Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dendrimers with Antibacterial Activities
2.3. Langmuir Monolayer Studies
2.4. FTIR-ATR Spectroscopy of MLVs
2.5. Laurdan Fluorescence Spectroscopy of LUVs
2.6. Zeta Potential and Size of LUVs
2.7. GUVs Electroformation and Video Microscopy
3. Results and Discussion
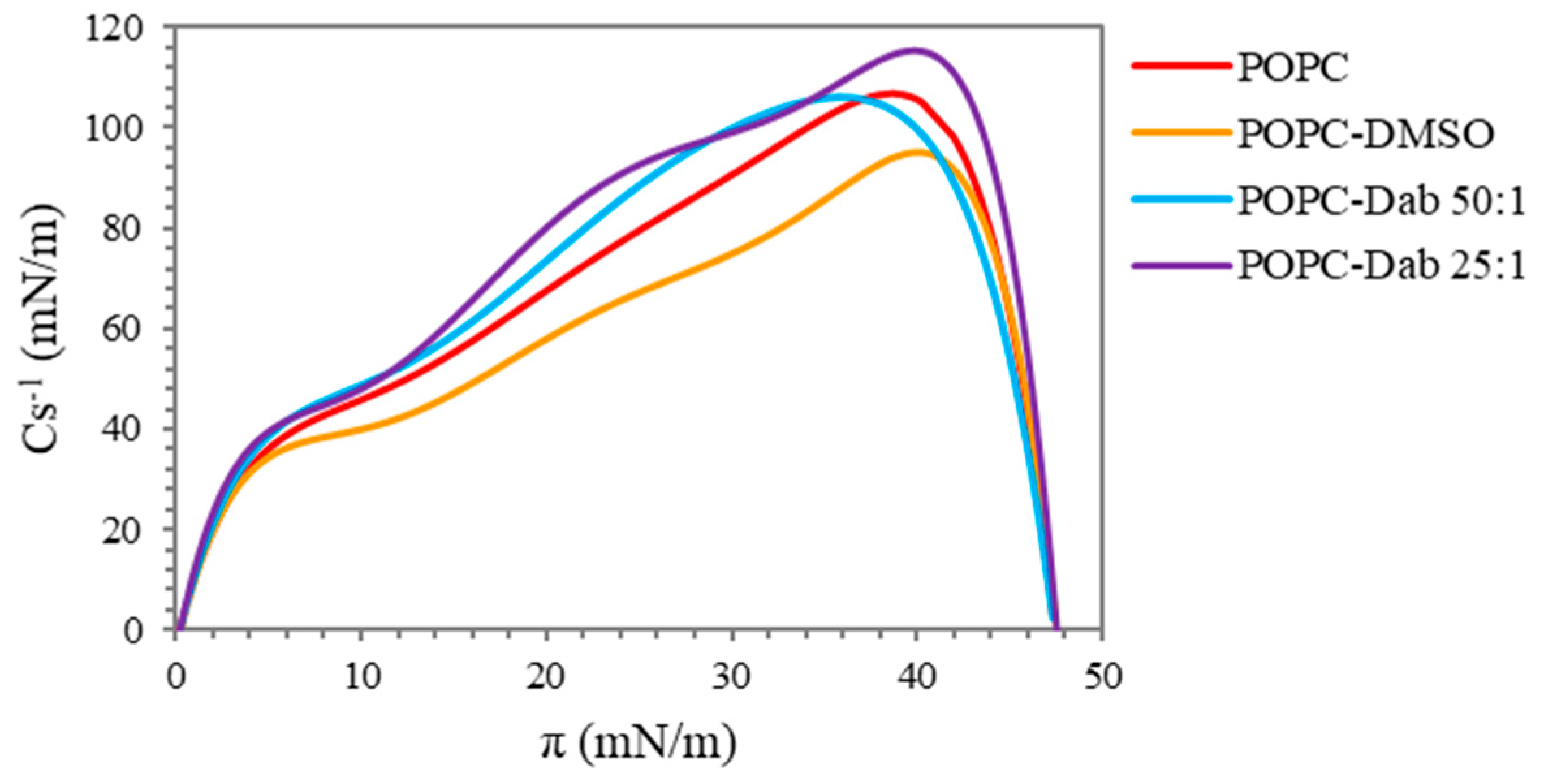
3.1. Langmuir Monolayer Studies of POPC Treated with Dendrimers
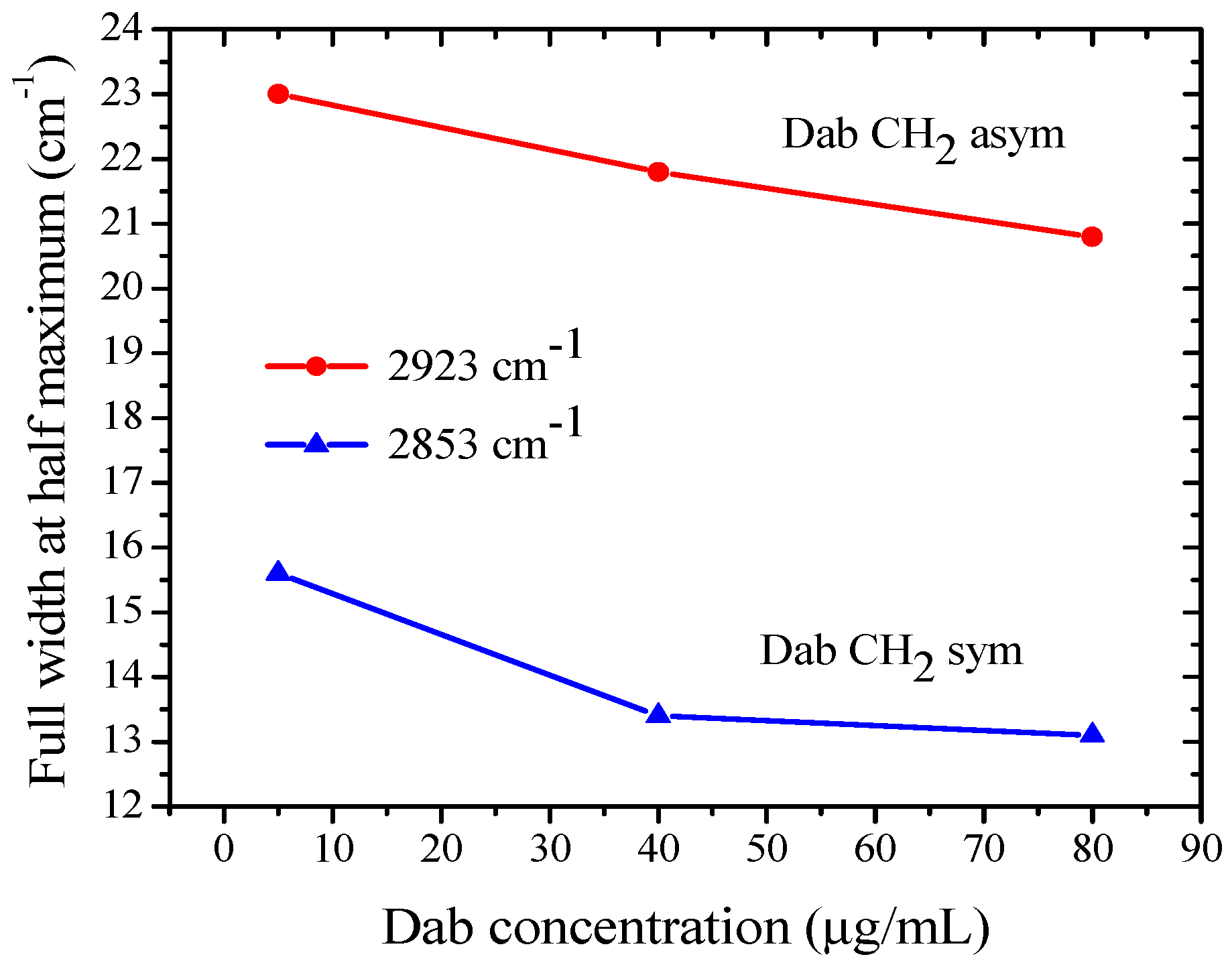
3.2. FTIR-ATR Spectroscopy of POPC Liposomes Containing Dab and Dab-Br Dendrimers
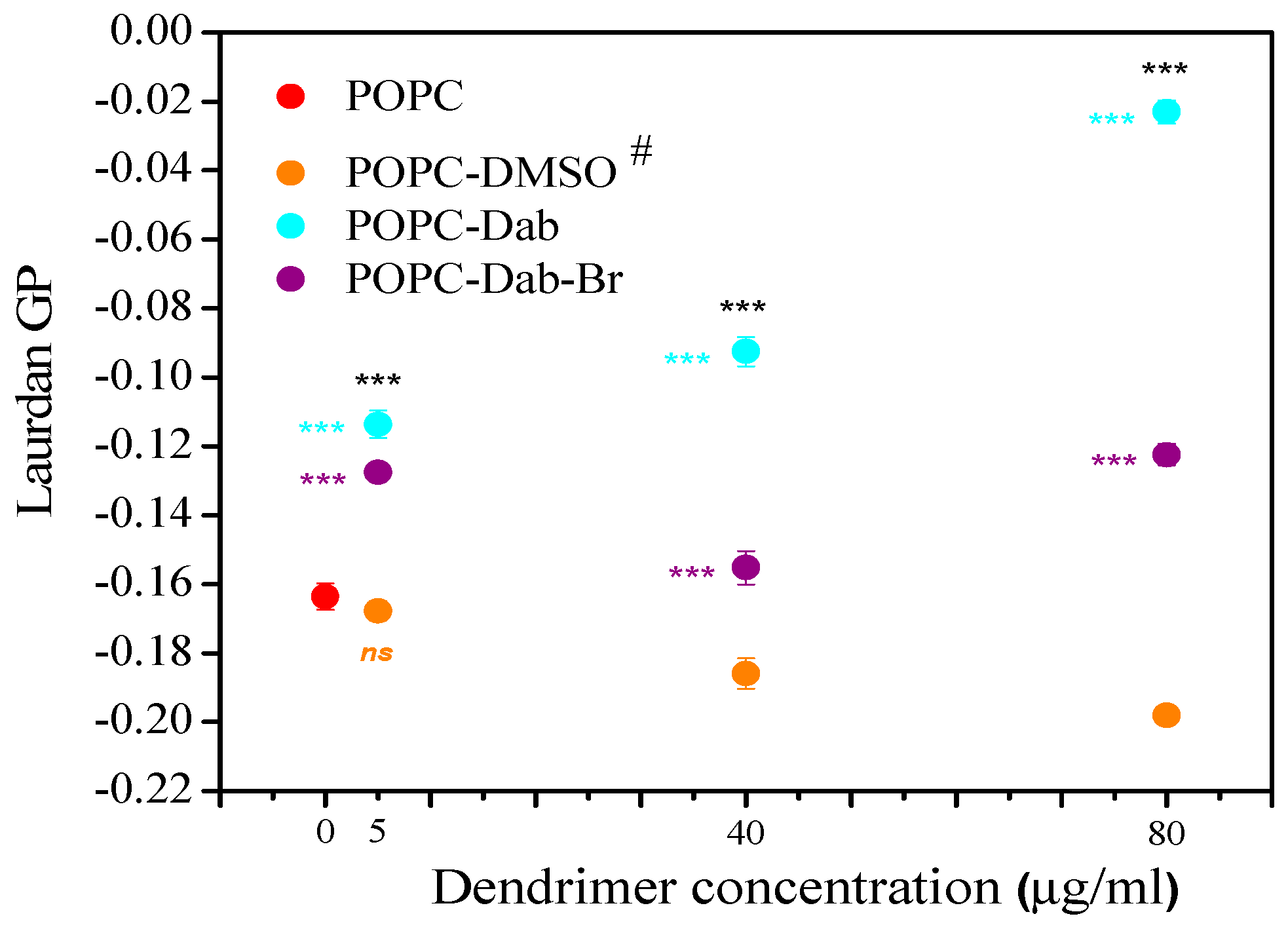
3.3. Membrane Lipid Order of LUVs Treated with Dendrimers
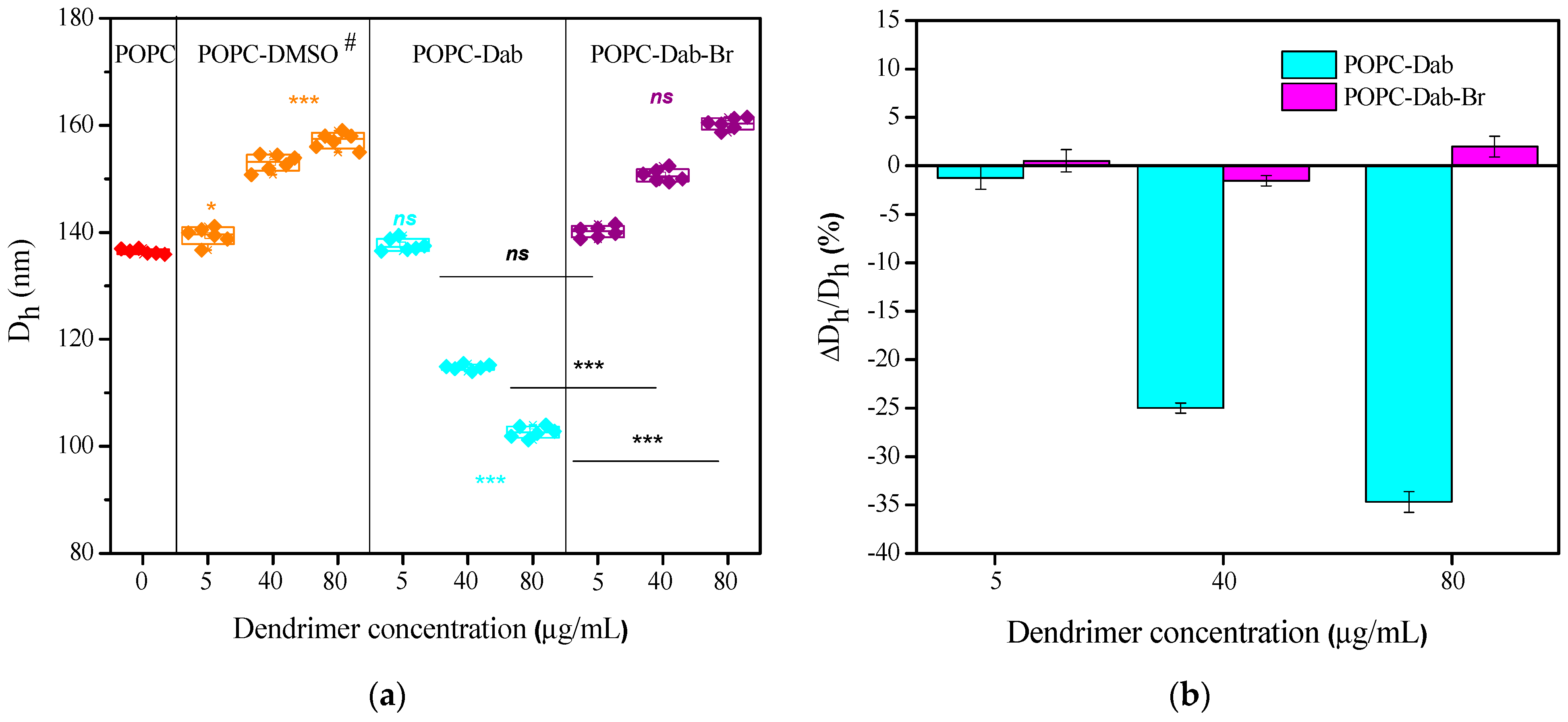
3.4. LUVs Zeta (ζ) Potential Changes and Size Distribution Under Dendrimer Treatment
3.5. Visualization of Dendrimer Action on GUVs’ Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef]
- Pandey, A.; Boros, E. Coordination Complexes to Combat Bacterial Infections: Recent Developments, Current Directions and Future Opportunities. Chemistry 2021, 27, 7340–7350. [Google Scholar] [CrossRef]
- Abd El-Aleam, R.H.; George, R.F.; Georgey, H.H.; Abdel-Rahman, H.M. Bacterial virulence factors: A target for heterocyclic compounds to combat bacterial resistance. RSC Adv. 2021, 11, 36459–36482. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12, 884. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.F.; et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty. 2022, 11, 92, Erratum in Infect. Dis. Poverty 2022, 11, 100. https://doi.org/10.1186/s40249-022-01027-2. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangku, D.; Hu, C.M.; Huang, C.M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Shlaes, D.M. Innovation, nontraditional antibacterial drugs, and clinical utility. ACS Infect. Dis. 2021, 7, 2027–2028. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. Cascade and Nonskid-chain-like Synthesis of Molecular Cavity Topologies. Synthesis 1978, 2, 155–158. [Google Scholar] [CrossRef]
- Maciejewski, M. Concepts of trapping topologically by shell molecules. J. Macromol. Sci. Chem. A 1982, 9, 689–703. [Google Scholar] [CrossRef]
- Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.J.; Kallos, G.; Martin, S.J.R.; Roeck, J.; Ryder, J.; Smith, P.B. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.K.; Gupta, T.; Batheja, S.; Goyal, A.K.; Gupta, U. Surface Engineered Dendrimers: A Potential Nanocarrier for the Effective Management of Glioblastoma Multiforme. Curr. Drug Metab. 2022, 23, 708–722. [Google Scholar] [CrossRef]
- Crintea, A.; Motofelea, A.C.; Șovrea, A.S.; Constantin, A.M.; Crivii, C.B.; Carpa, R.; Duțu, A.G. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A. Heterogeneous Dendrimer-Based Catalysts. Polymers 2022, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers—Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Poudel, D.P.; Taylor, R.T. A Model for Late-Stage Modification of Polyurethane Dendrimers Using Thiol-Ene Click Chemistry. ACS Omega 2021, 6, 12375–12381. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, V. An Overview on Surface Modified Dendrimers: Current Direction and Future Perspectives. Adv. Chem. Res. 2022, 2, 28–39. [Google Scholar] [CrossRef]
