Evaluation of the Method of Periodic Medium Renewal of Bacillus aryabhattai RAF 5 and Analysis of P(3HB) Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture Media and Growing Conditions
2.3. TEM Analysis of the Isolate
2.4. Extraction and Quantitative Determination of P(3HB) Using a Chemical Method
2.5. Extraction of P(3HB) from Biomass
2.6. IR-Fourier Spectroscopy to Determine the Characteristics of Extracted P(3HB)
2.7. Preparation of a P(3HB) Membrane
2.8. Mechanical Properties
2.8.1. Permeability of Water Vapor
2.8.2. Permeability of Oxygen
2.8.3. Permeability of Carbon Dioxide
2.8.4. Puncture Test
3. Results
3.1. Physiological and Biochemical Factors on Growth and P(3HB) Production
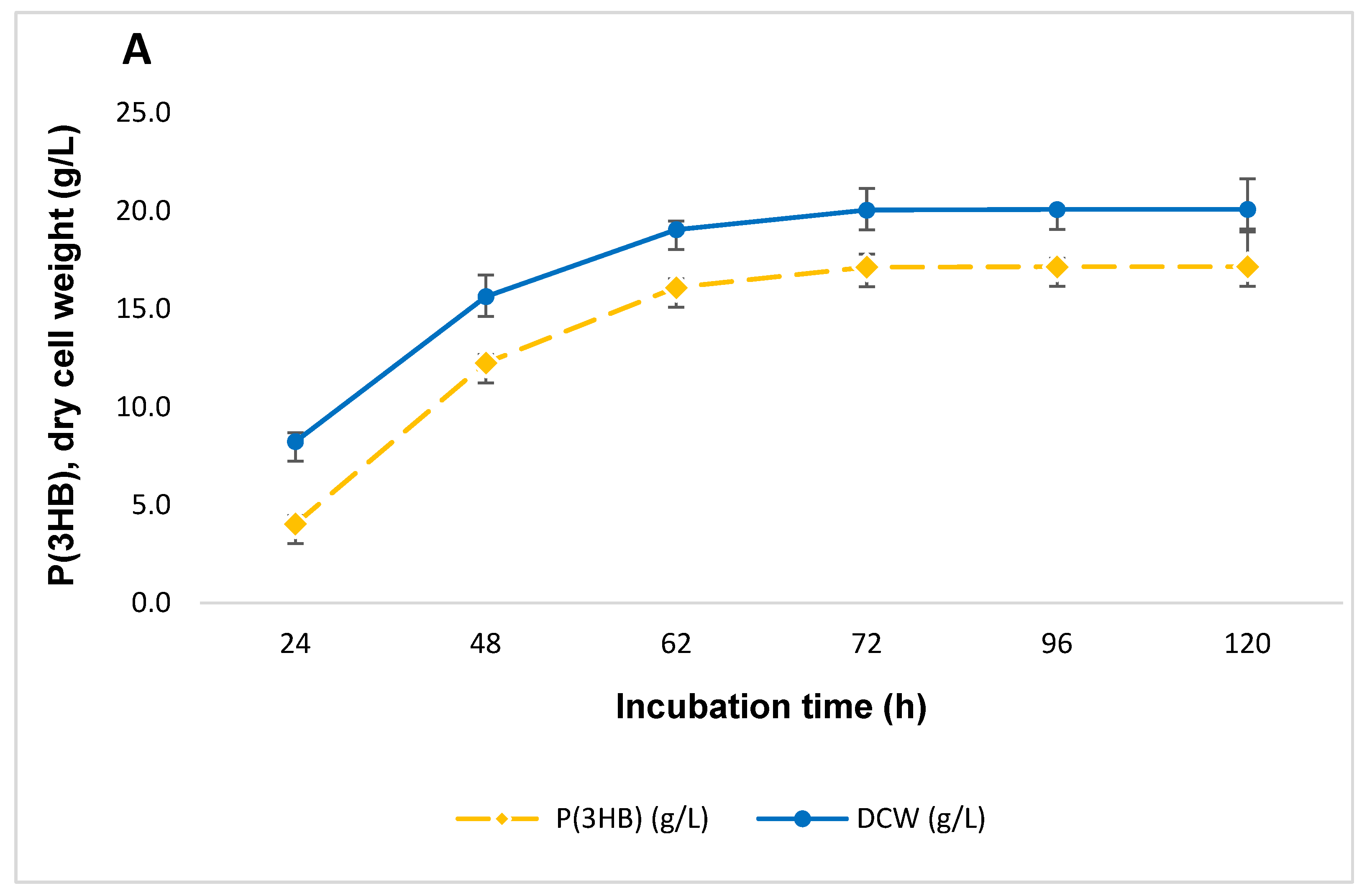
3.2. Effect of Periodic Medium Renewal on Biomass Growth and P(3HB) Production
3.3. TEM Analysis of Selected Isolates of Bacteria Producing P(3HB)
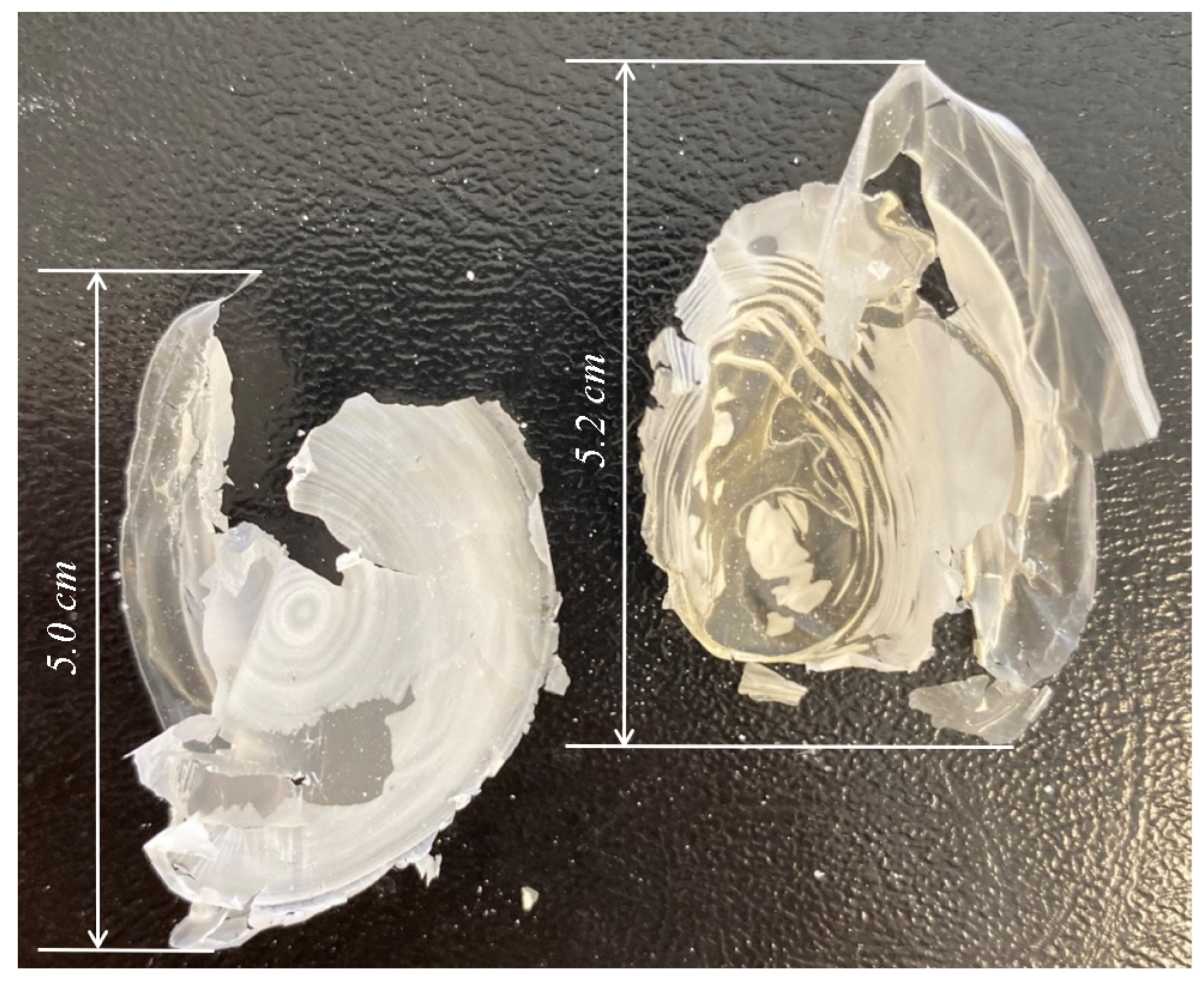
