Bottom and Top Internodes Subjected to Interactions with Genotype in Miscanthus: Impact of Biochemical Composition and Anatomy on Stem-Based Composites Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Climatic Conditions
2.2. Plant Material
2.3. Internode Sampling for Anatomical, Biochemical, and Mechanical Properties’ Analyses
2.4. Anatomical Analyses of Internodes
2.5. Preparation of Plant Samples for Biochemical Analyses and Composites Realization
2.6. Biochemical Analyses of Internodes
2.7. Mechanical Properties of Internodes-Based Composites
2.7.1. Preparation of the Polymeric Matrix
2.7.2. Composite Preparation
2.7.3. Mechanical Characterization
2.8. Data Analysis
3. Results
3.1. Internodes Displayed Great Effects in Interaction with Genotype and Year for Most Variables, Except for Tensile Strength
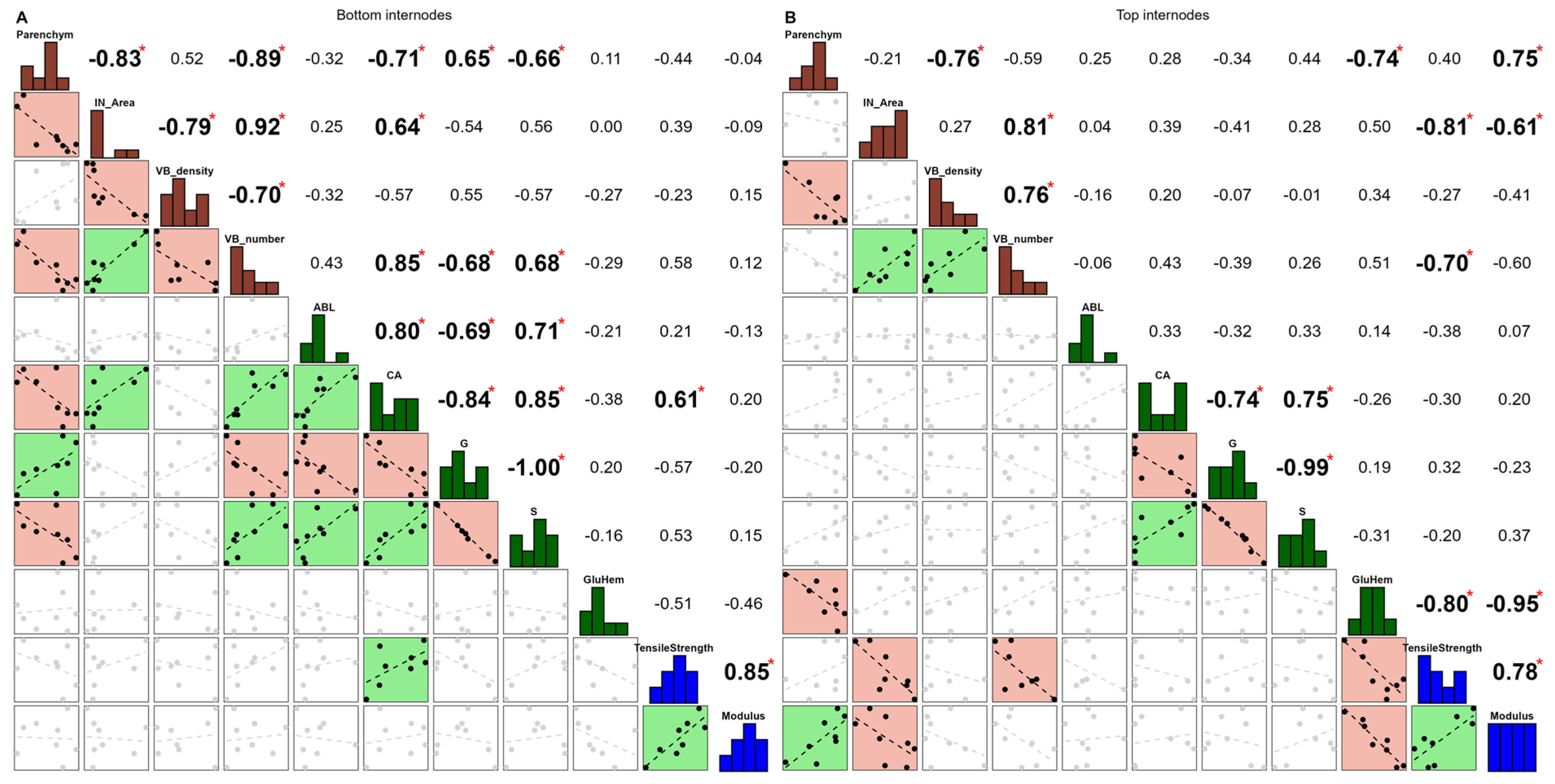
3.2. The Bottom and Top Internodes Showed Contrasted Correlations Between Tensile Strength and Some Anatomical Variables, While Stable Correlations Were Observed Between Years
3.3. A Principal Component Analysis Based on Variables Showing the Most Significant Internode x Genotype Interactions Clearly Separated the Bottom and Top Internodes
3.4. A Good Performance in Mechanical Properties Was Associated with the Highest Parenchym Values and Lowest VB_Number and IN_Area in the Top Internodes
4. Discussion
4.1. Bottom and Top Stem Internodes Displayed Some Contrasted Anatomical and Biochemical Characteristics, Which Impacted the Mechanical Properties of the Corresponding Internode-Based Composites
4.2. Differences Among the Internodes Were Much Smaller in the M. sinensis than in the M. × giganteus for Most Variables, Which Involved More Stable Mechanical Properties
4.3. Insight for Stem-Based Composites Preparation in Miscanthus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL | Lignin content according to acetyl bromide lignin method (% CW) |
| Area_Zext | Stem section rind fraction, in percentage of the internode section area |
| Arabinose | Amount of arabinose (mg/g CW) |
| Blue_Zint | Blue corresponding to less lignified cell wall (% of internal area) |
| bot | Bottom internodes |
| CA | Ester-linked p-coumaric acid (mg/g CW) |
| CW | Cell wall |
| EmptySpace_Zint | Empty space (% of internal area) |
| FA | Ferulic acid (mg/g CW) |
| G | Guaiacyl subunit (% of lignin) |
| Galactose Gen. | Amount of galactose (mg/g CW) Genotype |
| GluCell | Amount of glucose from cellulose (mg/g CW) |
