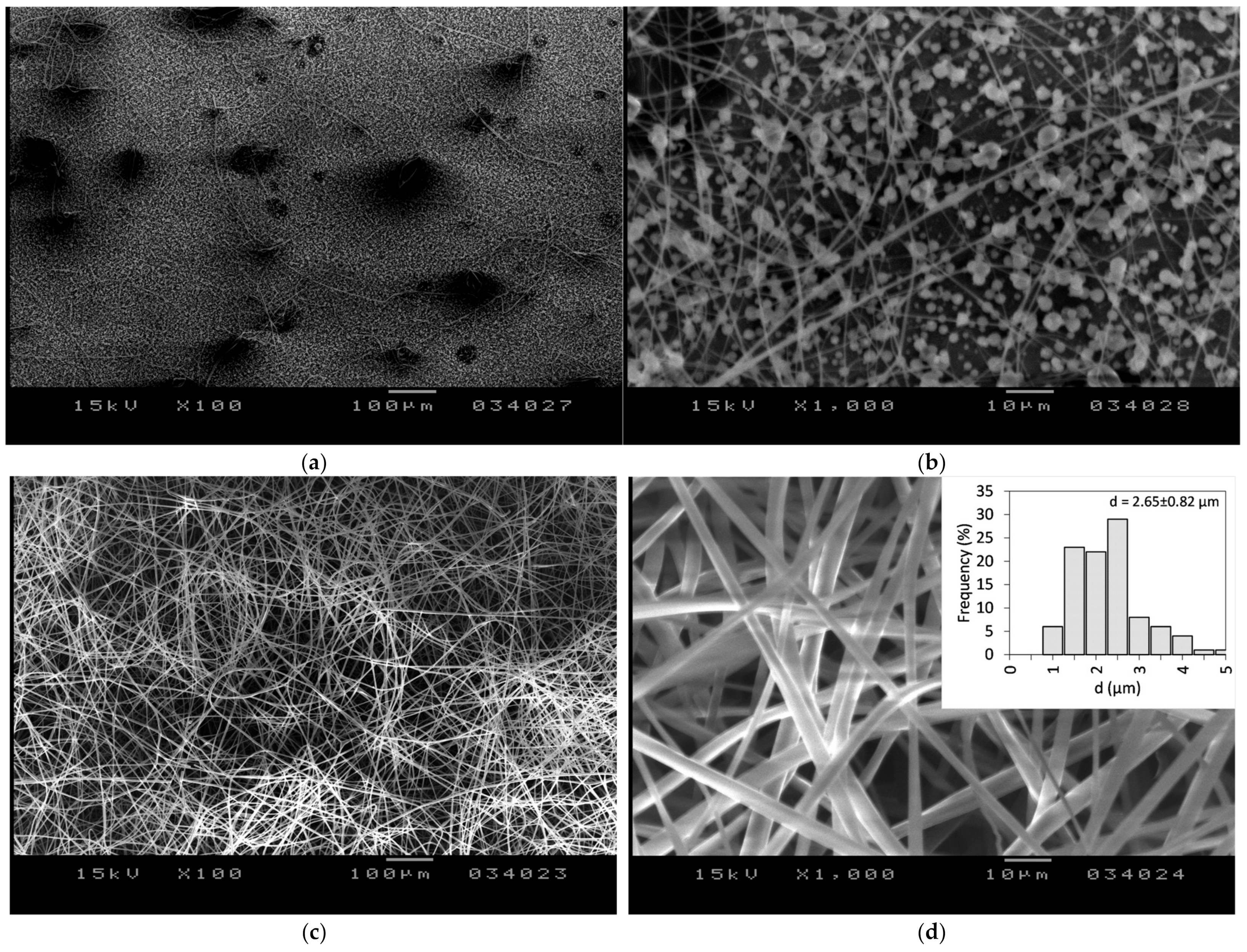
3.1. Fiber Morphology
3.2. Determination of the FEN Content of the Nanofibers
3.3. Thermoanalytical Studies
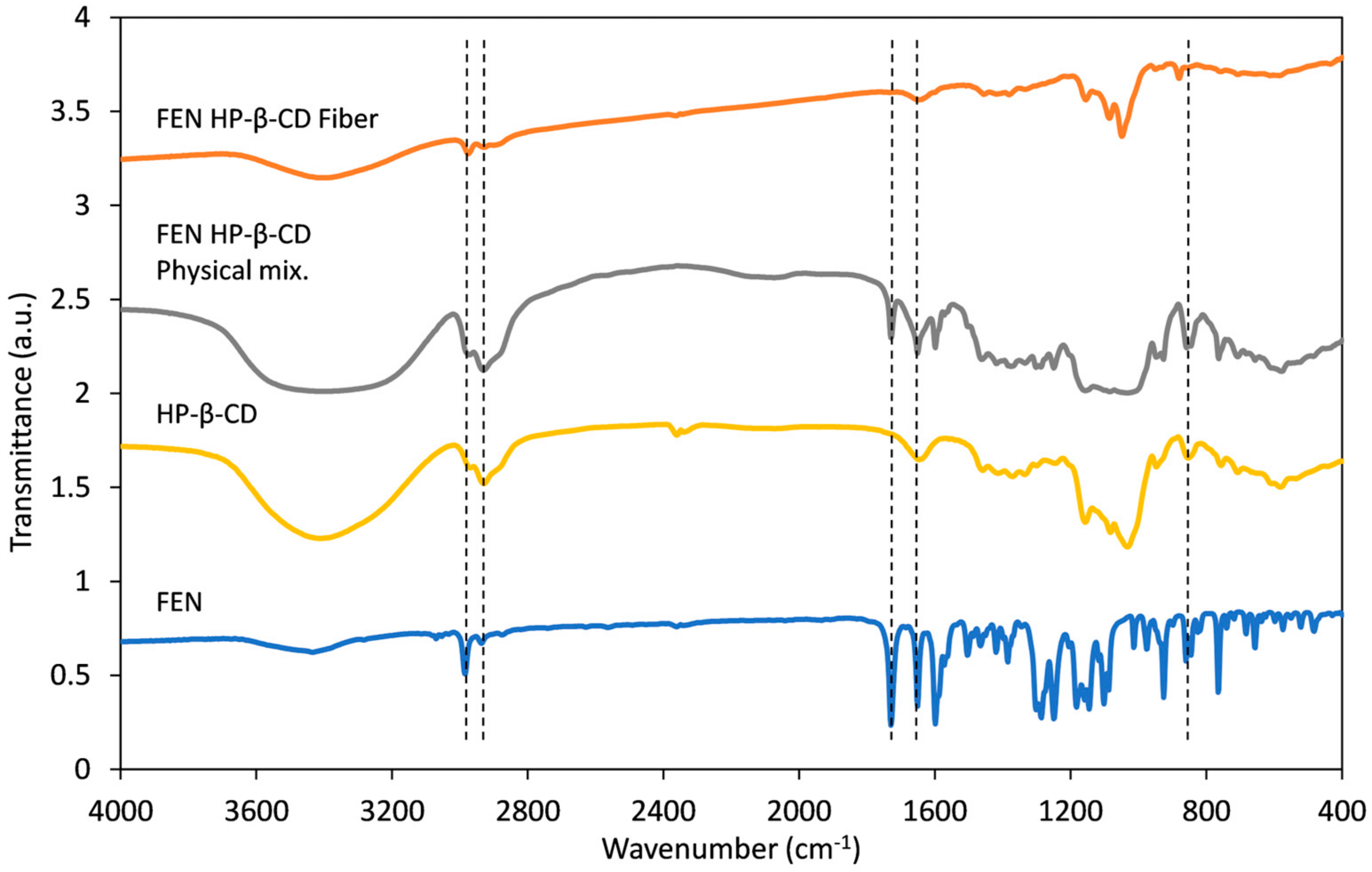
3.4. Fourier Transform Infrared (FT-IR) Spectroscopy Studies
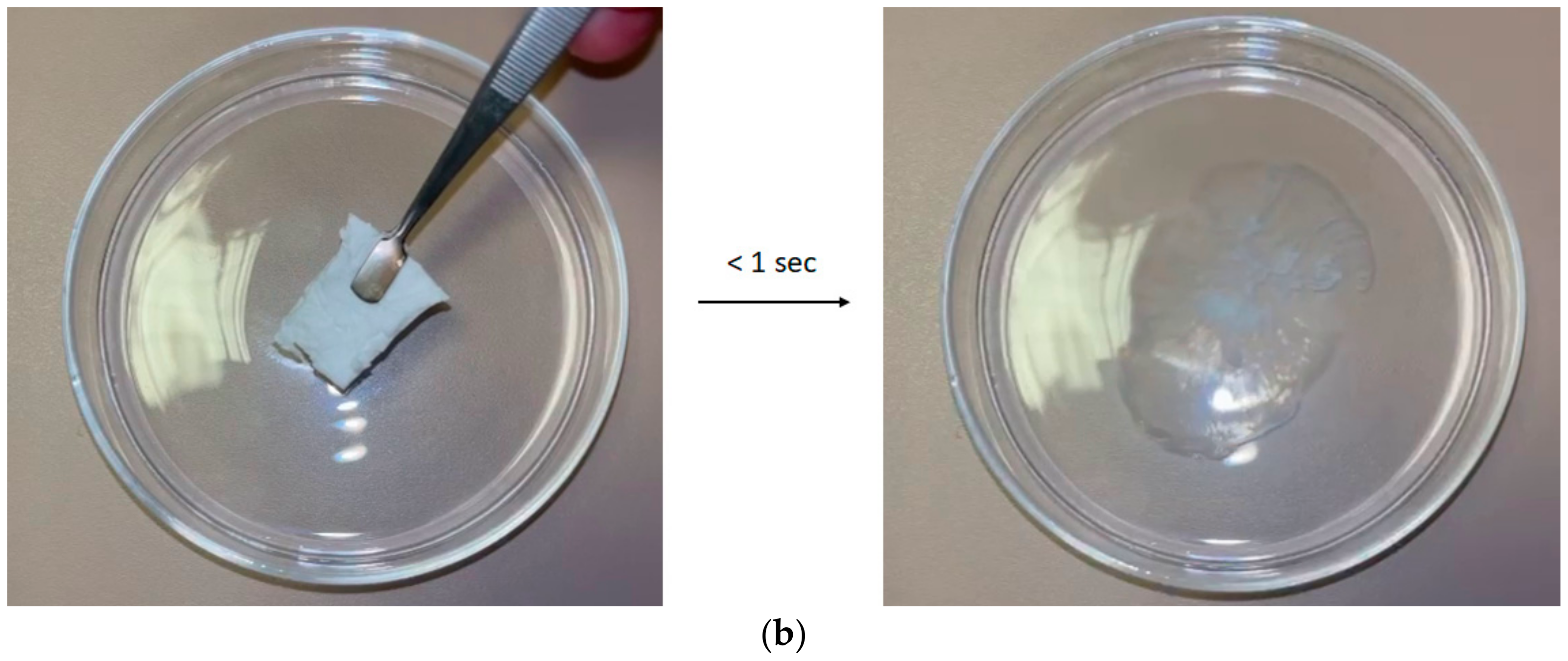
3.5. Disintegration Test
3.6. In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hann, M.M. Molecular Obesity, Potency and Other Addictions in Drug Discovery. In Multifaceted Roles of Crystallography in Modern Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 183–196. [Google Scholar]
- Vogt, M.; Kunath, K.; Dressman, J.B. Dissolution Enhancement of Fenofibrate by Micronization, Cogrinding and Spray-Drying: Comparison with Commercial Preparations. Eur. J. Pharm. Biopharm. 2008, 68, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S. Fenofibrate: Treatment of Hyperlipidemia and Beyond. Expert. Rev. Cardiovasc. Ther. 2008, 6, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.; Milionis, H.J. Treatment of Hyperlipidaemia with Fenofibrate and Related Fibrates. Expert. Opin. Investig. Drugs 2008, 17, 1599–1614. [Google Scholar] [CrossRef]
- Ling, H.; Luoma, J.T.; Hilleman, D. A Review of Currently Available Fenofibrate and Fenofibric Acid Formulations. Cardiol. Res. 2013, 4, 47. [Google Scholar] [CrossRef]
- Guay, D.R.P. Micronized Fenofibrate: A New Fibric Acid Hypolipidemic Agent. Ann. Pharmacother. 1999, 33, 1083–1103. [Google Scholar] [CrossRef]
- Kondoros, B.A.; Berkesi, O.; Tóth, Z.; Aigner, Z.; Ambrus, R.; Csóka, I. Cyclodextrin Complexation of Fenofibrate by Co-Grinding Method and Monitoring the Process Using Complementary Analytical Tools. Pharmaceutics 2022, 14, 1329. [Google Scholar] [CrossRef]