3.4. Extracted P(3HB) Film from the B. aryabhattai RAF 5 Strain
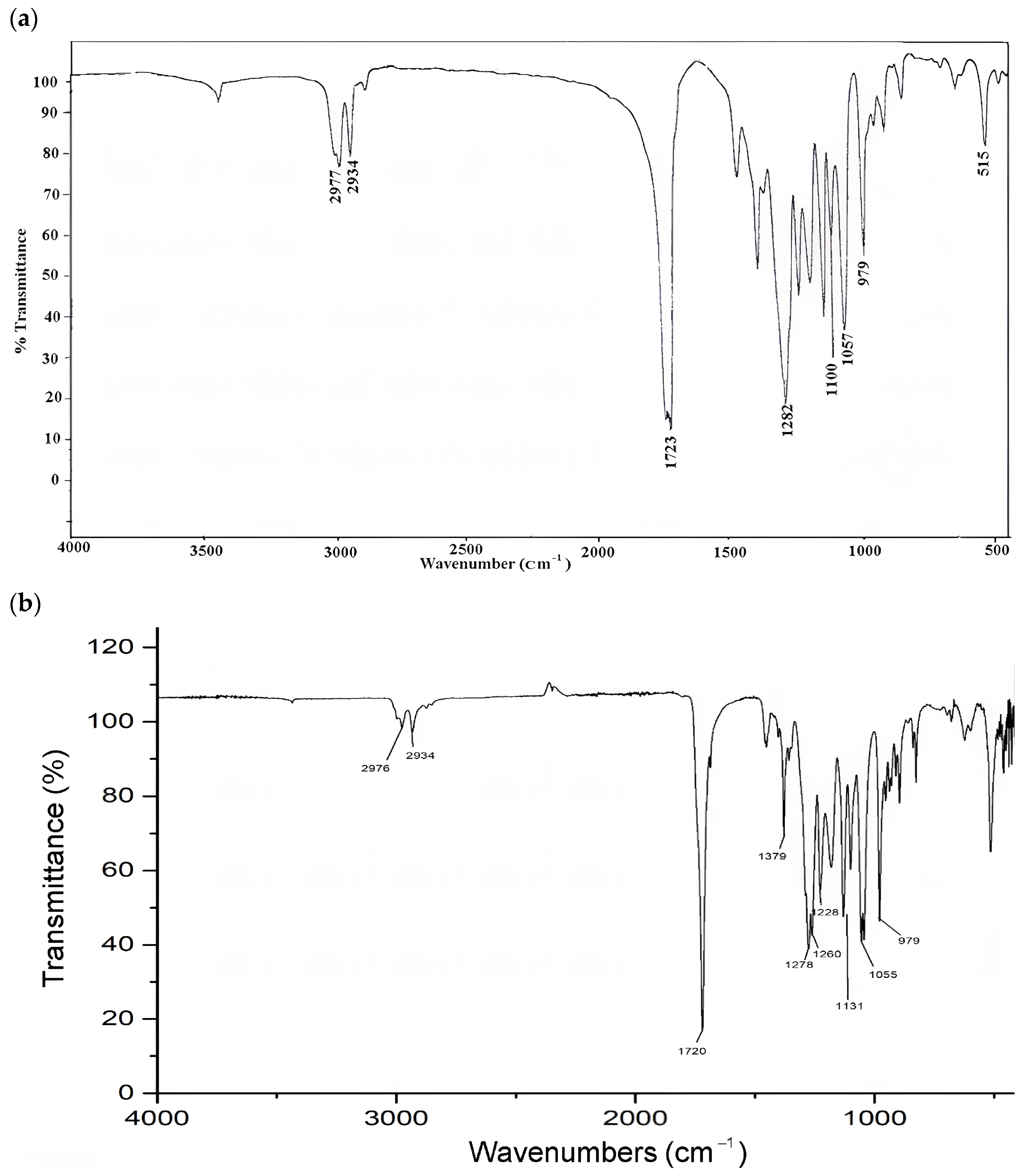
3.5. Characteristics of FTIR
3.6. Permeability of Water Vapor and Gases from the Prepared Membrane
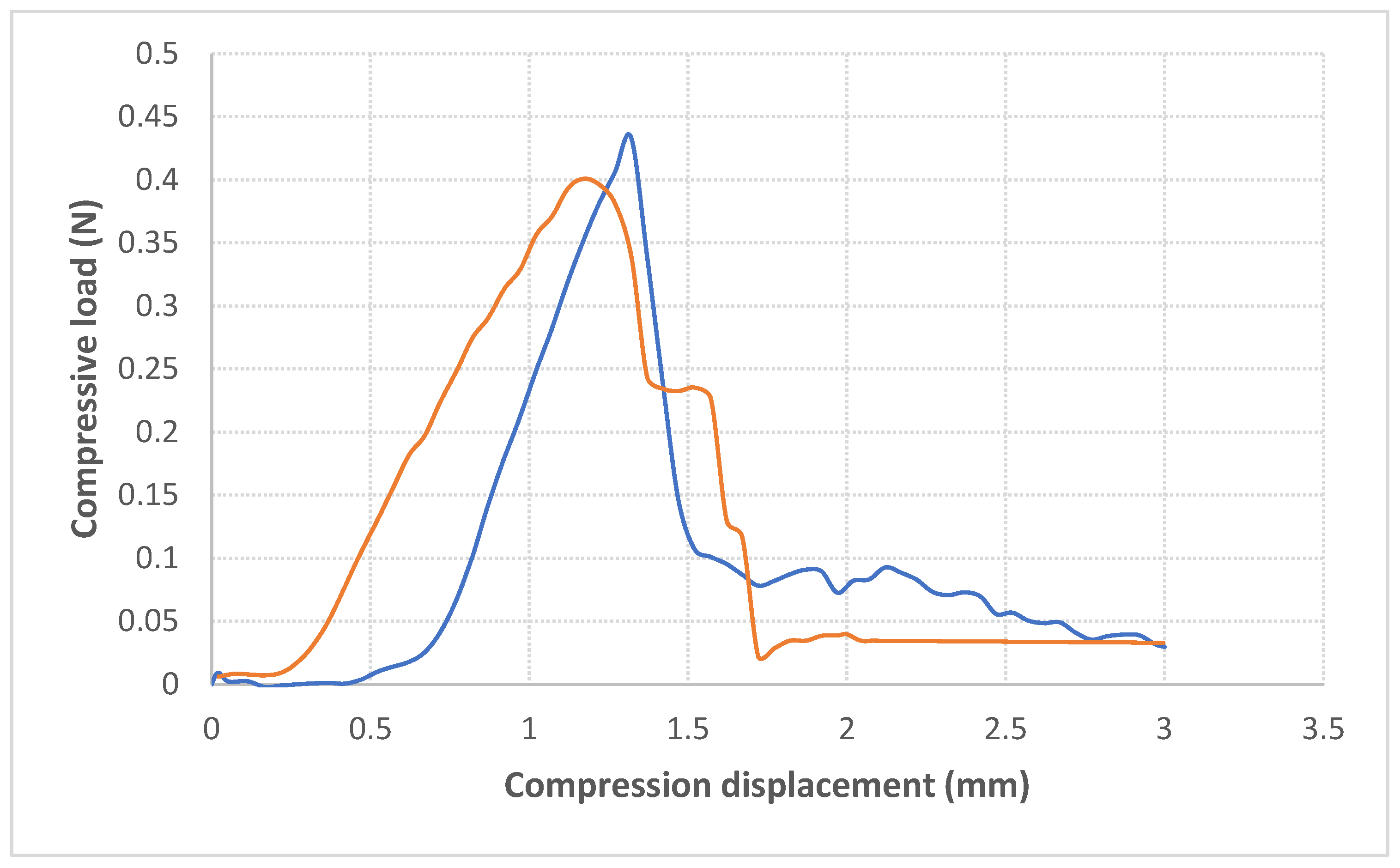
3.7. Puncture Test of Biopolymer
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohapatra, S.; Mohanta, P.R.; Sarkar, B.; Davar, A.; Kumar, K.; Samantarai, D.P. Production of polyhydroxyalkanoates (PHA) by Bacillus strain isolated from wastewater and its biochemical characteristics. Proceedings of the National Academy of Sciences, India Sect. B Biol. Sci. 2015, 87, 459–466. [Google Scholar] [CrossRef]
- Brigham, C.J.; Sinskey, A.J. Applications of Polyhydroxyalkanoates in the Medical Industry. Int. J. Biotechnol. Wellness Ind. 2012, 1, 53–60. [Google Scholar] [CrossRef]