| GluHem | Amount of glucose from hemicelluloses (mg/g CW) |
| H | p-hydroxyphenyl subunit (% of lignin) |
| IN_Area Intern. | Internode section area (mm2) Internode |
| Parenchym PCA | Parenchyma tissue (% of internal area) Principal component analysis |
| S | Syringyl subunit (% of lignin) |
| Scl_Zext | Sclerenchyma tissue (% of external area) |
| top | Top internodes |
| TotHem | Amount of hemicelluloses (mg/g CW) |
| VB_density | Density of vascular bundles in the internal area (nb/mm2) |
| VB_number | Number of vascular bundles in the internal and central areas |
| XylHem | Amount of xylose (mg/g CW) |
| Zext | External area (mg/g CW) |
| Zint | Internal area (mg/g CW) |
References
- Gallos, A.; Paës, G.; Allais, F.; Beaugrand, J. Lignocellulosic Fibers: A Critical Review of the Extrusion Process for Enhancement of the Properties of Natural Fiber Composites. RSC Adv. 2017, 7, 34638–34654. [Google Scholar] [CrossRef]
- Manu, T.; Nazmi, A.R.; Shahri, B.; Emerson, N.; Huber, T. Biocomposites: A Review of Materials and Perception. Mater. Today Commun. 2022, 31, 103308. [Google Scholar] [CrossRef]
- Strullu, L.; Cadoux, S.; Preudhomme, M.; Jeuffroy, M.H.; Beaudoin, N. Biomass Production and Nitrogen Accumulation and Remobilisation by Miscanthus × Giganteus as Influenced by Nitrogen Stocks in Belowground Organs. Field Crops Res. 2011, 121, 381–391. [Google Scholar] [CrossRef]
- Jones, M.; Walsh, M. Miscanthus for Energy and Fibre; James & James (Science Publishers) Ltd.: London, UK, 2001. [Google Scholar]
- Greef, J.M.; Deuter, M.; Jung, C.; Schondelmaier, J. Genetic Diversity of European Miscanthus Species Revealed by AFLP Fingerprinting. Genet. Resour. Crop Evol. 1997, 44, 185–195. [Google Scholar] [CrossRef]
- Glowacka, K.; Clark, L.V.; Adhikari, S.; Peng, J.; Stewart, J.R.; Nishiwaki, A.; Yamada, T.; Jørgensen, U.; Hodkinson, T.R.; Gifford, J.; et al. Genetic Variation in Miscanthus × Giganteus and the Importance of Estimating Genetic Distance Thresholds for Differentiating Clones. GCB Bioenergy 2015, 7, 386–404. [Google Scholar] [CrossRef]
- Kalinina, O.; Nunn, C.; Sanderson, R.; Hastings, A.F.S.; Van Der Weijde, T.; Özgüven, M.; Tarakanov, I.; Schüle, H.; Trindade, L.M.; Dolstra, O.; et al. Extending Miscanthus Cultivation with Novel Germplasm at Six Contrasting Sites. Front. Plant Sci. 2017, 8, 563. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, Q.; Yi, Z.-L.; Yang, Z.-R.; Zhou, F.-S. A Taxonomic Revision of Miscanthus s.l. (Poaceae) from China. Bot. J. Linn. Soc. 2010, 164, 178–220. [Google Scholar] [CrossRef]