- Kesharwani, P. Combination Drug Delivery Approach as an Effective Therapy for Various Diseases, 1st ed.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Xu, L.; Wang, H.; Xiao, W.; Zhang, W.; Stewart, C.; Huang, H.; Li, F.; Han, J. PAMAM dendrimer-based tongue rapidly identifies multiple antibiotics. Sens. Actuators B Chem. 2023, 382, 133519. [Google Scholar] [CrossRef]
- Contin, M.; Garcia, C.; Dobrecky, C.; Lucangioli, S.; D’Accorso, N. Advances in drug delivery, gene delivery and therapeutic agents based on dendritic materials. Future Med. Chem. 2019, 11, 1791–1810. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Hoyos, P.; Perona, A.; Juanes, O.; Rumbero, Á.; Hernáiz, M. Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity. Chem. A Eur. J. 2021, 27, 7593–7624. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, G.; Guo, Y.; Shen, M.; Li, G.; Shchabin, D.; Shi, X. Recent advances in PAMAM dendrimer-based CT contrast agents for molecular imaging and theranostics of cancer. Sens. Diagn. 2023, 2, 1145–1157. [Google Scholar] [CrossRef]
- Li, A.; Gao, Y.; Xiao, X.; Guo, H.; Wang, J.; Zhang, Z.; He, L.; Li, K.; Shcharbin, D.; Shi, X.; et al. Dendrimer-Cu(II) Complexes Mediate Enzyme Delivery for Lactate Depletion-Enhanced Combinational Treatment of Leukemia and Glioma. Adv. Funct. Mater. 2025, 2420825. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Krasnova, I.Y. Dendrimers: Potential Applications in Biomedicine. INEOS OPEN 2018, 1, 85–93. [Google Scholar] [CrossRef]
- Barman, S.R.; Nain, A.; Jain, S.; Punjabi, N.; Mukherji, S.; Satija, J. Dendrimer as a multifunctional capping agent for metal nanoparticles for use in bioimaging, drug delivery and sensor applications. J. Mater. Chem. B 2018, 6, 2368–2384. [Google Scholar] [CrossRef]
- Pooja, D.; Reddy, T.S.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Rawding, P.A.; Bu, J.; Wang, J.; Kim, D.W.; Drelich, A.J.; Kim, Y.; Hong, S. Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1752. [Google Scholar] [CrossRef]
- Kaurav, M.; Ruhi, S.; Al-Goshae, H.A.; Jeppu, A.K.; Ramachandran, D.; Sahu, R.K.; Sarkar, A.K.; Khan, J.; Ikbal, A.M.A. Dendrimer: An update on recent developments and future opportunities for the brain tumors diagnosis and treatment. Front. Pharmacol. 2023, 14, 1159131. [Google Scholar] [CrossRef]
- Falanga, A.; Del Genio, V.; Galdiero, S. Peptides and Dendrimers: How to Combat Viral and Bacterial Infections. Pharmaceutics 2021, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Grabchev, I. Dendrimer as antimicrobial agents. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 363–384. [Google Scholar] [CrossRef]
- Manov, H.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R.; Stoyanova, R.; Kukeva, R.; Stoyanov, S.; Grabchev, I. A New Cu(II) Complex of PAMAM Dendrimer Modified with 1,8-Naphthalimide: Antibacterial and Anticancer Activity. Biointerface Res. Appl. Chem. 2022, 12, 5534–5547. [Google Scholar] [CrossRef]