- Halami, P.M. Production of polyhydroxyalkanoate from starch by the native isolates Bacillus cereus CFR06. World J. Microbiol. Biotechnol. 2008, 24, 805–812. [Google Scholar] [CrossRef]
- Korneli, C.; Biedendieck, R.; David, F.; Jahn, D.; Wittmann, C. High yield production of extracellular recombinant levansucrase by Bacillus megaterium. Appl. Microbiol. Biotechnol. 2013, 97, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Schubert, P.; Steinbüchel, A.; Schlegel, H.G. Cloning of the Alcaligenes eutrophus gene for synthesis of poly-β-hydroxybutyric acid and synthesis of P(3HB) in Escherichia coli. J. Bacteriol. 1988, 170, 5837–5847. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, H.; Hu, G. Production of poly-3-hydroxybutyrate by Bacillus sp. JMa5 cultivated in molasses media. Antonie Van. Leeuwenhoek 2001, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Notification list. Notification that new names and new combinations have appeared in volume 70, part 11 of the IJSEM. Int. J. Syst. Evol. Microbiol. 2021, 71, 4645. [Google Scholar] [CrossRef]
- Saravanan, K.; Subramaniam, Y.; Kathirvel, P. Valorization of custard apple (Annona squamosa) waste through polyhydroxybutyrate (PHB) production by Bacillus megaterium MAPCS4: Optimization, characterization, and biodegradation studies. Biomass Conv. Bioref. 2024, 14, 26121–26137. [Google Scholar] [CrossRef]
- Martínez, A.L.M.; Yepes-Pérez, M.; Contreras, K.A.C.; Moreno, P.E.Z. Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Bacillus megaterium LVN01 Using Biogas Digestate. Appl. Microbiol. 2024, 4, 1057–1078. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Yaraguppi, D.A.; Bagewadi, Z.K.; Muddapur, U.M. Response surface methodology-based optimization of biosurfactant production from isolated Bacillus aryabhattai strain ZDY2. J. Petrol. Explor. Prod. Technol. 2020, 10, 2483–2498. [Google Scholar] [CrossRef]
- Rysbek, A.; Ramankulov, Y.; Kurmanbayev, A.; Richert, A.; Abeldenov, S. Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Kadouri, D.; Jurkevitch, E.; Okon, Y. Involvement of the reserve material poly-beta-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl. Environ. Microbiol. 2003, 69, 3244–3250. [Google Scholar] [CrossRef] [PubMed]
- Arzumanov, T.E.; Shishkanova, N.V.; Finogenova, T.V. Biosynthesis of citric acid by Yarrowia lipolytica repeated-batch culture on ethanol. Appl. Microbiol. Biotechnol. 2000, 53, 525–529. [Google Scholar] [CrossRef]
- Guidelines MU 1.3.3103-13 “Organization of Laboratory Work Using Electron and Atomic Force Microscopy Methods for Studying Microorganism Cultures of Pathogenicity Groups I-IV.” Federal Center of Hygiene and Epidemiology of Rospotrebnadzor. 2013. Available online: https://meganorm.ru/Data2/1/4293775/4293775584.pdf (accessed on 25 January 2025).
