Efficient Lignin Precipitation from Softwood Black Liquor Using Organic Acids for Sustainable Valorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Employed
2.2. Black Liquor Characterization
2.3. Lignin Precipitation Using Organic Acids
2.4. Lignins Characterization
2.4.1. Chemical Characterization
2.4.2. Thermal Characterization
3. Results and Discussion
3.1. Lignin Precipitation
3.2. Lignin Characterization
3.2.1. Chemical Characterization
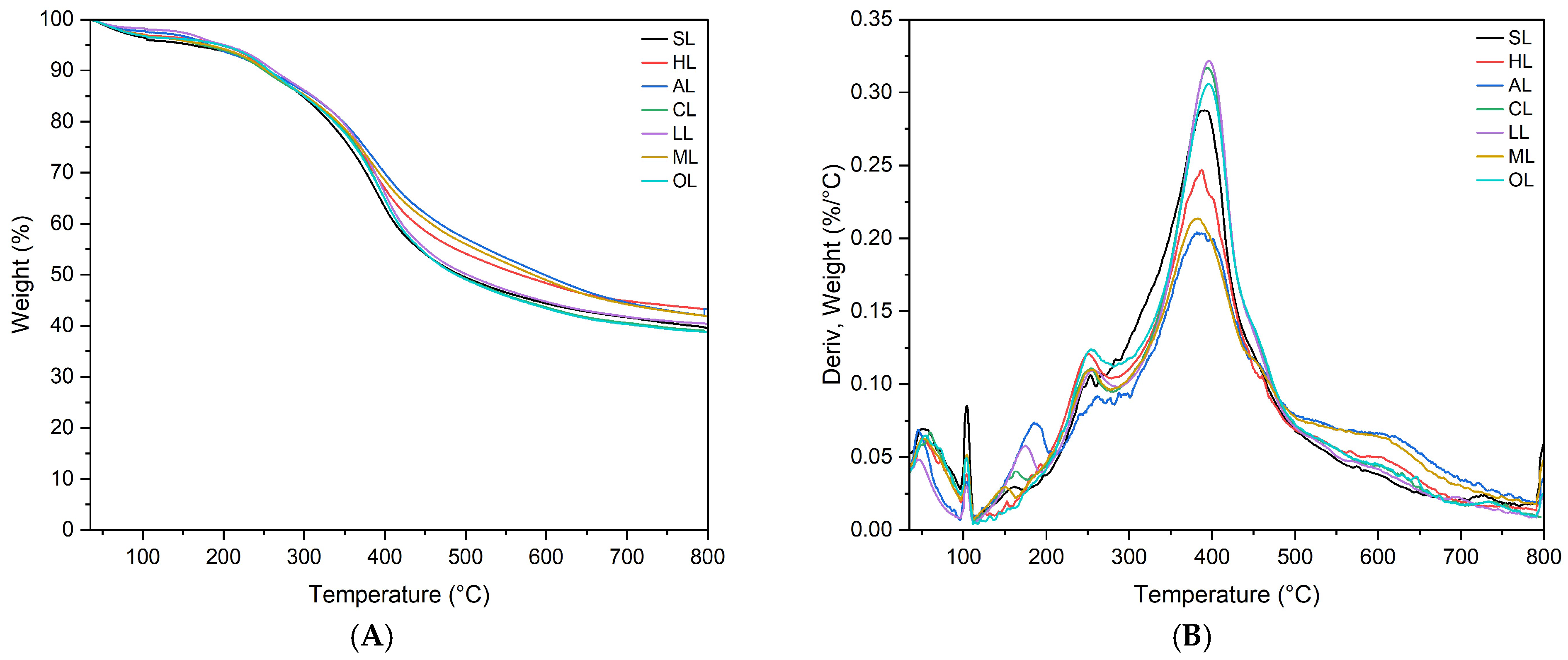
3.2.2. Thermal Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falcone, P.M.; Hiete, M. Exploring green and sustainable chemistry in the context of sustainability transition: The role of visions and policy. Curr. Opin. Green Sustain. Chem. 2019, 19, 66–75. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Huang, J.; Fu, S.; Gan, L. Lignin Chemistry and Applications; Huang, J., Fu, S., Gan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128139417. [Google Scholar]
- Morales, A.; Labidi, J.; Gullón, P. Impact of the lignin type and source on the characteristics of physical lignin hydrogels. Sustain. Mater. Technol. 2022, 31, e00369. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Erdocia, X.; Charrier-El Bouhtoury, F.; Prado, R.; Labidi, J. Multistage treatment of almonds waste biomass: Characterization and assessment of the potential applications of raw material and products. Waste Manag. 2018, 80, 40–50. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Naron, D.R.; Collard, F.-X.; Tyhoda, L.; Görgens, J.F. Characterisation of lignins from different sources by appropriate analytical methods: Introducing thermogravimetric analysis-thermal desorption-gas chromatography–mass spectroscopy. Ind. Crops Prod. 2017, 101, 61–74. [Google Scholar] [CrossRef]
- Haq, I.; Mazumder, P.; Kalamdhad, A.S. Recent advances in removal of lignin from paper industry wastewater and its industrial applications—A review. Bioresour. Technol. 2020, 312, 123636. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; Alriols, M.G.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2023, 2, 37–90. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Pu, Y.; Ragauskas, A. Cellulosic biorefineries-unleashing lignin opportunities. Curr. Opin. Environ. Sustain. 2010, 2, 383–393. [Google Scholar] [CrossRef]
- Li, W.; Wu, S. Depolymerization and product evolution of kraft lignin in a novel acid deep eutectic solvents (DESs). Ind. Crops Prod. 2025, 225, 120476. [Google Scholar] [CrossRef]
