Molecular Dynamics Simulation of Interfacial Effects in PBT-Based Azide Propellants Under Tensile Deformation
Abstract
1. Introduction
2. Materials and Methods
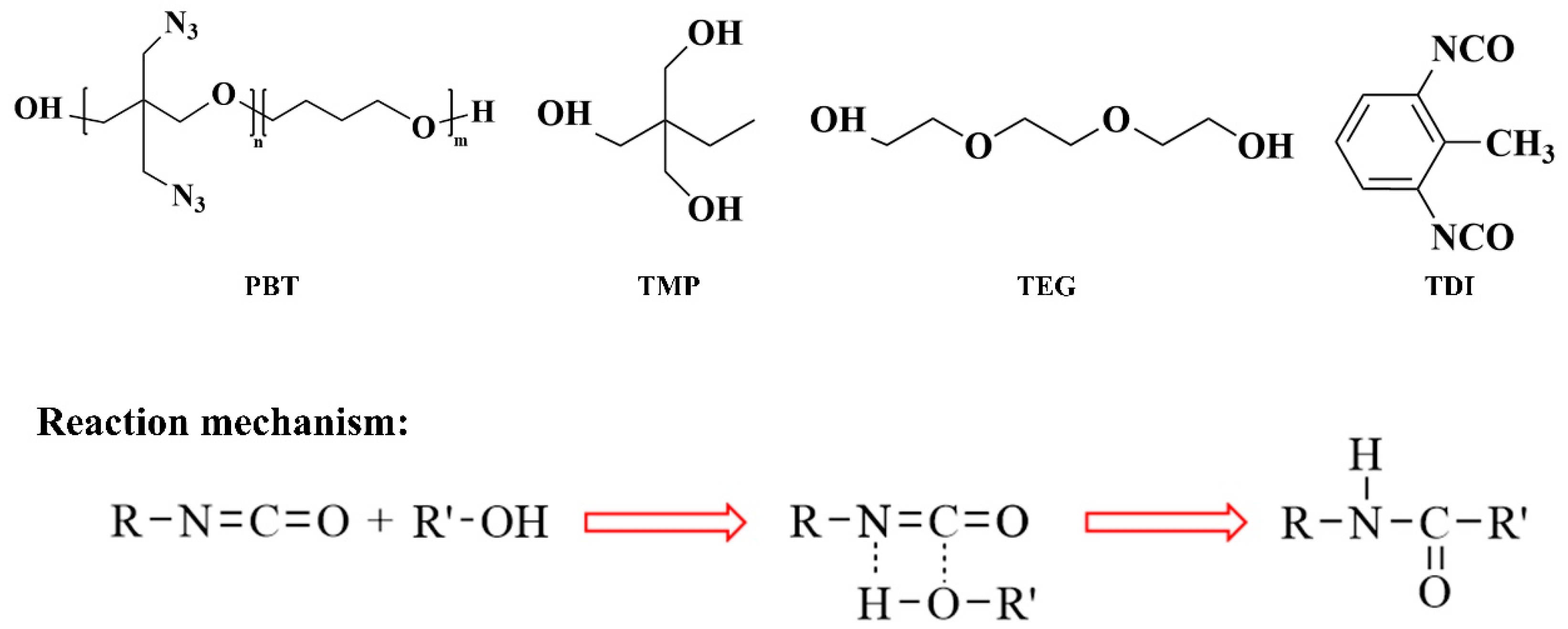
2.1. Initial Model