- Galanakou, C.; Dhumal, D.; Peng, L. Amphiphilic dendrimers against antibiotic resistance: Light at the end of the tunnel? Biomater. Sci. 2023, 11, 3379–3393. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Singhvi, G. Dendrimer: A Novel System in Pharmaceuticals. PharmaTutor 2014, 2, 83–97. [Google Scholar]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- An, H.; Deng, X.; Wang, F.; Xu, P.; Wang, N. Dendrimers as Nanocarriers for the Delivery of Drugs Obtained from Natural Products. Polymers 2023, 15, 2292. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Vilhelmova-Ilieva, N.; Nikolova, I.; Grabchev, I. Synthesis and photophysical characterisation of 3-bromo-4-dimethylamino-1, 8-naphthalimides and their evaluation as agents for antibacterial photodynamic therapy. J. Photochem. Photobiol. A Chem. 2020, 401, 112730. [Google Scholar] [CrossRef]
- Szoka, F., Jr.; Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Dimitrov, D. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Rojewska, M.; Smułek, W.; Kaczorek, E.; Prochaska, K. Langmuir Monolayer Techniques for the Investigation of Model Bacterial Membranes and Antibiotic Biodegradation Mechanisms. Membranes 2021, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Ciumac, D.; Gong, H.; Campbell, R.A.; Campana, M.; Xu, H.; Lu, J.R. Structural elucidation upon binding of antimicrobial peptides into binary mixed lipid monolayers mimicking bacterial membranes. J. Colloid Interface Sci. 2021, 598, 193–205. [Google Scholar] [CrossRef]
- Raghav, S.; Hitaishi, P.; Giri, R.P.; Mukherjee, A.; Sharma, V.K.; Ghosh, S.K. Selective assembly and insertion of ubiquicidin antimicrobial peptide in lipid monolayers. J. Mater. Chem. B. 2024, 12, 11731–11745. [Google Scholar] [CrossRef]
- Elderdfi, M.; Sikorski, A.F. Langmuir-monolayer methodologies for characterizing protein-lipid interactions. Chem. Phys. Lipids 2018, 212, 61–72. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef]
- Marrink, S.J.; Corradi, V.; Souza, P.C.T.; Ingólfsson, H.I.; Tieleman, D.P.; Sansom, M.S.P. Computational Modeling of Realistic Cell Membranes. Chem. Rev. 2019, 119, 6184–6226. [Google Scholar] [CrossRef] [PubMed]
- Wnętrzak, A.; Lipiec, E.; Łątka, K.; Kwiatek, W.; Dynarowicz-Łątka, P. Affinity of alkylphosphocholines to biological membrane of prostate cancer: Studies in natural and model systems. J. Membr. Biol. 2014, 247, 581–589. [Google Scholar] [CrossRef]
- Qiao, L.; Ge, A.; Liang, Y.; Ye, S. Oxidative Degradation of the Monolayer of 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine (POPC) in Low-Level Ozone. J. Phys. Chem. B. 2015, 119, 14188–14199. [Google Scholar] [CrossRef]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Anwar, J. Modulating the structure and properties of cell membranes: The molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Hughes, Z.E.; Mark, A.E.; Mancera, R.L. Molecular dynamics simulations of the interactions of DMSO with DPPC and DOPC phospholipid membranes. J. Phys. Chem. B 2012, 116, 11911–11923. [Google Scholar] [CrossRef]