- Idrus, A.; Dwiatmoko, A.A.; Maryati, Y. Lignin depolymerization using nickel-based catalysts: A mini review. Inorg. Chem. Commun. 2025, 174, 113901. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Jeffri, N.I.; Mohammad Rawi, N.F.; Mohamad Kassim, M.H.; Abdullah, C.K. Unlocking the potential: Evolving role of technical lignin in diverse applications and overcoming challenges. Int. J. Biol. Macromol. 2024, 274, 133506. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Gilarranz, M.A.; Rodríguez, F.; Oliet, M.; Revenga, J.A. Acid precipitation and purification of wheat straw lignin. Sep. Sci. Technol. 1998, 33, 1359–1377. [Google Scholar] [CrossRef]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Namane, M.; José García-Mateos, F.; Sithole, B.; Ramjugernath, D.; Rodríguez-Mirasol, J.; Cordero, T. Characteristics of Lignin Precipitated With Organic Acids As a Source for Valorisation of Carbon Products. Cellul. Chem. Technol. 2016, 50, 3–4. [Google Scholar]
- da Silva, S.H.F.; Gordobil, O.; Labidi, J. Organic acids as a greener alternative for the precipitation of hardwood kraft lignins from the industrial black liquor. Int. J. Biol. Macromol. 2020, 142, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, X.; Li, Y.; Li, L.; Huang, Y. Extraction of lignin from pulping black liquor by organic acid. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2009; Volume 620–622, pp. 571–574. [Google Scholar]
- Sharma, M.; Simões, A.; Alves, P.; Gando-Ferreira, L.M. Efficient Recovery of Lignin and Hemicelluloses from Kraft Black Liquor. In KnE Material Sciences; KnE Open: Malang, Indonesia, 2022. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Kamenská, L.; Vrška, M.; Šima, J. Deep Eutectic Solvents: Fractionation of Wheat Straw. BioResources 2015, 10, 8039–8047. [Google Scholar] [CrossRef]
- Cronin, D.J.; Zhang, X.; Bartley, J.; Doherty, W.O.S. Lignin Depolymerization to Dicarboxylic Acids with Sodium Percarbonate. ACS Sustain. Chem. Eng. 2017, 5, 6253–6260. [Google Scholar] [CrossRef]
- Santos, P.S.B.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics With Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Kumar, M.; Tyagi, I.; Kumar Pandey, A.; Park, J.; Raj, T.; Sirohi, R.; Kumar, V.; Kim, S.-H. Recent advances in black liquor valorization. Bioresour. Technol. 2022, 350, 126916. [Google Scholar] [CrossRef]
- Zhu, W.; Theliander, H. Precipitation of Lignin from Softwood Black Liquor: An Investigation of the Equilibrium and Molecular Properties of Lignin. BioResources 2015, 10, 1696–1714. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S.; Chen, Y. Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef]
- Namane, M. Precipitation and Valorisation of Lignin Obtained from South African Kraft Mill Black Liquor. University of Cape Town: Cape Town, South Africa, 2016. [Google Scholar]
- Hubbe, M.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Mohamad Ibrahim, M.; Chuah, S.; Wan Rosli, W.D. Characterization of Lignin Precipitated From The Soda Black Liquor of Oil Palm Empty Fruit Bunch Fibers by Various Mineral Acids. ASEAN J. Sci. Technol. Dev. 2017, 21, 57. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Espinosa, E.; Savy, D.; Rosal, A.; Rodriguez, A. Biorefinery Process Combining Specel® Process and Selective Lignin Precipitation using Mineral Acids. BioResources 2016, 11, 7061–7077. [Google Scholar]