- Law, J.; Slepecky, R.; Sun, Z.Y. Assay of poly-beta-hydroxybutyric acid. J. Bacteriol. 1961, 82, 33–36. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Osipov, E.V.; Chi, T.V.; Mai, P.T.T.; Topunov, A.F. Influence of Cultivation Conditions on the Synthesis of Poly-3-hydroxybutyrate by Nodulating Bacteria Rhizobium phaseoli. Appl. Biochem. Microbiol. 2020, 56, 60–68. [Google Scholar] [CrossRef]
- Moresi, M. Effect of glucose concentration on citric acid production by Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar] [CrossRef]
- Richert, A.; Olewnik-Kruszkowska, E.; Malinowski, R.; Kalwasińska, A.; Swiontek Brzezinska, M. Polycaprolactone-Based Films Incorporated with Birch Tar—Thermal, Physicochemical, Antibacterial, and Biodegradable Properties. Foods 2023, 12, 4244. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V.; Khramkova, A.V.; Ovchinnikov, V.A.; Krivandin, A.V. Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation. Polymers 2023, 15, 1930. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of tea tree essential oil andpoly(ethylene glycol) on antibacterial and physicochemical properties of polylactide-based films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef]
- ISO 15106-1: 2007; Plastics-Films and boards-Determination of Water Vapor Transmission Rate-Part 1: Moisture sensor Method. International Organization for Standardization: Geneva, Switzerland, 2007.
- ASTM F 1927-98; Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity Through Barrier Materials Using Coulometric Detector. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM F2476-20; Standard Test Method for the Determination of Carbon Dioxide Gas Transmission Rate (CO2TR) Through Barrier Materials Using an Infrared Detector. ASTM International: West Conshohocken, PA, USA, 2020.
- Hamdy, S.M.; Danial, A.W.; Gad El-Rab, S.M.F.; Shoreit, A.A.M.; Hesham, A.E. Production and optimization of bioplastic (Polyhydroxybutyrate) from Bacillus cereus strain SH-02 using response surface methodology. BMC Microbiol. 2022, 22, 183. [Google Scholar] [CrossRef]
- Djerrab, L.; Chekroud, Z.; Rouabhia, A.; Dems, M.A.; Attailia, I.; Garcia, L.I.R.; Smadi, M.A. Potential use of Bacillus paramycoides for the production of the biopolymer polyhydroxybutyrate from leftover carob fruit agro-waste. AIMS Microbiol. 2022, 8, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.K.; Swellam, A.E.; Omar, S.H. Production of P(3HB) by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol. Res. 2001, 156, 201–207. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (P(3HB)) accumulating bacteria using low-cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Jamil, N.; Ali, I.; Ayaz, M.H.; Hasnain, S. Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol. 2011, 61, 623–629. [Google Scholar] [CrossRef]
- Kojuri, S.A.; Issazadeh, K.; Heshmatipour, Z.; Mirpour, M.; Zarrabi, S. Production of Bioplastic (Polyhydroxybutyrate) with Local Bacillus megaterium Isolated from Petrochemical Wastewater. Iran. J. Biotechnol. 2021, 19, e2849. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Snoussi, M.; Badraoui, R.; Alreshidi, M.; Elasbali, A.M.; Al-Soud, W.A.; Alharethi, S.H.; Sachidanandan, M.; et al. Polyhydroxybutyrate (P(3HB))-Based Biodegradable Polymer from Agromyces indicus: Enhanced Production, Characterization, and Optimization. Polymers 2022, 14, 3982. [Google Scholar] [CrossRef]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lathwal, P.; Nehra, K.; Singh, M.; Rana, J.S. Characterization of Novel and Efficient Poly-3-hydroxybutyrate (P(3HB)) Producing Bacteria Isolated from Rhizospheric Soils. J. Polym. Environ. 2018, 26, 3437–3450. [Google Scholar] [CrossRef]
- Williamson, D.H.; Wilkinson, J.F. The isolation and estimation of the poly-β-hydroxy- butyrate inclusions of Bacillus species. J. Gen. Microbiol. 1958, 19, 198–200. [Google Scholar] [CrossRef]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 2006, 71, 423–431. [Google Scholar] [CrossRef]