2.2. Establishment of the PBT-AP Interface Model
2.3. Uniaxial Tension Simulation
3. Results and Discussion
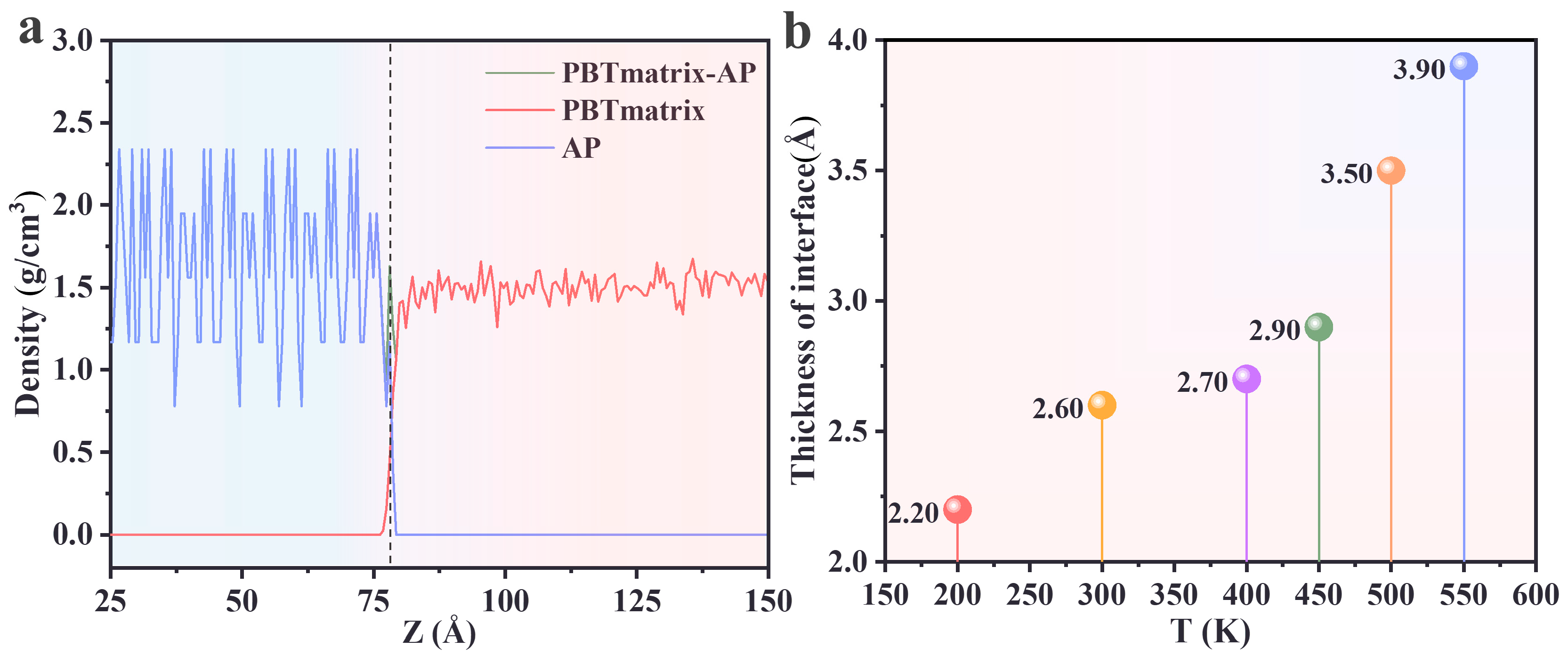
3.1. Determination of Interface Area
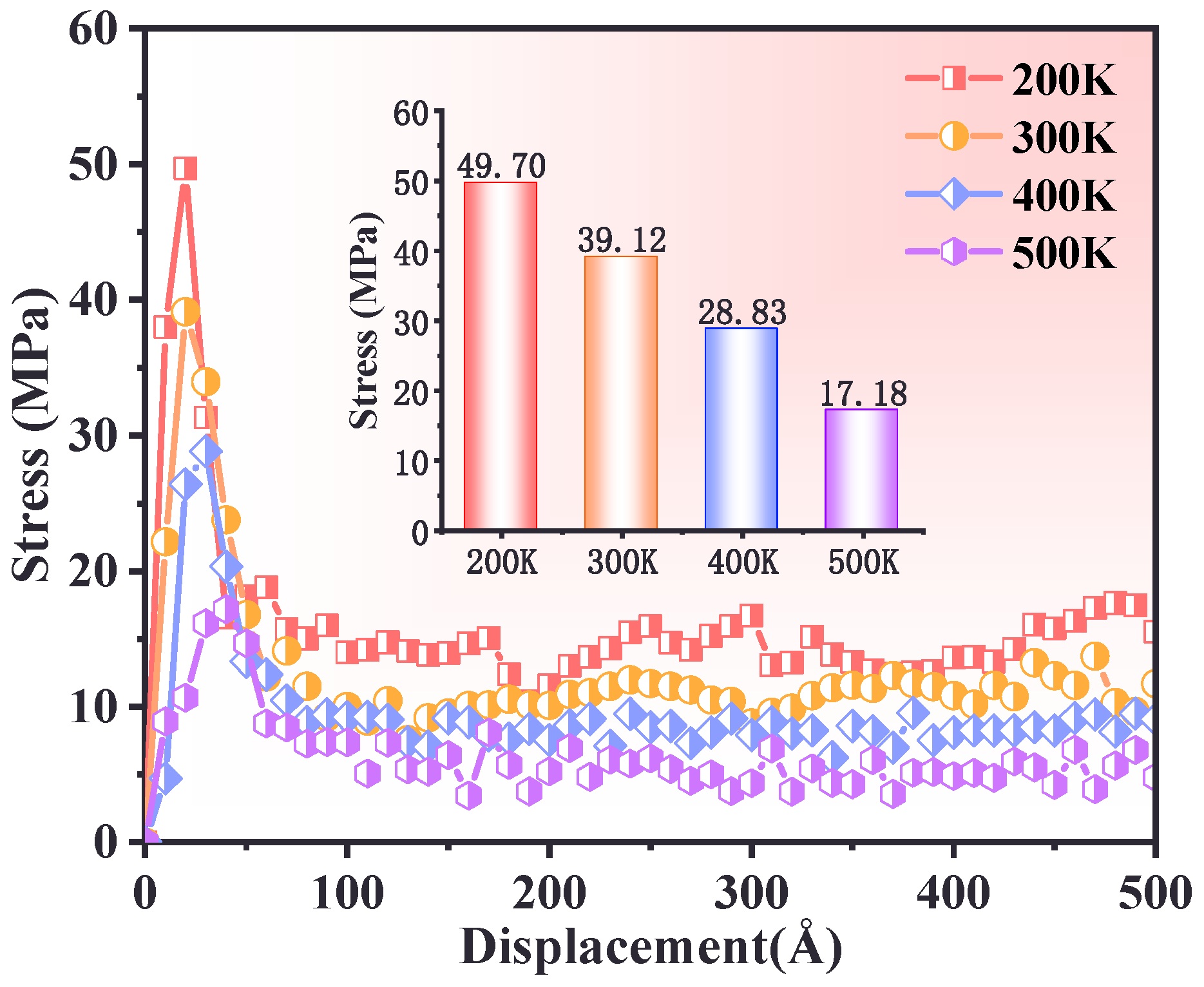