- Sharma, M.; Marques, J.; Simões, A.; Donato, M.M.; Cardoso, O.; Gando-Ferreira, L.M. Optimization of lignin precipitation from black liquor using organic acids and its valorization by preparing lignin nanoparticles. Int. J. Biol. Macromol. 2024, 269, 131881. [Google Scholar] [CrossRef]
- Perez-arce, J. A New Route Toward Lignin-Based Polyols—A Highway for Lignin Exploitation. Ph.D. Thesis, Universidad del País Vasco-Euskal Herriko Unibertsitatea, Biscay, Spain, 2020. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Yuan, Q.L.; Huang, F. Study of Chemical Modification of Alkaline Lignin by the Glyoxalation Reaction. BioResources 2011, 6, 4523–4536. [Google Scholar]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Jonas Ragauskas, A. Fractionation of Organosolv Lignin Using Acetone:Water and Properties of the Obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; He, J.; Xu, F.; Sun, R.C. Fractionation and physico-chemical analysis of degraded lignins from the black liquor of Eucalyptus pellita KP-AQ pulping. Polym. Degrad. Stab. 2009, 94, 1142–1150. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Alriols, M.G.; Tejado, A.; Blanco, M.; Mondragon, I.; Labidi, J. Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem. Eng. J. 2009, 148, 106–114. [Google Scholar] [CrossRef]
- Yasuda, S.; Fukushima, K.; Kakehi, A. Formation and chemical structures of acid-soluble lignin I: Sulfuric acid treatment time and acid-soluble lignin content of hardwood. J. Wood Sci. 2001, 47, 69–72. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Lloyd Jones, G. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Kamble, S.V.; Bhattacharyulu, Y.C. Selective separation of biomass from black liquor waste by inorganic and organic acids. Int. J. Adv. Res. 2015, 3, 684–692. [Google Scholar]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodríguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crops Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]



| Parameter | Value | Unit |
|---|---|---|
| Density | 1.14 ± 0.00 | g/mL |
| pH | 12.77 ± 0.46 | - |
| Total dry solid | 21.89 ± 0.06 | (%) |
| Acid-soluble lignin | 6.66 ± 0.04 | (%) |
| Acid-insoluble lignin | 27.31 ± 0.36 | (%) |
| Carbohydrates | 10.97 ± 0.13 | (%) |
| Volatiles | 27.13 ± 0.31 | (%) |
| Fixed carbon | 21.71 ± 1.47 | (%) |
| Ash | 47.16 ± 0.85 | (%) |
| g of Lignin Obtained/ 100 mL of Black Liquor | OH Content (mmol OH/g Dry Lignin) | IC50 (µg/mL) | Total Phenolic Content (µg GAE/g Dry Lignin) | |
|---|---|---|---|---|
| SL | 7.41 ± 0.09 | 0.67 ± 0.06 | 36.82 ± 1.17 | 285.01 ± 25.40 |
| HL | 8.07 ± 0.75 | 0.78 ± 0.03 | 28.72 ± 0.32 | 333.40 ± 12.33 |
| AL | 6.26 ± 0.56 | 0.70 ± 0.02 | 27.35 ± 0.24 | 298.38 ± 7.74 |
| CL | 7.45 ± 0.32 | 0.79 ± 0.00 | 29.95 ± 0.34 | 336.34 ± 1.30 |
| LL | 7.01 ± 0.49 | 0.82 ± 0.02 | 31.93 ± 0.35 | 348.39 ± 8.77 |
| ML | 7.62 ± 0.39 | 0.78 ± 0.02 | 32.65 ± 0.09 | 331.84 ± 10.17 |