- Muhammadi; Shabina; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Gottschalk, G.; von Bartta, R. Formation and utilization of poly-β-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature 1961, 191, 463–465. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Narsico, J.; Kobayashi, T.; Inoue, A.; Ojima, T. Production of poly(3-hydroyxybutylate) by a novel alginolytic bacterium Hydrogenophaga sp. strain UMI-18 using alginate as a sole carbon source. J. Biosci. Bioeng. 2019, 128, 203–208. [Google Scholar] [CrossRef]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.; Vijayendra, S.V.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate co-polymers and evaluation of their blends by Fourier transform infrared spectroscopy and scanning electron microscopy. Indian. J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Łabuzek, S.; Radecka, I. Biosynthesis of P3HB tercopolymer by Bacillus cereus UW85. J. Appl. Microbiol. 2001, 90, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sithole, B.; Lekha, P. Optimization of cultivation medium and cyclic fed-batch fermentation strategy for enhanced polyhydroxyalkanoate production by Bacillus thuringiensis using a glucose-rich hydrolyzate. Bioresour. Bioprocess. 2021, 8, 11. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial synthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Zhao, J.; Geuskens, G. Surface modification of polymers VI. Thermal and radiochemical grafting of acrylamide on polyethylene and polystyrene. Eur. Polym. J. 1999, 35, 2115–2123. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Rehm, H.J. Citric acid production from glucose by yeast Candida oleophila ATCC 20177 under batch, continuous and repeated batch cultivation. Electron. J. Biotechnol. 2006, 9, 26–39. [Google Scholar] [CrossRef]
- Kamzolova, S.V. A Review on Citric Acid Production by Yarrowia lipolytica Yeast: Past and Present Challenges and Developments. Processes 2023, 11, 3435. [Google Scholar] [CrossRef]
- Aneesh Balakrishna Pillai, Arjun Jaya Kumar, Kavitha Thulasi, Harikrishnan Kumarapillai, Evaluation of short-chain-length polyhydroxyalkanoate accumulation in Bacillus aryabhatta. Braz. J. Microbiol. 2017, 48, 451–460. [CrossRef] [PubMed]
- Balakrishna Pillai, A.; Jaya Kumar, A.; Kumarapillai, H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) in Bacillus aryabhattai and cytotoxicity evaluation of PHBV/poly(ethylene glycol) blends. 3 Biotech 2020, 10, 32. [Google Scholar] [CrossRef]
- Chanasit, W.; Sueree, L.; Hodgson, B.; Umsakul, K. The production of poly(3-hydroxybutyrate) [P(3HB)] by a newly isolated Bacillus sp. ST1C using liquid waste from biodiesel production. Ann. Microbiol. 2014, 64, 1157–1166. [Google Scholar] [CrossRef]
- Parra, D.F.; Fusaro, J.A.; Ponce, P. Biodegradable Polymeric Films of PHB from Burkholderia saccharia in Presence of Polyethyleneglycol. Pak. J. Biol. Sci. 2005, 8, 1041–1044. [Google Scholar] [CrossRef]



| Sample | Pv, g/m2·24 h | Po, mL/m2·24 h | PD, mL/m2·24 h |
|---|---|---|---|
| P(3HB) | 57 ± 0.50 | 140 ± 0.30 | 705 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysbek, A.; Jankiewicz, U.; Pogorzelska-Nowicka, E.; Wyrwisz, J.; Abeldenov, S.; Mirończuk, A.M.; Richert, A. Evaluation of the Method of Periodic Medium Renewal of Bacillus aryabhattai RAF 5 and Analysis of P(3HB) Production. Polymers 2025, 17, 968. https://doi.org/10.3390/polym17070968
Rysbek A, Jankiewicz U, Pogorzelska-Nowicka E, Wyrwisz J, Abeldenov S, Mirończuk AM, Richert A. Evaluation of the Method of Periodic Medium Renewal of Bacillus aryabhattai RAF 5 and Analysis of P(3HB) Production. Polymers. 2025; 17(7):968. https://doi.org/10.3390/polym17070968
Chicago/Turabian StyleRysbek, Aidana, Urszula Jankiewicz, Ewelina Pogorzelska-Nowicka, Jarosław Wyrwisz, Sailau Abeldenov, Aleksandra M. Mirończuk, and Agnieszka Richert. 2025. "Evaluation of the Method of Periodic Medium Renewal of Bacillus aryabhattai RAF 5 and Analysis of P(3HB) Production" Polymers 17, no. 7: 968. https://doi.org/10.3390/polym17070968
APA StyleRysbek, A., Jankiewicz, U., Pogorzelska-Nowicka, E., Wyrwisz, J., Abeldenov, S., Mirończuk, A. M., & Richert, A. (2025). Evaluation of the Method of Periodic Medium Renewal of Bacillus aryabhattai RAF 5 and Analysis of P(3HB) Production. Polymers, 17(7), 968. https://doi.org/10.3390/polym17070968