- Truong, D.H.; Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Lee, H.H.; Moon, C.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of Solid Self-Emulsifying Formulation for Improving the Oral Bioavailability of Erlotinib. AAPS PharmSciTech 2016, 17, 466–473. [Google Scholar] [CrossRef]
- Kanaujia, P.; Ng, W.K.; Tan, R.B.H. Solid Self-Emulsifying Drug Delivery System (S-SEDDS) for Improved Dissolution Rate of Fenofibrate. J. Microencapsul. 2014, 31, 293–298. [Google Scholar] [CrossRef]
- Sauron, R.; Wilkins, M.; Jessent, V.; Dubois, A.; Maillot, C.; Weil, A. Absence of a Food Effect with a 145 Mg Nanoparticle Fenofibrate Tablet Formulation. Int. J. Clin. Pharmacol. Ther. 2006, 44, 64–70. [Google Scholar] [CrossRef]
- Bhakay, A.; Vizzotti, E.; Li, M.; Davé, R.; Bilgili, E. Incorporation of Fenofibrate Nanoparticles Prepared by Melt Emulsification into Polymeric Films. J. Pharm. Innov. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Patil, H.; Feng, X.; Ye, X.; Majumdar, S.; Repka, M.A. Continuous Production of Fenofibrate Solid Lipid Nanoparticles by Hot-Melt Extrusion Technology: A Systematic Study Based on a Quality by Design Approach. AAPS J. 2015, 17, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated β-Cyclodextrin-Loaded Eudragit® RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef] [PubMed]
- Guivarc’h, P.H.; Vachon, M.G.; Fordyce, D. A New Fenofibrate Formulation: Results of Six Single-Dose, Clinical Studies of Bioavailability under Fed and Fasting Conditions. Clin. Ther. 2004, 26, 1456–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams, R.O. Formulation and Delivery of Improved Amorphous Fenofibrate Solid Dispersions Prepared by Thin Film Freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating Small Molecules or Biologics into Nanofibers for Optimized Drug Release: A Review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun Amorphous Solid Dispersions of Poorly Water-Soluble Drugs: A Review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef]