| OL | 7.62 ± 0.03 | 0.73 ± 0.08 | 32.50 ± 0.91 | 331.84 ± 32.51 |
| Mn (g/mol) | Mw (g/mol) | Đ | |
|---|---|---|---|
| SL | 2042 | 14,024 | 6.87 |
| HL | 2781 | 15,415 | 5.54 |
| AL | 3012 | 17,130 | 5.69 |
| CL | 2667 | 16,025 | 6.01 |
| LL | 2875 | 16,160 | 5.62 |
| ML | 3148 | 17,358 | 5.51 |
| OL | 2770 | 15,620 | 5.64 |
| AIL (%) | ASL (%) | Carbohydrates (%) | Volatiles (%) | Fixed Carbon (%) | Ash (%) | |
|---|---|---|---|---|---|---|
| SL | 77.66 ± 7.63 | 1.68 ± 0.06 | 3.25 ± 0.13 | 44.83 ± 1.20 | 51.53 ± 1.35 | 3.28 ± 0.07 |
| HL | 90.87 ± 0.61 | 1.63 ± 0.04 | 3.58 ± 0.02 | 45.56 ± 1.27 | 52.71 ± 1.51 | 0.31 ± 0.04 |
| AL | 89.76 ± 0.40 | 0.65 ± 0.09 | 3.76 ± 0.11 | 45.13 ± 0.27 | 54.08 ± 0.64 | 0.17 ± 0.02 |
| CL | 90.35 ± 1.01 | 1.77 ± 0.33 | 3.04 ± 0.02 | 48.16 ± 0.73 | 51.07 ± 0.38 | 0.32 ± 0.02 |
| LL | 85.20 ± 5.24 | 1.05 ± 0.13 | 3.37 ± 0.25 | 40.49 ± 2.86 | 54.95 ± 2.3 | 0.10 ± 0.02 |
| ML | 92.00 ± 0.99 | 1.11 ± 0.17 | 3.53 ± 0.13 | 52.14 ± 2.55 | 52.14 ± 2.55 | 0.49 ± 0.02 |
| OL | 92.43 ± 0.20 | 1.27 ± 0.05 | 3.38 ± 0.09 | 46.02 ± 0.91 | 47.60 ± 0.87 | 0.45 ± 0.03 |
| SL | HL | AL | CL | LL | ML | OL | ||
|---|---|---|---|---|---|---|---|---|
| OH types (mmol/g) | Aliphatic | 1.57 | 1.97 | 1.41 | 2.70 | 1.91 | 1.59 | 1.86 |
| C5-substituted | 2.57 | 2.93 | 1.75 | 3.35 | 2.84 | 2.71 | 2.6 | |
| Guaiacyl | 3.21 | 3.70 | 1.69 | 3.63 | 3.33 | 3.27 | 3.24 | |
| p-hydroxyphenyl | 0.03 | 0.08 | 0.09 | 0.11 | 0.07 | 0.06 | 0.02 | |
| Syringyl | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 | 0.04 | 0.05 | |
| Phenolic | 5.81 | 6.70 | 3.53 | 7.08 | 6.24 | 5.81 | 5.85 | |
| Carboxylic acid | 0.27 | 0.31 | 0.15 | 0.28 | 0.19 | 0.27 | 0.20 | |
| S/G ratio | 0.009 | 0.011 | 0.018 | 0.008 | 0.006 | 0.012 | 0.015 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duret, E.; de Moura, L.C.R.; Morales, A.; Labidi, J.; Robles, E.; Charrier-El Bouhtoury, F. Efficient Lignin Precipitation from Softwood Black Liquor Using Organic Acids for Sustainable Valorization. Polymers 2025, 17, 926. https://doi.org/10.3390/polym17070926
Duret E, de Moura LCR, Morales A, Labidi J, Robles E, Charrier-El Bouhtoury F. Efficient Lignin Precipitation from Softwood Black Liquor Using Organic Acids for Sustainable Valorization. Polymers. 2025; 17(7):926. https://doi.org/10.3390/polym17070926
Chicago/Turabian StyleDuret, Elsa, Luanna C. R. de Moura, Amaia Morales, Jalel Labidi, Eduardo Robles, and Fatima Charrier-El Bouhtoury. 2025. "Efficient Lignin Precipitation from Softwood Black Liquor Using Organic Acids for Sustainable Valorization" Polymers 17, no. 7: 926. https://doi.org/10.3390/polym17070926
APA StyleDuret, E., de Moura, L. C. R., Morales, A., Labidi, J., Robles, E., & Charrier-El Bouhtoury, F. (2025). Efficient Lignin Precipitation from Softwood Black Liquor Using Organic Acids for Sustainable Valorization. Polymers, 17(7), 926. https://doi.org/10.3390/polym17070926