- Kirwan, K.; Johnson, R.M.; Jacobs, D.K.; Smith, G.F.; Shepherd, L.; Tucker, N. Enhancing Properties of Dissolution Compounded Miscanthus Giganteus Reinforced Polymer Composite Systems—Part 1.: Improving Flexural Rigidity. Ind. Crops Prod. 2007, 26, 14–27. [Google Scholar] [CrossRef]
- Park, H.J.; Park, K.-H.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, S.H.; Park, Y.-K. Production of Phenolics and Aromatics by Pyrolysis of Miscanthus. Fuel 2012, 97, 379–384. [Google Scholar] [CrossRef]
- Ragoubi, M.; George, B.; Molina, S.; Bienaime, D.; Merlin, A.; Hiver, J.-M.; Dahoun, A. Effect of Corona Discharge Treatment on Mechanical and Thermal Properties of Composites Based on Miscanthus Fibres and Polylactic Acid or Polypropylene Matrix. Compos. Part A-Appl. Sci. Manuf. 2012, 43, 675–685. [Google Scholar] [CrossRef]
- Girones, J.; Vo, L.; Arnoult, S.; Brancourt-Hulmel, M.; Navard, P. Miscanthus Stem Fragment—Reinforced Polypropylene Composites: Development of an Optimized Preparation Procedure at Small Scale and Its Validation for Differentiating Genotypes. Polym. Test. 2016, 55, 166–172. [Google Scholar] [CrossRef]
- Allison, G.G.; Morris, C.; Clifton-Brown, J.; Lister, S.J.; Donnison, I.S. Genotypic Variation in Cell Wall Composition in a Diverse Set of 244 Accessions of Miscanthus. Biomass Bioenergy 2011, 35, 4740–4747. [Google Scholar] [CrossRef]
- Chupin, L.; de Ridder, D.; Clement-Vidal, A.; Soutiras, A.; Gineau, E.; Mouille, G.; Arnoult, S.; Brancourt-Hulmel, M.; Lapierre, C.; Pot, D.; et al. Influence of the Radial Stem Composition on the Thermal Behaviour of Miscanthus and Sorghum Genotypes. Carbohydr. Polym. 2017, 167, 12–19. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Martins, R.C.; Brendle, C.; Lafon-Pham, D.; Sonnier, R. Enhancing Insight into Photochemical Weathering of Flax and Miscanthus: Exploring Diverse Chemical Compositions and Composite Materials. Molecules 2024, 29, 3945. [Google Scholar] [CrossRef]
- Brancourt-Hulmel, M.; Arnoult, S.; Cezard, L.; El Hage, F.; Gineau, E.; Girones, J.; Griveau, Y.; Jacquemont, M.-P.; Jaffuel, S.; Mignot, E.; et al. A Comparative Study of Maize and Miscanthus Regarding Cell-Wall Composition and Stem Anatomy for Conversion into Bioethanol and Polymer Composites. Bioenergy Res. 2022, 15, 777–791. [Google Scholar] [CrossRef]