3.2. Effect of Temperature
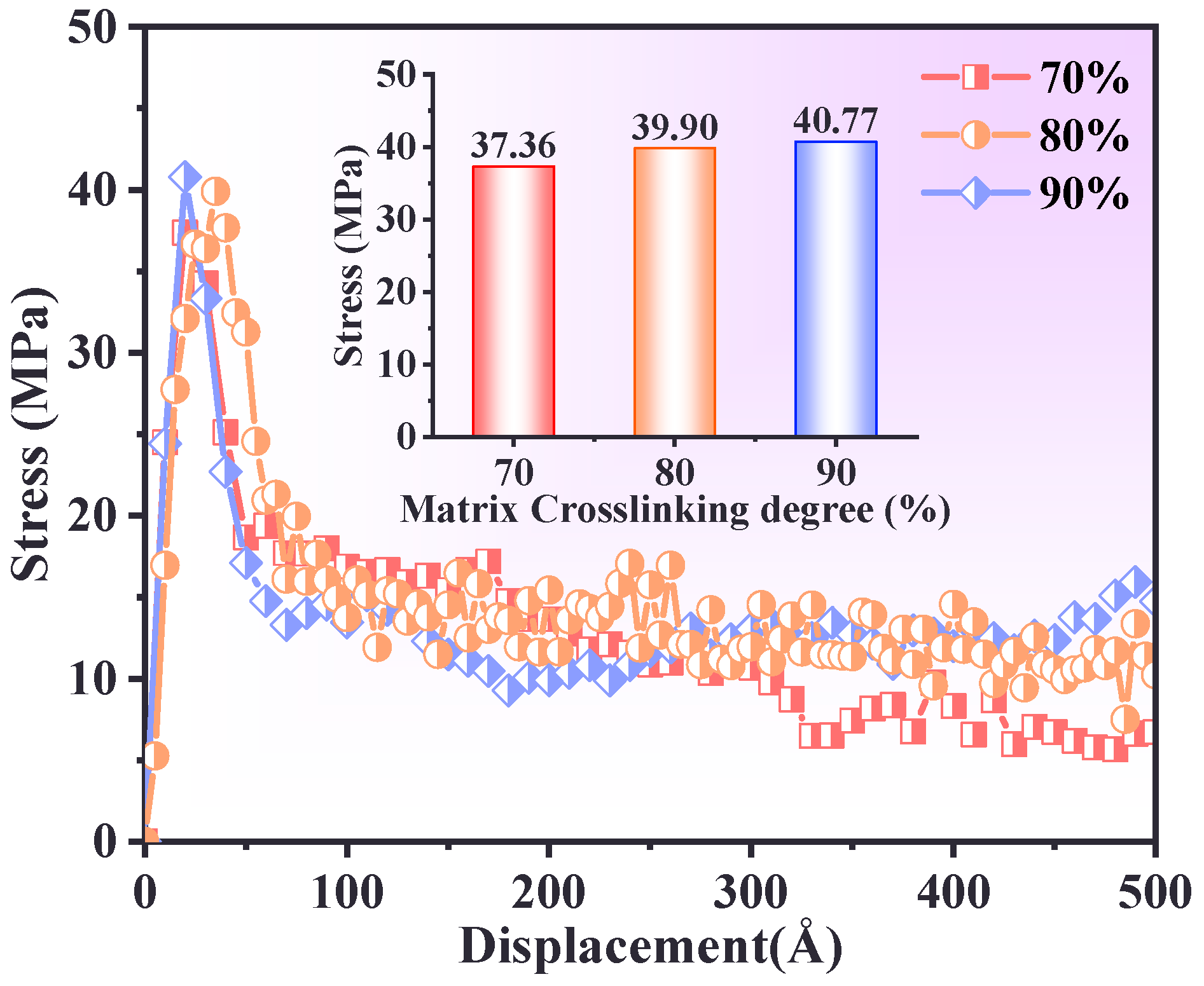
3.3. Effect of Crosslinking Degree of PBT Matrix
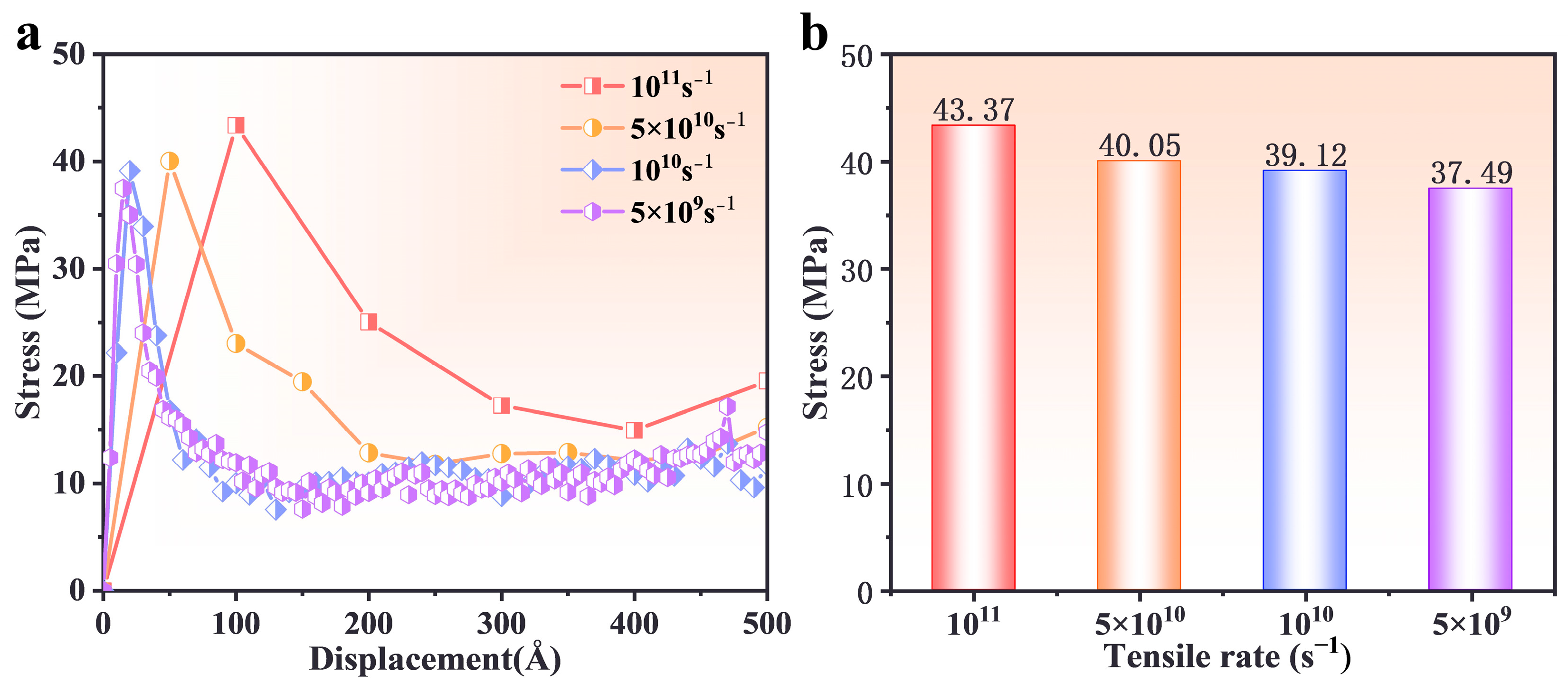
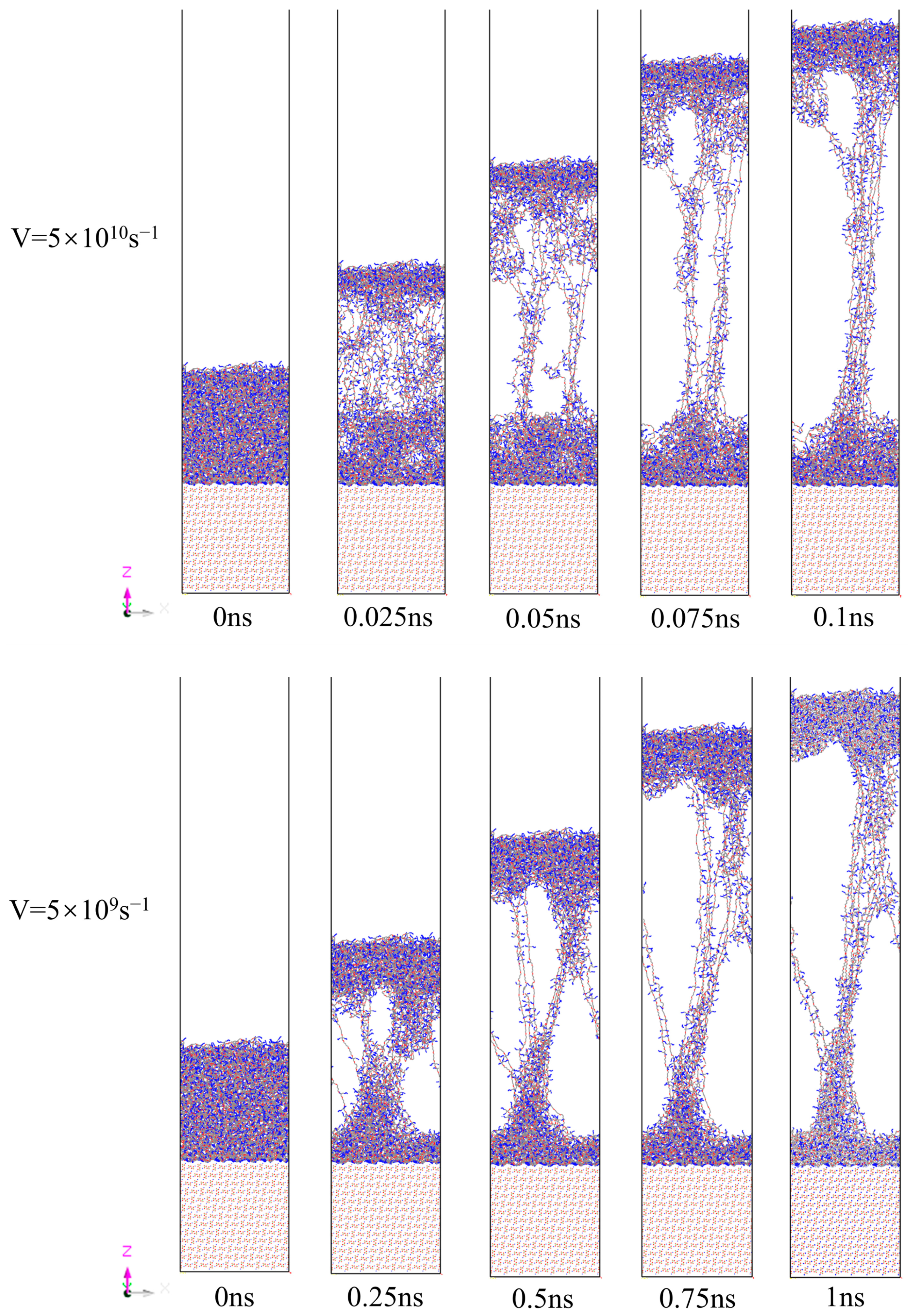
3.4. Effect of Strain Rate