- Malajczuk, C.J.; Hughes, Z.E.; Mancera, R.L. Molecular dynamics simulations of the interactions of DMSO, mono- and polyhydroxylated cryosolvents with a hydrated phospholipid bilayer. Biochim. Biophys. Acta 2013, 1828, 2041–2055. [Google Scholar] [CrossRef]
- Raju, R.; Torrent-Burgués, J.; Bryant, G. Interactions of cryoprotective agents with phospholipid membranes—A Langmuir monolayer study. Chem. Phys. Lipids 2020, 231, 104949. [Google Scholar] [CrossRef]
- Cancino, J.; Nobre, T.M.; Oliveira, O.N., Jr.; Machado, S.A.; Zucolotto, V. A new strategy to investigate the toxicity of nanomaterials using Langmuir monolayers as membrane models. Nanotoxicology 2013, 7, 61–70. [Google Scholar] [CrossRef]
- Wilde, M.; Green, R.J.; Sanders, M.R.; Greco, F. Biophysical studies in polymer therapeutics: The interactions of anionic and cationic PAMAM dendrimers with lipid monolayers. J. Drug Target. 2017, 25, 910–918. [Google Scholar] [CrossRef]
- Miñones, J., Jr.; Dynarowicz-Latka, P.; Conde, O.; Miñones, J.; Iribarnegaray, E.; Casas, M. Interactions of amphotericin B with saturated and unsaturated phosphatidylcholines at the air/water interface. Colloids Surf. B 2003, 29, 205–215. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Wiącek, A.E. Langmuir monolayer study of phospholipid DPPC on the titanium dioxide–chitosan–hyaluronic acid subphases. Adsorption 2019, 25, 469–476. [Google Scholar] [CrossRef]
- Maget-Dana, R. The monolayer technique: A potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim. Biophys. Acta 1999, 1462, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Golonka, I.; Łukasiewicz, I.W.; Sebastiańczyk, A.; Greber, K.E.; Sawicki, W.; Musiał, W. The Influence of the Amphiphilic Properties of Peptides on the Phosphatidylinositol Monolayer in the Presence of Ascorbic Acid. Int. J. Mol. Sci. 2024, 25, 12484. [Google Scholar] [CrossRef]
- Yu, Z.W.; Jin, J.; Cao, Y. Characterization of the liquid-expanded to liquid-condensed phase transition of monolayers by means of compressibility. Langmuir 2002, 18, 4530–4531. [Google Scholar] [CrossRef]
- Mildner, J.; Wnętrzak, A.; Dynarowicz-Latka, P. Cholesterol and Cardiolipin Importance in Local Anesthetics-Membrane Interactions: The Langmuir Monolayer Study. J. Membr. Biol. 2019, 252, 31–39. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena, 2nd ed.; Academic Press: New York, NY, USA, 1963. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Kyzioł, A. Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: I. DPPG. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 349–358. [Google Scholar] [CrossRef]
- Tian, F.; Lin, X.; Valle, R.P.; Zuo, Y.Y.; Gu, N. Poly(amidoamine) Dendrimer as a Respiratory Nanocarrier: Insights from Experiments and Molecular Dynamics Simulations. Langmuir 2019, 35, 5364–5371. [Google Scholar] [CrossRef]
- Korkmaz, F.; Köster, S.; Yildiz, O.; Mäntele, W. The Role of Lipids for the Functional Integrity of Porin: An FTIR Study Using Lipid and Protein Reporter Groups. Biochemistry 2008, 47, 12126–12134. [Google Scholar] [CrossRef]