- Kaack, K.; Schwarz, K.; Brander, P. Variation in Morphology, Anatomy and Chemistry of Stems of Miscanthus Genotypes Differing in Mechanical Properties. Ind. Crops Prod. 2003, 17, 131–142. [Google Scholar] [CrossRef]
- Slupska, M.; Dyjakon, A.; Stopa, R. Determination of Strength Properties of Energy Plants on the Example of Miscanthus × giganteus, Rosa multiflora and Salix viminalis. Energies 2019, 12, 3660. [Google Scholar] [CrossRef]
- Francik, S.; Knapik, P.; Lapczynska-Kordon, B.; Francik, R.; Slipek, Z. Values of Selected Strength Parameters of Miscanthus × giganteus Stalk Depending on Water Content and Internode Number. Materials 2022, 15, 1480. [Google Scholar] [CrossRef]
- Joly, D.; Brossard, T.; Cardot, H.; Cavailhes, J.; Hilal, M.; Wavresky, P. Types of Climates on Continental France, a Spatial Construction. Cybergeo 2010, 501, 1–23. [Google Scholar] [CrossRef]
- Delannoy, D.; Maury, O.; Décome, J. CLIMATIK: Système d’information Pour Les Données Du Réseau Agroclimatique INRAE. Rech. Data Gouv V1 2022. [Google Scholar] [CrossRef]
- Tolivia, D.; Tolivia, J. Fasga—A new polychromatic method for simultaneous and differential staining of plant-tissues. J. Microsc. 1987, 148, 113–117. [Google Scholar] [CrossRef]
- Sibout, R.; Le Bris, P.; Legee, F.; Cezard, L.; Renault, H.; Lapierre, C. Structural Redesigning Arabidopsis Lignins into Alkali-Soluble Lignins through the Expression of p-Coumaroyl-CoA: Monolignol Transferase PMT. Plant Physiol. 2016, 170, 1358–1366. [Google Scholar] [CrossRef]
- Mechin, V.; Laluc, A.; Legee, F.; Cezard, L.; Denoue, D.; Barriere, Y.; Lapierre, C. Impact of the Brown-Midrib Bm5 Mutation on Maize Lignins. J. Agric. Food Chem. 2014, 62, 5102–5107. [Google Scholar] [CrossRef] [PubMed]
- Ho-Yue-Kuang, S.; Alvarado, C.; Antelme, S.; Bouchet, B.; Cezard, L.; Le Bris, P.; Legee, F.; Maia-Grondard, A.; Yoshinaga, A.; Saulnier, L.; et al. Mutation in Brachypodium Caffeic Acid O-Methyltransferase 6 Alters Stem and Grain Lignins and Improves Straw Saccharification without Deteriorating Grain Quality. J. Exp. Bot. 2016, 67, 227–237. [Google Scholar] [CrossRef] [PubMed]
- ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding Andextrusion Plastics. ISO International Organization for Standardization: Geneva, Switzerland, 2012.
- ASTM D618-21; Standard Practice for Conditioning Plastics for Testing. Advancing Standart Advancing Markets (ASTM): West Conshohocken, PA, USA, 2021.