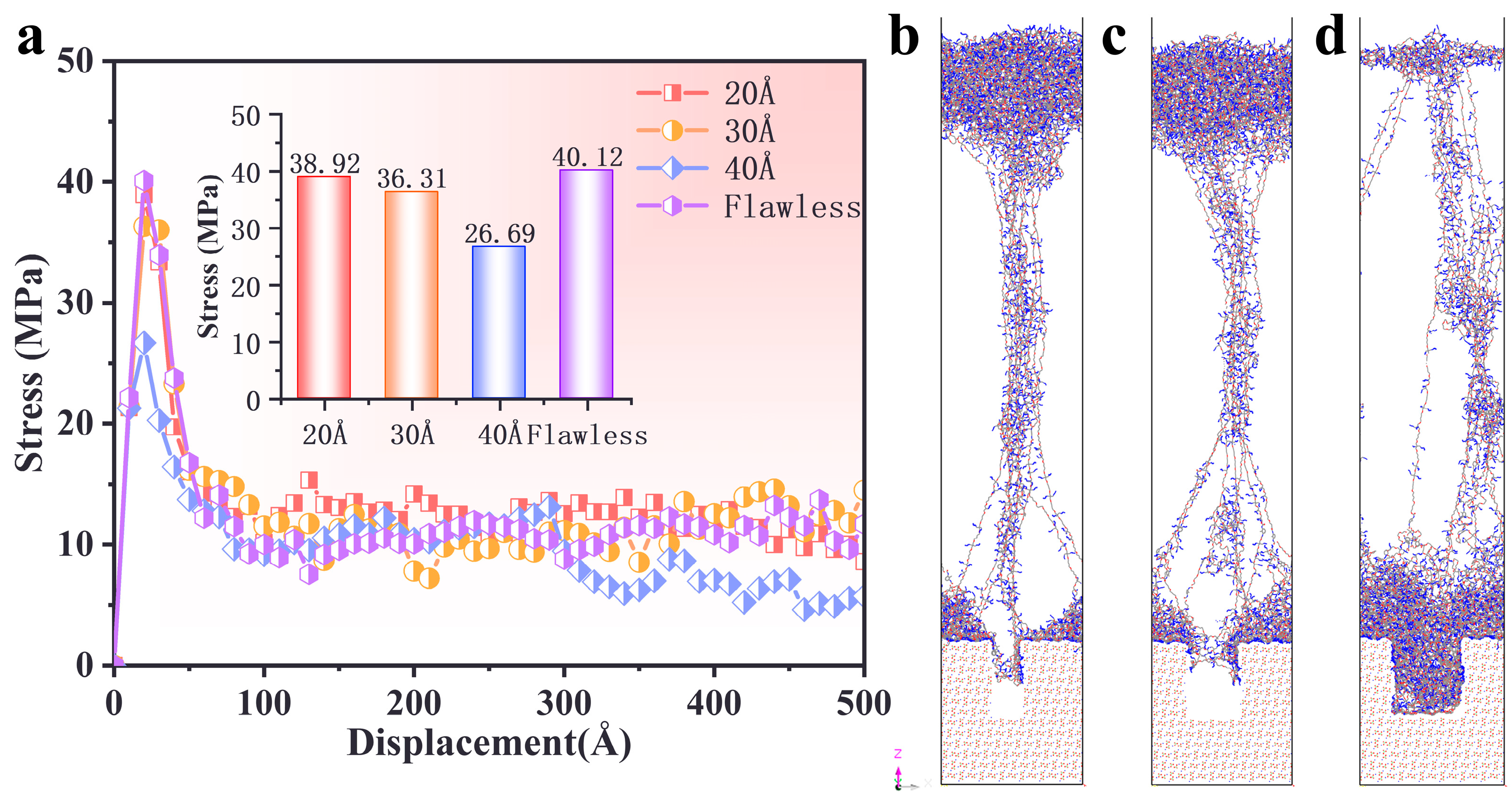
3.5. Effect of AP Defect
4. Conclusions
- (1)
- At low temperatures (e.g., 200 K), the PBT–AP interface exhibited high stress peaks and brittleness characteristics due to restricted molecular chain mobility. In contrast, when at low temperatures (e.g., 500 K), the molecular chain dynamics increased, the stress peak decreased, the plastic flow was prolonged, and there was a transition to a viscoelastic dominated ductile deformation.