- Peng, B.; Ding, X.; Sun, C.; Yang, Y.; Gao, Y.; Zhao, X. The chain order of binary unsaturated lipid bilayers modulated by aromatic-residue-containing peptides: An ATR-FTIR spectroscopy study. RSC Adv. 2017, 7, 29386. [Google Scholar] [CrossRef]
- Yakimov, I.D.; Kolmogorov, I.M.; Le-Deygen, I.M. Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies. Biophysica 2023, 3, 501–512. [Google Scholar] [CrossRef]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta 1984, 779, 381–401. [Google Scholar] [CrossRef]
- Arrondo, J.L.; Goñi, F.M.; Macarulla, J.M. Infrared spectroscopy of phosphatidylcholines in aqueous suspension. A study of the phosphate group vibrations. Biochim. Biophys. Acta 1984, 794, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Nawagamuwage, S.; Parshin, I.; Richard, M.; Burin, A.; Rubtsov, I. Probing the Hydrophobic Region of a Lipid Bilayer at Specific Depths Using Vibrational Spectroscopy. J. Am. Chem. Soc. 2023, 145, 26363–26373. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Mukamel, S. Vibrational sum-frequency generation spectroscopy at the water/lipid interface: Molecular dynamics simulation study. J. Am. Chem. Soc. 2010, 132, 6434–6442. [Google Scholar] [CrossRef] [PubMed]
- Ohto, T.; Backus, E.H.; Hsieh, C.S.; Sulpizi, M.; Bonn, M.; Nagata, Y. Lipid carbonyl groups terminate the hydrogen bond network of membrane-bound water. J. Phys. Chem. Lett. 2015, 6, 4499–4503. [Google Scholar] [CrossRef]
- Lorenz-Fonfria, V.A. Infrared Difference Spectroscopy of Proteins: From Bands to Bonds. Chem. Rev. 2020, 120, 3466–3576. [Google Scholar] [CrossRef]
- Fringeli, U.P.; Günthard, H.H. Infrared Membrane Spectroscopy. In Membrane Spectroscopy; Grell, E., Ed.; Springer: Berlin, Germany, 1981; pp. 270–332. [Google Scholar] [CrossRef]
- Chen, X.; Al-Mualem, Z.A.; Baiz, C.R. Lipid Landscapes: Vibrational Spectroscopy for Decoding Membrane Complexity. Annu. Rev. Phys. Chem. 2024, 75, 283–305. [Google Scholar] [CrossRef]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Schmid, M.; Wölk, C.; Giselbrecht, J.; Chan, K.L.A.; Harvey, R.D. A combined FTIR and DSC study on the bilayer-stabilising effect of electrostatic interactions in ion paired lipids. Colloids Surf. B Biointerfaces 2018, 169, 298–304. [Google Scholar] [CrossRef]
- Watkins, E.B.; Miller, C.E.; Liao, W.P.; Kuhl, T.L. Equilibrium or quenched: Fundamental differences between lipid monolayers, supported bilayers, and membranes. ACS Nano 2014, 8, 3181–3191. [Google Scholar] [CrossRef]
- Pinto, O.A.; Disalvo, E.A. A new model for lipid monolayer and bilayers based on thermodynamics of irreversible processes. PLoS ONE 2019, 14, e0212269. [Google Scholar] [CrossRef]
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and use of model membranes to study biomolecular interactions using complementary surface-sensitive techniques. Adv. Colloid Interface Sci. 2020, 277, 102118. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Devoisselle, J.; Chopineau, J. The relevance of membrane models to understand nanoparticles-cell membrane interactions. Nanoscale 2016, 8, 44780–44798. [Google Scholar] [CrossRef]