- Froissard, J. Permutation Tests for Regression, (Repeated Measures) ANOVA/ANCOVA and Comparison of Signals. J. Stat. Softw. 2022, 99, 1–32. [Google Scholar]
- Chen, L.; Auh, C.; Chen, F.; Cheng, X.; Aljoe, H.; Dixon, R.; Wang, Z. Lignin Deposition and Associated Changes in Anatomy, Enzyme Activity, Gene Expression, and Ruminal Degradability in Stems of Tall Fescue at Different Developmental Stages. J. Agric. Food Chem. 2002, 50, 5558–5565. [Google Scholar] [CrossRef]
- Schnurr, J.A.; Jung, H.-J.G.; Samac, D.A. A Comparative Study of Alfalfa and Medicago truncatula Stem Traits:: Morphology, Chemical Composition, and Ruminal Digestibility. Crop Sci. 2007, 47, 1672–1680. [Google Scholar] [CrossRef]
- Beaugrand, J.; Nottez, M.; Konnerth, J.; Bourmaud, A. Multi-Scale Analysis of the Structure and Mechanical Performance of Woody Hemp Core and the Dependence on the Sampling Location. Ind. Crops Prod. 2014, 60, 193–204. [Google Scholar] [CrossRef]
- El Hage, F.; Legland, D.; Borrega, N.; Jacquemot, M.-P.; Griveau, Y.; Coursol, S.; Mechin, V.; Reymond, M. Tissue Lignification, Cell Wall p-Coumaroylation and Degradability of Maize Stems Depend on Water Status. J. Agric. Food Chem. 2018, 66, 4800–4808. [Google Scholar] [CrossRef]
- Xie, D.; Chen, F.; Wang, J.; Liu, L. Exploring the Constituents and Thermal Characteristics of Miscanthus Floridulus for Biomass Composites. Ind. Crops Prod. 2024, 222, 119777. [Google Scholar] [CrossRef]
- Zapater, M.; Catterou, M.; Mary, B.; Ollier, M.; Fingar, L.; Mignot, E.; Ferchaud, F.; Strullu, L.; Dubois, F.; Brancourt-Hulmel, M. A Single and Robust Critical Nitrogen Dilution Curve for Miscanthus × Giganteus and Miscanthus Sinensis. Bioenergy Res. 2017, 10, 115–128. [Google Scholar] [CrossRef]
- Arnoult, S.; Obeuf, A.; Bethencourt, L.; Mansard, M.-C.; Brancourt-Hulmel, M. Miscanthus Clones for Cellulosic Bioethanol Production: Relationships between Biomass Production, Biomass Production Components, and Biomass Chemical Composition. Ind. Crops Prod. 2015, 63, 316–328. [Google Scholar] [CrossRef]
- Mangold, A.; Lewandowski, I.; Möhring, J.; Clifton-Brown, J.; Krzyzak, J.; Mos, M.; Pogrzeba, M.; Kiesel, A. Harvest Date and Leaf:Stem Ratio Determine Methane Hectare Yield of Miscanthus Biomass. GCB Bioenergy 2018, 11, 21–33. [Google Scholar] [CrossRef]