- (2)
- The interfacial adhesion strength and tensile resistance of the PBT matrix were strongly influenced by cross-linking density. A 90% cross-linking degree formed a robust molecular network with strong intermolecular interactions, yielding the highest stress peak (39 MPa) and optimal resistance to interfacial debonding. In contrast, a 70% cross-linking degree resulted in rapid post-peak stress decay due to a loosely connected network, while an 80% cross-linking degree exhibited delayed stress accumulation due to inefficient interchain stress transfer.
- (3)
- Higher strain rates (5 × 1010 s−1) increased peak stress and reduced deformation time, as molecular chains had limited time for relaxation and rearrangement. Lower strain rates (5 × 109 s−1) facilitated gradual slippage and reorientation of molecular chains, enabling more uniform deformation and higher residual stress retention.
- (4)
- Defect size significantly influenced the interfacial failure mechanism. Smaller defects (20–30 Å) preserved stress distribution patterns similar to those of the defect-free interface, while larger defects (40 Å) disrupted stress transfer, shifting failure from the interface to the PBT matrix and reducing peak stress by approximately 32%.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, S.; Wang, S.; Zhou, H.; Mao, C.; Wang, J. Molecular dynamics simulation of molecular network structure and mechanical properties of polymer matrix in PBT propellant. Mater. Today Commun. 2023, 35, 105723. [Google Scholar]
- Grégoire, C.M.; Mathieu, O.; Kalman, J.; Petersen, E.L. Review and assessment of the ammonium perchlorate chemistry in AP/HTPB composite propellant gas-phase chemical kinetics mechanisms. Prog. Energy Combust. Sci. 2025, 106, 101195. [Google Scholar]
- Kang, L.; Liu, J.; Yao, Y.; Wu, X.; Zhang, J.; Zhu, C.; Xu, F.; Xu, S. Enhancing risk/safety management of HAN-based liquid propellant as a green space propulsion fuel: A study of its hazardous characteristics. Process Saf. Environ. Prot. 2023, 177, 921–931. [Google Scholar]
- Swami, U.; Kumbhakarna, N.; Chowdhury, A. Green hypergolic ionic liquids: Future rocket propellants. J. Ion. Liq. 2022, 2, 100039. [Google Scholar]
- Jamil, N.A.B.M.; Mohamaddiah, A.H.B.; Azami, M.H.B.; Nordin, N.H. High entropy alloy as metal additive for hybrid rocket propellant. Mater. Today Proc. 2023, 75, 140–146. [Google Scholar]
- Nath, S.; Laso, I.; Mallick, L.; Sobe, Z.; Koffler, S.; Blumer-Ganon, B.; Borzin, E.; Libis, N.; Lefkowitz, J.K. Comprehensive ignition characterization of a non-toxic hypergolic hybrid rocket propellant. Proc. Combust. Inst. 2023, 39, 3361–3370. [Google Scholar]
- Okninski, A.; Kopacz, W.; Kaniewski, D.; Sobczak, K. Hybrid rocket propulsion technology for space transportation revisited—Propellant solutions and challenges. FirePhysChem 2021, 1, 260–271. [Google Scholar]
- Zhao, X.; Zhu, W. Optimization and design for the curing process of solid azide propellant: Influence of typical components on the curing reactions of PBT binders with TDI. J. Chin. Chem. Soc. 2022, 69, 419–439. [Google Scholar]
- Hou, Y.; Xu, J.; Zhou, C.; Chen, X. Microstructural simulations of debonding, nucleation, and crack propagation in an HMX-MDB propellant. Mater. Des. 2021, 207, 109854. [Google Scholar]
- Lei, M.; Ren, S.; Chen, E.; Zhang, Z.; Xiao, J.; Wen, L.; Hou, X. Correlation between solid propellant failure and interface debonding in solid rocket motors. Polym. Test. 2022, 115, 107755. [Google Scholar]
- Liu, B.; Wang, S.; Zhan, M.; Wang, H.; Zhang, G.; Jian, Q. Optical nondestructive evaluation for minor debonding defects and interfacial adhesive strength of solid propellant. Measurement 2022, 194, 111066. [Google Scholar]
- Wubuliaisana, M.; Wu, Y.; Hou, X.; Duan, H.; Huang, F. Viscoelastic debbonding criterion-based interface for modeling the mechanical behavior of solid propellants subjected to large deformation. Eur. J. Mech.-A/Solids 2023, 98, 104873. [Google Scholar]
- Wubuliaisan, M.; Wu, Y.; Hou, X.; Yang, K.; Duan, H.; Yin, X. Effect of neutral polymeric bonding agent on tensile mechanical properties and damage evolution of NEPE propellant. Def. Technol. 2024, 32, 357–367. [Google Scholar]
- Dong, Z.; Xiang, L.; Xin, S.; Zhi, W.; Ning, W. Effect of pre-strain during ageing on the maximum elongation of composite solid propellants and its modelling. Polym. Test. 2016, 50, 200–207. [Google Scholar]