- Nabika, H.; Unoura, K. Interaction between nanoparticles and cell membrane. In Surface Chemistry of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 231–263. [Google Scholar] [CrossRef]
- Bunea, A.I.; Harloff-Helleberg, S.; Taboryski, R.; Nielsen, H.M. Membrane interactions in drug delivery: Model cell membranes and orthogonal techniques. Adv. Colloid Interface Sci. 2020, 281, 102177. [Google Scholar] [CrossRef]
- Claessens, M.M.; Van Oort, B.F.; Leermakers, F.A.; Hoekstra, F.A.; Stuart, M.A.C. Charged lipid vesicles: Effects of salts on bending rigidity, stability, and size. Biophys. J. 2004, 87, 3882–3893. [Google Scholar] [CrossRef]
- Tayebi, L.; Vashaee, D.; Parikh, A.N. Stability of uni- and multillamellar spherical vesicles. Chemphyschem 2012, 13, 314–322. [Google Scholar] [CrossRef]
- Åkesson, A.K.L.; Lundgaard, C.V.; Ehrlich, N.; Günther-Pomorski, T.; Stamou, D.; Cárdenas, M. Induced dye leakage by PAMAM G6 does not imply dendrimer entry into vesicle lumen. Soft Matter 2012, 8, 8972–8980. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—Cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Surewicz, W.K. Membrane actions of water-soluble fusogens: Effect of dimethyl sulfoxide, glycerol and sucrose on lipid bilayer order and fluidity. Chem. Phys. Lipids 1984, 34, 363–372. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Rangel, M.; Bastos, M. Effect of DMSO on Structural Properties of DMPC and DPPC Liposome Suspensions. J. Funct. Biomater. 2024, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Bellocco, E.; Laganà, G.; Barreca, D.; Magazù, S.; Wanderlingh, U.; Kiselev, M.A. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. Biochim. Biophys. Acta 2016, 1858, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef]
- Xie, H.; Li, L.; Sun, Y.; Wang, Y.; Gao, S.; Tian, Y.; Ma, X.; Guo, C.; Bo, F.; Zhang, L. An Available Strategy for Nasal Brain Transport of Nanocomposite Based on PAMAM Dendrimers via In Situ Gel. Nanomaterials 2019, 24, 147. [Google Scholar] [CrossRef]
- Milowska, K.; Rodacka, A.; Melikishvili, S.; Buczkowski, A.; Pałecz, B.; Waczulikova, I.; Hianik, T.; Majoral, J.P.; Ionov, M.; Bryszewska, M. Dendrimeric HIV-peptide delivery nanosystem affects lipid membranes structure. Sci. Rep. 2021, 11, 16810. [Google Scholar] [CrossRef]
- Smith, P.E.; Brender, J.R.; Dürr, U.H.; Xu, J.; Mullen, D.G.; Banaszak Holl, M.M.; Ramamoorthy, A. Solid-state NMR reveals the hydrophobic-core location of poly(amidoamine) dendrimers in biomembranes. J. Am. Chem. Soc. 2010, 132, 8087–8097. [Google Scholar] [CrossRef]
- Kelly, C.V.; Liroff, M.G.; Triplett, L.D.; Leroueil, P.R.; Mullen, D.G.; Wallace, J.M.; Meshinchi, S.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Stoichiometry and Structure of Poly(amidoamine) Dendrimer-Lipid Complexes. ACS Nano 2009, 3, 1886–1896. [Google Scholar] [CrossRef]