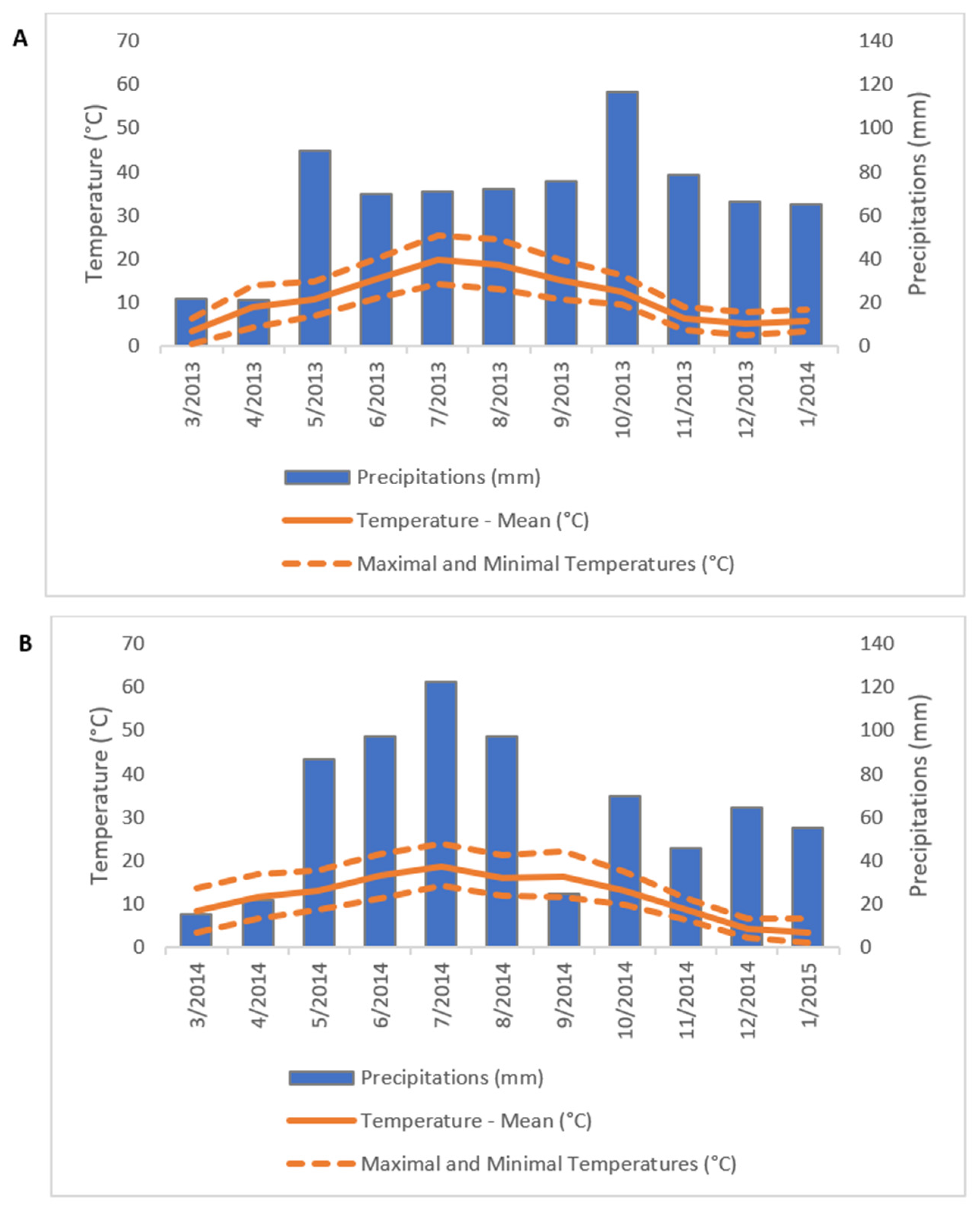





| Trait | Canopy Height at the End of Season (m) | Stem Section (mm) | Heading Date | Biomass Production (t DM/ha) | Mean Height of the Stems Sampled (m) | Mean Length of the Internodes Sampled (cm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Mean of the years 2011 to 2013 (Fourth to sixth years of cultivation) | Mean of the years 2011 to 2013 (Fourth to sixth years of cultivation) | 2013 (sixth year of cultivation) | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | |
| Genotype | FLO | 2.9 | 10 | No heading appearance | 28.4 | 40.6 | 2.4 ± 0.01 | 3.1 ± 0.2 | Bot: 29.5 ± 4.4 Top: 19.7 ± 2.0 | Bot 15.8 ± 5.3 Top: 12.4 ± 2.1 |
| GIG_B | 2.8 | 10 | No heading appearance | 26.1 | 34.2 | 2.4 ± 0.1 | 2.9 ± 0.2 | Bot: 25.9 ± 2.4 Top: 12.3 ± 0.9 | Bot: 18.3 ± 3.1 Top: 10.7 ± 1.2 | |
| GOL | 1.9 | 7 | 29/08/2012 | 22.8 | 20.5 | 1.9 ± 0.3 | 1.8 ± 0.1 | Bot: 9.5 ± 2.8 Top: 14.9 ± 0.7 | Bot: 9.3 ± 1.2 Top: 15.1 ± 2.2 | |
| MAL | 1.4 | 6 | 20/08/2012 | 12.1 | 10.9 | 1.3 ± 0.05 | 1.4 ± 0.06 | Bot: 7.8 ± 1.0 Top: 11.8 ± 0.4 | Bot: 9.6 ± 3.7 Top: 11.6 ± 3.1 | |
| Year | Genotype | Internode | Year x Genotype | Year x Internode | Gen. x Internode | Year x Gen. x Internode | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | 1 | 3 | 1 | 3 | 1 | 3 | 3 | ||||||||
| (a) | Tensile_Strength | 74.9 | *** | 33.4 | *** | 0.5 | ns | 12.6 | *** | 2.2 | ns | 52.2 | *** | 10.7 | *** |
| Modulus | 3.2 | ns | 13.8 | *** | 107.5 | *** | 0.4 | ns | 18.6 | *** | 4.9 | ** | 10.7 | *** | |
| (b) | IN_Area (mm2) | 4.8 | * | 38.4 | *** | 72.9 | *** | 2.6 | ns | 0.7 | ns | 15.0 | *** | 2.5 | ns |
| Parenchym (% of Zint area) | 0.9 | ns | 10.3 | *** | 298.2 | *** | 2.2 | ns | 9.1 | ** | 9.5 | *** | 1.7 | ns | |
| EmptySpace_Zint (% of Zint ar.) | 3.2 | ns | 5.2 | ** | 111.5 | *** | 1.9 | ns | 5.5 | * | 7.9 | *** | 1.6 | ns | |
| Area_Zext (% of section area) | 5.2 | * | 3.4 | * | 49.1 | *** | 1.9 | ns | 0.0 | ns | 2.9 | ns | 4.0 | * | |
| Scl_Zext (% of Zext area) | 23.1 | *** | 7.6 | ** | 10.0 | ** | 1.2 | ns | 1.6 | ns | 1.0 | ns | 2.3 | ns | |
| VB_density (nb/m2 in Zint) | 5.6 | * | 7.4 | *** | 29.7 | *** | 5.4 | ** | 0.0 | ns | 9.2 | *** | 2.4 | ns | |
| VB_number (number) | 6.1 | * | 53.7 | *** | 0.1 | ns | 4.4 | ** | 4.3 | * | 4.6 | ** | 3.0 | * | |