- Guo, X.; Zhang, J.; Zhang, M.; Liu, L.; Zhai, P.-C.; Zhang, Q.-J. Effects of liner properties on the stress and strain along liner/propellant interface in solid rocket motor. Aerosp. Sci. Technol. 2016, 58, 594–600. [Google Scholar]
- Wu, C.; Jiang, M.; Lu, Y.; Qu, H.; Li, H.; Hu, S. Experimental and numerical investigation into the mechanical behavior of composite solid propellants subject to uniaxial tension. Materials 2023, 16, 6695. [Google Scholar] [CrossRef]
- Zou, Z.; Qiang, H.; Li, Y.; Wang, X. Review on the dewetting of the particle-matrix interface in composite solid propellants. Propellants Explos. Pyrotech. 2023, 48, e202200270. [Google Scholar]
- Yu, T.; Yan, Y.; Wang, X. Advancements in Multiscale Numerical Simulation of Composite Solid Propellant Interfaces. Chin. J. Energetic Mater. 2024, 32, 554–569. [Google Scholar]
- Zou, Z.; Qiang, H.; Zhang, F.; Wang, X.; Li, Y. Research on mechanical behavior of particle/matrix interface in composite solid propellant. European J. Mech.-A/Solids 2025, 109, 105498. [Google Scholar]
- Pei, S.; Qiang, H.; Wang, X.; Li, S. Mesoscopic failure behavior of HTPB propellant bonding interface under multi-angle pull-and-shear loading. Polym. Test. 2024, 132, 108365. [Google Scholar]
- Yeager, J.D.; Ramos, K.J.; Pesce-Rodriguez, R.A.; Piraino, S.M. Microstructural effects of processing in the plastic-bonded explosive Composition A-3. Mater. Chem. Phys. 2013, 139, 305–313. [Google Scholar]
- Lei, M.; Wang, J.; Cheng, J.; Xiao, J.; Wen, L.; Lu, H.; Hou, X. A constitutive model of the solid propellants considering the interface strength and dewetting. Compos. Sci. Technol. 2020, 185, 107893. [Google Scholar]
- Gu, X.; Liu, X.; Dong, C.; Zhang, G.; Zhang, L.; Zhang, F. The mesoscopic numerical simulation of GAP/CL20/AP composite solid propellant based on MPM and FEM. Appl. Sci. 2023, 13, 4552. [Google Scholar] [CrossRef]
- Zhang, P.; Pang, A.; Tang, G.; Deng, J. Molecular dynamics simulation study on the mechanism of NPBA enhancing interface strength of NEPE propellant. Appl. Surf. Sci. 2019, 123, 131–138. [Google Scholar]
- Long, Y.; Liu, Y.G.; Nie, F.D.; Chen, J. Theoretical study of breaking and slipping processes for HMX/graphite interface. Appl. Surf. Sci. 2012, 123, 2384–2392. [Google Scholar]
- Pan, A.; Wang, W.; Xie, J.; Zhang, H.; Hao, S. Molecular dynamics simulations of interface structure and deformation mechanisms in metal/ceramic composites under tension. Mech. Mater. 2023, 123, 104688. [Google Scholar]
- Attarian, S.; Xiao, S. Investigating the strength of Ti/TiB interfaces at multiple scales using density functional theory, molecular dynamics, and cohesive zone modeling. Ceram. Int. 2022, 48, 33185–33199. [Google Scholar]
- Zhang, J.; Huang, W.; Su, Y.; Luo, P.; Shi, L. Unraveling and in-situ observation on the interfacial mechanical behavior of the NEPE propellant reinforced by NPBA via molecular dynamics simulation. Chem. Eng. Sci. 2024, 299, 120463. [Google Scholar]
- Lv, L.; Yang, M.; Long, Y.; Chen, J. Molecular dynamics simulation of structural and mechanical features of a polymer-bonded explosive interface under tensile deformation. Appl. Surf. Sci. 2021, 557, 149823. [Google Scholar]
- Dong, G.; Liu, H.; Deng, L.; Yu, H.; Zhou, X.; Tang, X.; Li, W. Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation. e-Polymers 2022, 22, 264–275. [Google Scholar]
- He, J.; Arab, A.; Zhang, C. Molecular dynamics study of temperature and defects on mechanical properties of Gr (GO)/C-S-H composites. J. Non-Cryst. Solids 2024, 639, 123094. [Google Scholar]
- Chen, X.; Zhang, J.; Han, L.; Tang, Z. Molecular dynamics study of the effect of point defects on the stress at the Si/Ge interface. Appl. Surf. Sci. 2018, 456, 43–48. [Google Scholar]
- Chen, L.; Lei, K.; Wang, Q.; Wang, L.; Gong, H. Molecular dynamics study of defect evolutions and mechanical properties of W/Fe semi-coherent interfaces under irradiation. Vacuum 2023, 207, 111618. [Google Scholar]
- Cui, B.; Wang, H. Molecular modeling of asphalt-aggregate debonding potential under moisture environment and interface defect. Appl. Surf. Sci. 2022, 606, 154858. [Google Scholar]
- Li, C.; Yang, W.; Gan, Q.; Wang, Y.; Liang, L.; Zhang, W.; Zhu, S.; Feng, C. Molecular Dynamics Simulation of Shock Response of CL-20 Co-crystals Containing Void Defects. Def. Technol. 2024, 31, 364–374. [Google Scholar]
- Long, Z.; You, L.; Tang, X.; Ma, W.; Ding, Y.; Xu, F. Analysis of interfacial adhesion properties of nano-silica modified asphalt mixtures using molecular dynamics simulation. Constr. Build. Mater. 2020, 254, 119332. [Google Scholar]