- Szabó, P.; Sebe, I.; Stiedl, B.; Kállai-Szabó, B.; Zelkó, R. Tracking of Crystalline-Amorphous Transition of Carvedilol in Rotary Spun Microfibers and Their Formulation to Orodispersible Tablets for in Vitro Dissolution Enhancement. J. Pharm. Biomed. Anal. 2015, 115, 359–367. [Google Scholar] [CrossRef][Green Version]
- Kim, H.S.; Yoo, H.S. Therapeutic Application of Electrospun Nanofibrous Meshes. Nanomedicine 2014, 9, 517–533. [Google Scholar] [CrossRef]
- Hirsch, E.; Vass, P.; Demuth, B.; Petho, Z.; Bitay, E.; Andersen, S.K.; Vigh, T.; Verreck, G.; Molnar, K.; Nagy, Z.K.; et al. Electrospinning Scale-up and Formulation Development of PVA Nanofibers Aiming Oral Delivery of Biopharmaceuticals. Express Polym. Lett. 2019, 13, 590–603. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending Instability in Electrospinning of Nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Stanger, J.; Tucker, N.; Kirwan, K.; Staiger, M.P. Effect of Charge Density on the Taylor Cone in Electrospinning. Int. J. Mod. Phys. B 2009, 23, 1956–1961. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor Cone and Jetting from Liquid Droplets in Electrospinning of Nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef]
- Sipos, E.; Csatári, T.; Kazsoki, A.; Gergely, A.; Bitay, E.; Szabó, Z.I.; Zelkó, R. Preparation and Characterization of Fenofibrate-Loaded PVP Electrospun Microfibrous Sheets. Pharmaceutics 2020, 12, 612. [Google Scholar] [CrossRef]
- Bitay, E.; Szabó, Z.-I.; Kántor, J.; Molnar, K.; Gergely, A.L. Scale-up and Optimization of Fenofibrate-Loaded Fibers Electrospun by Corona-Electrospinning. Express Polym. Lett. 2021, 15, 375–387. [Google Scholar] [CrossRef]