- Veliskova, M.; Zvarik, M.; Suty, S.; Jacko, J.; Mydla, P.; Cechova, K.; Dzubinska, D.; Morvova, M.; Ionov, M.; Terehova, M.; et al. In Vitro Interactions of Amphiphilic Phosphorous Dendrons with Liposomes and Exosomes—Implications for Blood Viscosity Changes. Pharmaceutics 2022, 14, 1596. [Google Scholar] [CrossRef]
- Efimova, A.A.; Sorokina, S.A.; Trosheva, K.S.; Yaroslavov, A.A.; Shifrina, Z.B. Complexes of Cationic Pyridylphenylene Dendrimers with Anionic Liposomes: The Role of Dendrimer Composition in Membrane Structural Changes. Int. J. Mol. Sci. 2023, 24, 2225. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Garaiová, Z.; Waczulíková, I.; Wrobel, D.; Pędziwiatr-Werbicka, E.; Gómez-Ramírez, R.; de la Mata, F.J.; Klajnert, B.; Hianik, T.; Bryszewska, M. siRNA carriers based on carbosilane dendrimers affect zeta potential and size of phospholipid vesicles. Biochim. Biophys. Acta 2012, 1818, 2209–2216. [Google Scholar] [CrossRef]
- Ionov, M.; Ciepluch, K.; Garaiova, Z.; Melikishvili, S.; Michlewska, S.; Balcerzak, Ł.; Glińska, S.; Miłowska, K.; Gomez-Ramirez, R.; de la Mata, F.J.; et al. Dendrimers complexed with HIV-1 peptides interact with liposomes and lipid monolayers. Biochim. Biophys. Acta 2015, 1848, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Trosheva, K.S.; Sorokina, S.A.; Efimova, A.A.; Semenyuk, P.I.; Berkovich, A.K.; Yaroslavov, A.A.; Shifrina, Z.B. Interaction of multicomponent anionic liposomes with cationic pyridylphenylene dendrimer: Does the complex behavior depend on the liposome composition? Biochim. Biophys. Acta Biomembr. 2021, 1863, 183761. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.; Akesson, A.; Chapuis, P.Y.; Skrzynski Nielsen, C.A.; Monopoli, M.P.; Dawson, K.A.; Pomorski, T.G.; Cárdenas, M. The dendrimer impact on vesicles can be tuned based on the bilayer charge and the presence of albumin. Soft Matter 2013, 9, 8862–8870. [Google Scholar] [CrossRef]
- Aden, S.; Snoj, T.; Anderluh, G. The use of giant unilamellar vesicles to study functional properties of pore-forming toxins. Methods Enzymol. 2021, 649, 219–251. [Google Scholar] [CrossRef]
- Dolder, N.; Müller, P.; von Ballmoos, C. Experimental platform for the functional investigation of membrane proteins in giant unilamellar vesicles. Soft Matter 2022, 18, 5877–5893. [Google Scholar] [CrossRef]
- Chang, P.K.C.; Prestidge, C.A.; Bremmell, K.E. PAMAM versus PEI complexation for siRNA delivery: Interaction with model lipid membranes and cellular uptake. Pharm. Res. 2022, 39, 1151–1163. [Google Scholar] [CrossRef]
- Roy, B.; Guha, P.; Chang, C.H.; Nahak, P.; Karmakar, G.; Bykov, A.G.; Akentiev, A.V.; Noskov, B.A.; Patra, A.; Dutta, K.; et al. Effect of cationic dendrimer on membrane mimetic systems in the form of monolayer and bilayer. Chem. Phys. Lipids 2024, 258, 105364. [Google Scholar] [CrossRef]
- Mecke, A.; Uppuluri, S.; Sassanella, T.M.; Lee, D.K.; Ramamoorthy, A.; Baker, J.R., Jr.; Orr, B.G.; Holl, M.M.B. Direct observation of lipid bilayer disruption by poly(amidoamine) dendrimers. Chem. Phys. Lipids 2004, 132, 3–14. [Google Scholar] [CrossRef]