- Jia, X.; Tang, L.; Liu, R.; Liao, H.; Cao, L.; Tang, X.; Cao, P. Analysis of interfacial adhesion properties between PBT azide propellant matrix and defective AP fillers using molecular dynamics simulations. Polymers 2024, 16, 3497. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Tang, W.; Xu, X.; Zhao, S.; Xuan, F. A multiscale model for predicting mechanical properties of polymer composites. Chem. Eng. Sci. 2023, 282, 119352. [Google Scholar]
- Huang, X.; Wang, X.; Wang, Q.; Xu, X.; Zhao, S. Promoting Cross-Link Reaction of Polymers by the Matrix–Filler Interface Effect: Role of Coupling Agents and Intermediate Linkers. J. Phys. Chem. B 2024, 128, 5534–5541. [Google Scholar]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar]
- Li, M.; Li, F.; Shen, R.; Guo, X. Molecular dynamics study of the structures and properties of RDX/GAP propellant. J. Hazard. Mater. 2011, 186, 2031–2036. [Google Scholar] [PubMed]
- Yang, J.; Gong, X.; Wang, G. Theoretical studies on the plasticizing effect of DIANP on NC with various esterification degrees. Comput. Mater. Sci. 2014, 95, 129–135. [Google Scholar]
- Sun, T.; Xiao, J.; Liu, Q.; Zhao, F.; Xiao, H.M. Comparative study on structure, energetic and mechanical properties of a ε-CL-20/HMX cocrystal and its composite with molecular dynamics simulation. J. Mater. Chem. A 2014, 2, 13898–13904. [Google Scholar]
- Taylor, D.E.; Strawhecker, K.E.; Shanholtz, E.R.; Sorescu, D.C.; Sausa, R.C. Investigations of the intermolecular forces between RDX and polyethylene by force distance spectroscopy and molecular dynamics simulations. J. Phys. Chem. A 2014, 118, 5083–5097. [Google Scholar]
- Zhao, Y.; Zhang, X.; Zhang, W.; Xu, H.; Xie, W.; Du, J.; Liu, Y. Simulation and experimental on the solvation interaction between the GAP matrix and insensitive energetic plasticizers in solid propellants. J. Phys. Chem. A 2016, 120, 765–770. [Google Scholar]
- Tang, Q.; Fan, X.; Li, J.; Bi, F.; Fu, X.; Zhai, L. Experimental and theoretical studies on stability of new stabilizers for N-methyl-P-nitroaniline derivative in CMDB propellants. J. Hazard. Mater. 2017, 327, 187–196. [Google Scholar]
- Choi, C.S.; Prask, H.J.; Prince, E. Crystal structure of NH4ClO4 at 298, 78, and 10 °K by neutron diffraction. J. Chem. Phys. 1974, 61, 3523–3529. [Google Scholar]
- Wang, J.; Qiang, H.; Wang, Z.; Li, S. Numerical study of mechanical properties of composite solid propellant with initial defects. J. Phys. Conf. Ser. 2020, 1634, 012146. [Google Scholar]
- Ren, H.; Li, W.; Ning, J.; Liu, Y. The influence of initial defects on impact ignition of aluminum/polytetrafluoroethylene reactive material. Adv. Eng. Mater. 2020, 22, 1901234. [Google Scholar]
- Peng, C.; Hu, F.M.; Liu, J.D. Effect of pH value on adsorption of dodecylamine by montmorillonite: Insights from experiments and molecular dynamics simulations. Appl. Surf. Sci. 2017, 425, 996–1005. [Google Scholar]
- Sha, Y.; Zhang, X. Impact sensitivity and moisture adsorption on the surface of CL20/TNT cocrystal by molecular dynamics simulation. Appl. Surf. Sci. 2019, 483, 91–97. [Google Scholar]
- Zhang, J.; Shi, L.; Luo, P.; Zhou, J. Mechanical properties and deformation behaviors of the hydroxyl-terminated polybutadiene and ammonium perchlorate interface by molecular dynamics simulation. Comput. Mater. Sci. 2023, 221, 112077. [Google Scholar]
- Delasoudas, I.; Kallivokas, S.V.; Kostopoulos, V. Fracture of epoxy networks using atomistic simulations. J. Phys. Chem. B 2024, 128, 1234–1245. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.; Lv, J.; Cao, P.; Cao, L.; Huang, R.; Tang, X. Molecular Dynamics Simulation of Interfacial Effects in PBT-Based Azide Propellants Under Tensile Deformation. Polymers 2025, 17, 885. https://doi.org/10.3390/polym17070885
Liao H, Lv J, Cao P, Cao L, Huang R, Tang X. Molecular Dynamics Simulation of Interfacial Effects in PBT-Based Azide Propellants Under Tensile Deformation. Polymers. 2025; 17(7):885. https://doi.org/10.3390/polym17070885
Chicago/Turabian StyleLiao, Hongjun, Jiangyan Lv, Peng Cao, Liang Cao, Renlong Huang, and Xianqiong Tang. 2025. "Molecular Dynamics Simulation of Interfacial Effects in PBT-Based Azide Propellants Under Tensile Deformation" Polymers 17, no. 7: 885. https://doi.org/10.3390/polym17070885
APA StyleLiao, H., Lv, J., Cao, P., Cao, L., Huang, R., & Tang, X. (2025). Molecular Dynamics Simulation of Interfacial Effects in PBT-Based Azide Propellants Under Tensile Deformation. Polymers, 17(7), 885. https://doi.org/10.3390/polym17070885