- Bitay, E.; Gergely, A.L.; Balint, I.; Molnar, K.; Fulop, I.; Fogarasi, E.; Szabo, Z.I. Preparation and Characterization of Lapatinib-Loaded PVP Nanofiber Amorphous Solid Dispersion by Electrospinning. Express Polym. Lett. 2021, 15, 1041–1050. [Google Scholar] [CrossRef]
- Nam, S.; Lee, J.J.; Lee, S.Y.; Jeong, J.Y.; Kang, W.S.; Cho, H.J. Angelica Gigas Nakai Extract-Loaded Fast-Dissolving Nanofiber Based on Poly(Vinyl Alcohol) and Soluplus for Oral Cancer Therapy. Int. J. Pharm. 2017, 526, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-Dissolving Electrospun Gelatin Nanofibers Encapsulating Ciprofloxacin/Cyclodextrin Inclusion Complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mano, F.; Martins, M.; Sá-Nogueira, I.; Barreiros, S.; Borges, J.P.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Production of Electrospun Fast-Dissolving Drug Delivery Systems with Therapeutic Eutectic Systems Encapsulated in Gelatin. AAPS PharmSciTech 2017, 18, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Potrč, T.; Baumgartner, S.; Kocbek, P.; Kristl, J. Formulation and Evaluation of Chitosan/Polyethylene Oxide Nanofibers Loaded with Metronidazole for Local Infections. Eur. J. Pharm. Sci. 2016, 95, 152–160. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast Dissolving Oral Films for Drug Delivery Prepared from Chitosan/Pullulan Electrospinning Nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K. Chitosan—Locust Bean Gum Interpenetrating Polymeric Network Nanocomposites for Delivery of Aceclofenac. Int. J. Biol. Macromol. 2017, 102, 878–884. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug Delivery System Based on Cyclodextrin-Naproxen Inclusion Complex Incorporated in Electrospun Polycaprolactone Nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Monteiro, A.P.F.; Rocha, C.M.S.L.; Oliveira, M.F.; Gontijo, S.M.L.; Agudelo, R.R.; Sinisterra, R.D.; Cortés, M.E. Nanofibers Containing Tetracycline/β-Cyclodextrin: Physico-Chemical Characterization and Antimicrobial Evaluation. Carbohydr. Polym. 2017, 156, 417–426. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-Free Cyclodextrin and Natural Polymer-Cyclodextrin Electrospun Nanofibers: A Comprehensive Review on Current Applications and Future Perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Narayanan, G.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Analytical Techniques for Characterizing Cyclodextrins and Their Inclusion Complexes with Large and Small Molecular Weight Guest Molecules. Polym. Test. 2017, 62, 402–439. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Qiu, N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers 2023, 15, 1400. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and Derivatives in Drug Delivery: New Developments, Relevant Clinical Trials, and Advanced Products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Recent Advances in Cyclodextrin-Based Nanoscale Drug Delivery Systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2024, 16, e1995. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2018, 11, 6. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the Design and Development of “Fast-Dissolving” Electrospun Nanofibers Based Drug Delivery Systems—A Systematic Review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Cyclodextrin Nanofibers by Electrospinning. Chem. Commun. 2010, 46, 6903–6905. [Google Scholar] [CrossRef]