- Shcharbin, D.; Drapeza, A.; Loban, V.; Lisichenok, A.; Bryszewska, M. The breakdown of bilayer lipid membranes by dendrimers. Cell. Mol. Biol. Lett. 2006, 11, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Larson, R.G. Multiscale Modeling of Dendrimers and Their Interactions with Bilayers and Polyelectrolytes. Molecules 2009, 14, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Domański, D.M.; Klajnert, B.; Bryszewska, M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochem 2004, 63, 189–191. [Google Scholar] [CrossRef]
- Ziemba, B.; Matuszko, G.; Bryszewska, M.; Klajnert, B. Influence of dendrimers on red blood cells. Cell. Mol. Biol. Lett. 2012, 17, 21–35. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, R.; Li, C.; Wang, Q.; Liu, Z.; Xue, W. Effects of poly(amidoamine) dendrimers on the structure and function of key blood components. J. Bioact. Compat. Polym. 2014, 29, 165–179. [Google Scholar] [CrossRef]
- Sideratou, Z.; Foundis, J.; Tsiourvas, D.; Nezis, I.P.; Papadimas, G.; Paleos, C.M. A Novel Dendrimeric “Glue” for Adhesion of Phosphatidyl Choline-Based Liposomes. Langmuir 2002, 18, 5036–5039. [Google Scholar] [CrossRef]
- Roy, B.; Guha, P.; Nahak, P.; Karmakar, G.; Maiti, S.; Mandal, A.K.; Bykov, A.G.; Akentiev, A.V.; Noskov, B.A.; Tsuchiya, K.; et al. Biophysical Correlates on the Composition, Functionality, and Structure of Dendrimer-Liposome Aggregates. ACS Omega 2018, 3, 12235–12245. [Google Scholar] [CrossRef]










| (a) | (b) | |||
|---|---|---|---|---|
| DMSO (µL) | POPC + DMSO | Dendrimers (µg/mL) | POPC + Dab | POPC + Dab-Br |
| 0 | 0.12 ± 0.04 | 0 | 0.12 ± 0.04 | 0.12 ± 0.04 |
| 5 | 0.14 ± 0.02 | 5 | 0.14 ± 0.03 | 0.12 ± 0.02 |
| 40 | 0.16 ± 0.03 | 40 | 0.20 ± 0.03 | 0.17 ± 0.03 |
| 80 | 0.19 ± 0.02 | 80 | 0.24 ± 0.01 | 0.20 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordanova, A.; Tsanova, A.; Stoimenova, E.; Minkov, I.; Kostadinova, A.; Hazarosova, R.; Angelova, R.; Antonova, K.; Vitkova, V.; Staneva, G.; et al. Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes. Polymers 2025, 17, 929. https://doi.org/10.3390/polym17070929
Jordanova A, Tsanova A, Stoimenova E, Minkov I, Kostadinova A, Hazarosova R, Angelova R, Antonova K, Vitkova V, Staneva G, et al. Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes. Polymers. 2025; 17(7):929. https://doi.org/10.3390/polym17070929
Chicago/Turabian StyleJordanova, Albena, Asya Tsanova, Emilia Stoimenova, Ivan Minkov, Aneliya Kostadinova, Rusina Hazarosova, Ralitsa Angelova, Krassimira Antonova, Victoria Vitkova, Galya Staneva, and et al. 2025. "Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes" Polymers 17, no. 7: 929. https://doi.org/10.3390/polym17070929
APA StyleJordanova, A., Tsanova, A., Stoimenova, E., Minkov, I., Kostadinova, A., Hazarosova, R., Angelova, R., Antonova, K., Vitkova, V., Staneva, G., & Grabchev, I. (2025). Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes. Polymers, 17(7), 929. https://doi.org/10.3390/polym17070929