| (c) | ABL (%CW) | 4.9 | * | 9.0 | ** | 98.1 | *** | 2.4 | ns | 1.0 | ns | 10.3 | *** | 4.1 | * |
| Yd_ABL | 1062.5 | *** | 4.0 | * | 66.9 | *** | 0.9 | ns | 47.4 | *** | 4.5 | * | 3.3 | ns | |
| CA (mg/g CW) | 17.7 | *** | 65.6 | *** | 11.9 | ** | 12.1 | *** | 25.5 | *** | 55.6 | *** | 11.0 | *** | |
| FA (mg/g CW) | 1.4 | ns | 101.2 | *** | 222.8 | *** | 0.1 | ns | 4.3 | ns | 6.2 | ** | 1.5 | ns | |
| Rhamnose (mg/g CW) | 87.0 | *** | 0.8 | ns | 49.9 | *** | 3.4 | * | 1.7 | ns | 5.6 | ** | 0.5 | ns | |
| H lignin subunit (% of lignin) | 11.0 | ** | 3.3 | ns | 52.8 | *** | 1.8 | ns | 4.9 | ns | 2.7 | ns | 2.7 | ns | |
| G lignin subunit (% of lignin) | 88.6 | *** | 37.5 | *** | 6.3 | * | 7.1 | ** | 14.3 | ** | 20.3 | *** | 0.1 | ns | |
| S lignin subunit (% of lignin) | 74.9 | *** | 19.8 | *** | 18.1 | *** | 6.5 | ** | 13.4 | ** | 15.9 | *** | 0.5 | ns | |
| Galactose (mg/g CW) | 4.7 | * | 2.2 | ns | 30.9 | *** | 3.6 | * | 1.1 | ns | 3.0 | ns | 1.0 | ns | |
| GlucHemCel (mg/g CW) | 15.9 | ** | 0.9 | ns | 3.0 | ns | 5.2 | ** | 8.6 | ** | 9.2 | *** | 1.1 | ns | |
| Arabinose (mg/g CW) | 0.02 | ns | 21.2 | *** | 128.1 | *** | 5.0 | * | 2.2 | ns | 4.6 | * | 1.8 | ns | |
| XylHemCel (mg/g CW) | 7.7 | * | 16.9 | *** | 106.6 | *** | 2.9 | ns | 0.2 | ns | 2.1 | ns | 1.1 | ns | |
| TotHemCel (mg/g CW) | 7.2 | * | 16.0 | *** | 106.8 | *** | 3.5 | * | 0.5 | ns | 2.9 | ns | 1.1 | ns | |
| GlucCel (mg/g CW) | 31.8 | *** | 0.7 | ns | 15.8 | ** | 0.1 | ns | 1.2 | ns | 2.9 | ns | 0.3 | ns | |
| XylCel (mg/g CW) | 0.03 | ns | 2.9 | ns | 0.1 | ns | 0.2 | ns | 0.0 | ns | 0.3 | ns | 0.1 | ns | |
| TotCel (mg/g CW) | 23.8 | *** | 0.4 | ns | 12.1 | ** | 0.1 | ns | 0.9 | ns | 2.2 | ns | 0.2 | ns | |
| Mean | CV (%) | Bot Mean | Top Mean | ||
|---|---|---|---|---|---|
| (a) | Tensile_Strength | 36.4 | 1.3 | 36.4 | 36.5 |
| Modulus | 3096.3 | 3.3 | 3202.5 | 2987.6 | |
| (b) | IN_Area (mm2) | 43.0 | 25.8 | 56.6 | 29.3 |
| Parenchym (% of Zint area) | 61.3 | 10.1 | 45.9 | 76.7 | |
| EmptySpace_Zint (% of Zint area) | 12.1 | 46.3 | 20.6 | 3.5 | |
| Area_Zext (% of section area) | 21.2 | 21.9 | 25.9 | 16.5 | |
| Scl_Zext (% of Zext area) | 50.5 | 22.6 | 55.8 | 45.3 | |
| VB_density (nb/m2 in Zint) | 3.3 | 18.8 | 2.8 | 3.8 | |
| VB_number (number) | 101.3 | 18.8 | 102.0 | 100.5 | |
| (c) | ABL (%CW) | 23.5 | 2.9 | 24.8 | 22.1 |
| Yd_ABL | 1039.7 | 8.2 | 1241.3 | 838.0 | |