- Kelly, A.; Ahmed, J.; Edirisinghe, M. Manufacturing Cyclodextrin Fibers Using Water. Macromol. Mater. Eng. 2022, 307, 2100891. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Uyar, T. Polymer-Free Electrospun Nanofibers from Sulfobutyl Ether7-Beta-Cyclodextrin (SBE7-β-CD) Inclusion Complex with Sulfisoxazole: Fast-Dissolving and Enhanced Water-Solubility of Sulfisoxazole. Int. J. Pharm. 2017, 531, 550–558. [Google Scholar] [CrossRef]
- Balogh, A.; Horváthová, T.; Fülöp, Z.; Loftsson, T.; Harasztos, A.H.; Marosi, G.; Nagy, Z.K. Electroblowing and Electrospinning of Fibrous Diclofenac Sodium-Cyclodextrin Complex-Based Reconstitution Injection. J. Drug Deliv. Sci. Technol. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Vass, P.; Démuth, B.; Farkas, A.; Hirsch, E.; Szabó, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous Alternative to Freeze Drying: Manufacturing of Cyclodextrin-Based Reconstitution Powder from Aqueous Solution Using Scaled-up Electrospinning. J. Control. Release 2019, 298, 120–127. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Metronidazole/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Nanofibrous Webs as Fast-Dissolving Oral Drug Delivery System. Int. J. Pharm. 2019, 572, 118828. [Google Scholar] [CrossRef] [PubMed]
- Bitay, E.; Gergely, A.L.; Szabó, Z.-I. One-Step Preparation of Fiber-Based Chlorzoxazone Solid Dispersion by Centrifugal Spinning. Polymers 2023, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Bitay, E.; Gergely, A.L.; Szabó, Z.I. Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning. Pharmaceutics 2023, 15, 2256. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Jagdale, S.K.; Dehghan, M.H.; Paul, N.S. Enhancement of Dissolution of Fenofibrate Using Complexation with Hydroxy Propyl β-Cyclodextrin. Turk. J. Pharm. Sci. 2019, 16, 48–53. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, H. Characterization of Hydroxypropyl-β-Cyclodextrins with Different Substitution Patterns via FTIR, GC-MS, and TG-DTA. Carbohydr. Polym. 2015, 118, 36–40. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, M.; Lu, J.; Zhu, X. Preparation and Evaluation of Binary and Ternary Inclusion Complexes of Fenofibrate/Hydroxypropyl-β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 17–24. [Google Scholar] [CrossRef]
- Sailaja, U.; Thayyil, M.S.; Kumar, N.S.K.; Govindaraj, G. Molecular Dynamics of Amorphous Pharmaceutical Fenofibrate Studied by Broadband Dielectric Spectroscopy. J. Pharm. Anal. 2016, 6, 165–170. [Google Scholar] [CrossRef]
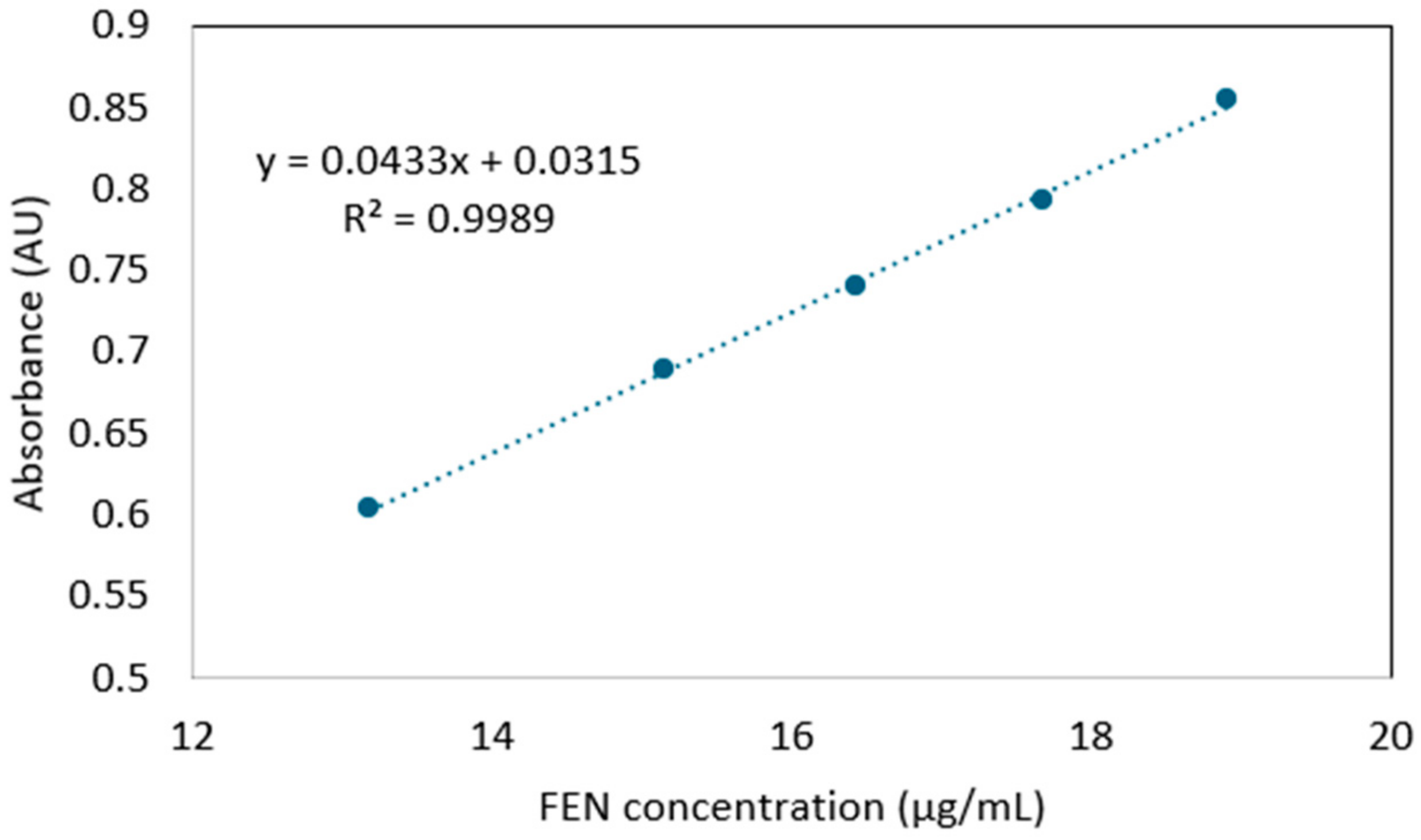






| Sample No. | Measured FEN Content (%) | Theoretical FEN Content (%) | Loading Efficiency (%) | Average Loading Efficiency (%) | Standard Deviation |
|---|---|---|---|---|---|
| 1 | 5.82 | 6.04 | 96.3 | 97.2 | 1.10 |
| 2 | 5.85 | 96.8 | |||
| 3 | 5.95 | 98.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitay, E.; Szabó, Z.-I.; Gergely, A.L. Preparation and Characterization of Fenofibrate-Loaded Fibers Based on 2-Hydroxylpropyl-β-Cyclodextrin. Polymers 2025, 17, 1037. https://doi.org/10.3390/polym17081037
Bitay E, Szabó Z-I, Gergely AL. Preparation and Characterization of Fenofibrate-Loaded Fibers Based on 2-Hydroxylpropyl-β-Cyclodextrin. Polymers. 2025; 17(8):1037. https://doi.org/10.3390/polym17081037
Chicago/Turabian StyleBitay, Enikő, Zoltán-István Szabó, and Attila Levente Gergely. 2025. "Preparation and Characterization of Fenofibrate-Loaded Fibers Based on 2-Hydroxylpropyl-β-Cyclodextrin" Polymers 17, no. 8: 1037. https://doi.org/10.3390/polym17081037
APA StyleBitay, E., Szabó, Z.-I., & Gergely, A. L. (2025). Preparation and Characterization of Fenofibrate-Loaded Fibers Based on 2-Hydroxylpropyl-β-Cyclodextrin. Polymers, 17(8), 1037. https://doi.org/10.3390/polym17081037