| CA (mg/g CW) | 18.0 | 3.4 | 18.7 | 17.2 | |
| FA (mg/g CW) | 4.4 | 5.1 | 3.8 | 5.0 | |
| Rhamnose (mg/g CW) | 0.4 | 12.5 | 0.3 | 0.4 | |
| H lignin subunit (% of lignin) | 2.4 | 16.2 | 1.7 | 3.1 | |
| G lignin subunit (% of lignin) | 58.9 | 1.8 | 57.9 | 59.8 | |
| S lignin subunit (% of lignin) | 38.7 | 3.3 | 40.3 | 37.1 | |
| Galactose (mg/g CW) | 2.8 | 10.6 | 2.5 | 3.2 | |
| GlucHemCel (mg/g CW) | 6.0 | 12.6 | 5.5 | 6.5 | |
| Arabinose (mg/g CW) | 20.5 | 6.7 | 17.1 | 23.8 | |
| XylHemCel (mg/g CW) | 170.9 | 6.2 | 148.3 | 193.4 | |
| TotHemCel (mg/g CW) | 200.5 | 6.1 | 173.6 | 227.4 | |
| GlucCel (mg/g CW) | 419.5 | 5.5 | 441.0 | 398.0 | |
| XylCel (mg/g CW) | 40.9 | 12.3 | 41.3 | 40.6 | |
| TotCel (mg/g CW) | 460.4 | 5.9 | 482.3 | 438.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancourt-Hulmel, M.; Arnoult, S.; Girones, J.; Jaffuel, S.; Vo, T.T.L.; Gineau, E.; Mouille, G.; Dubois, S.; Navard, P. Bottom and Top Internodes Subjected to Interactions with Genotype in Miscanthus: Impact of Biochemical Composition and Anatomy on Stem-Based Composites Mechanical Properties. Polymers 2025, 17, 966. https://doi.org/10.3390/polym17070966
Brancourt-Hulmel M, Arnoult S, Girones J, Jaffuel S, Vo TTL, Gineau E, Mouille G, Dubois S, Navard P. Bottom and Top Internodes Subjected to Interactions with Genotype in Miscanthus: Impact of Biochemical Composition and Anatomy on Stem-Based Composites Mechanical Properties. Polymers. 2025; 17(7):966. https://doi.org/10.3390/polym17070966
Chicago/Turabian StyleBrancourt-Hulmel, Maryse, Stéphanie Arnoult, Jordi Girones, Sylvie Jaffuel, Thi To Loan Vo, Emilie Gineau, Gregory Mouille, Sophie Dubois, and Patrick Navard. 2025. "Bottom and Top Internodes Subjected to Interactions with Genotype in Miscanthus: Impact of Biochemical Composition and Anatomy on Stem-Based Composites Mechanical Properties" Polymers 17, no. 7: 966. https://doi.org/10.3390/polym17070966
APA StyleBrancourt-Hulmel, M., Arnoult, S., Girones, J., Jaffuel, S., Vo, T. T. L., Gineau, E., Mouille, G., Dubois, S., & Navard, P. (2025). Bottom and Top Internodes Subjected to Interactions with Genotype in Miscanthus: Impact of Biochemical Composition and Anatomy on Stem-Based Composites Mechanical Properties. Polymers, 17(7), 966. https://doi.org/10.3390/polym17070966








