PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst
Abstract
1. Introduction
2. Experimental Materials
2.1. Materials and Sample Preparation
2.2. Characterization
2.3. Cell Culture Assay
2.4. Quantum Mechanical Calculations
3. Results and Discussion
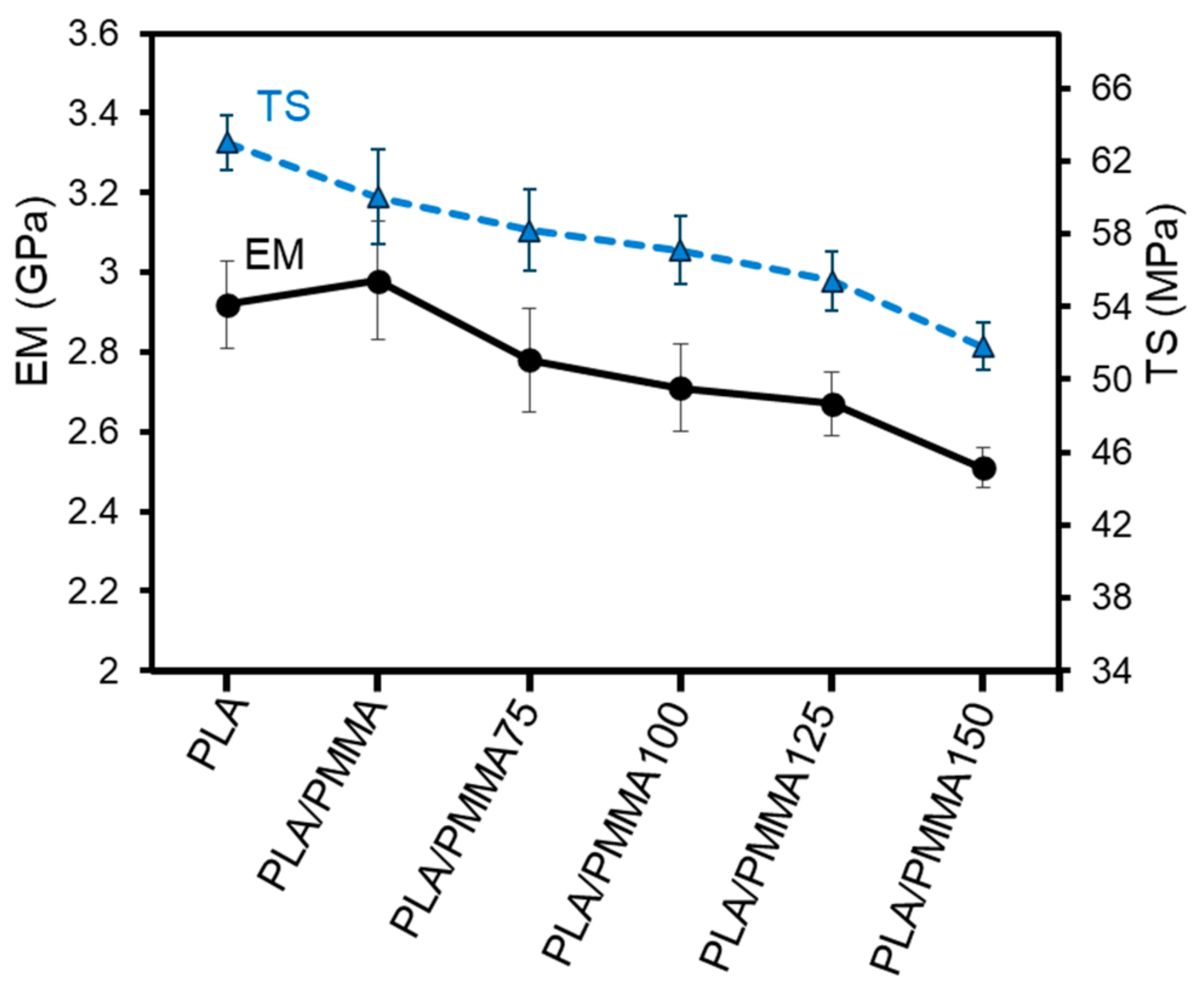
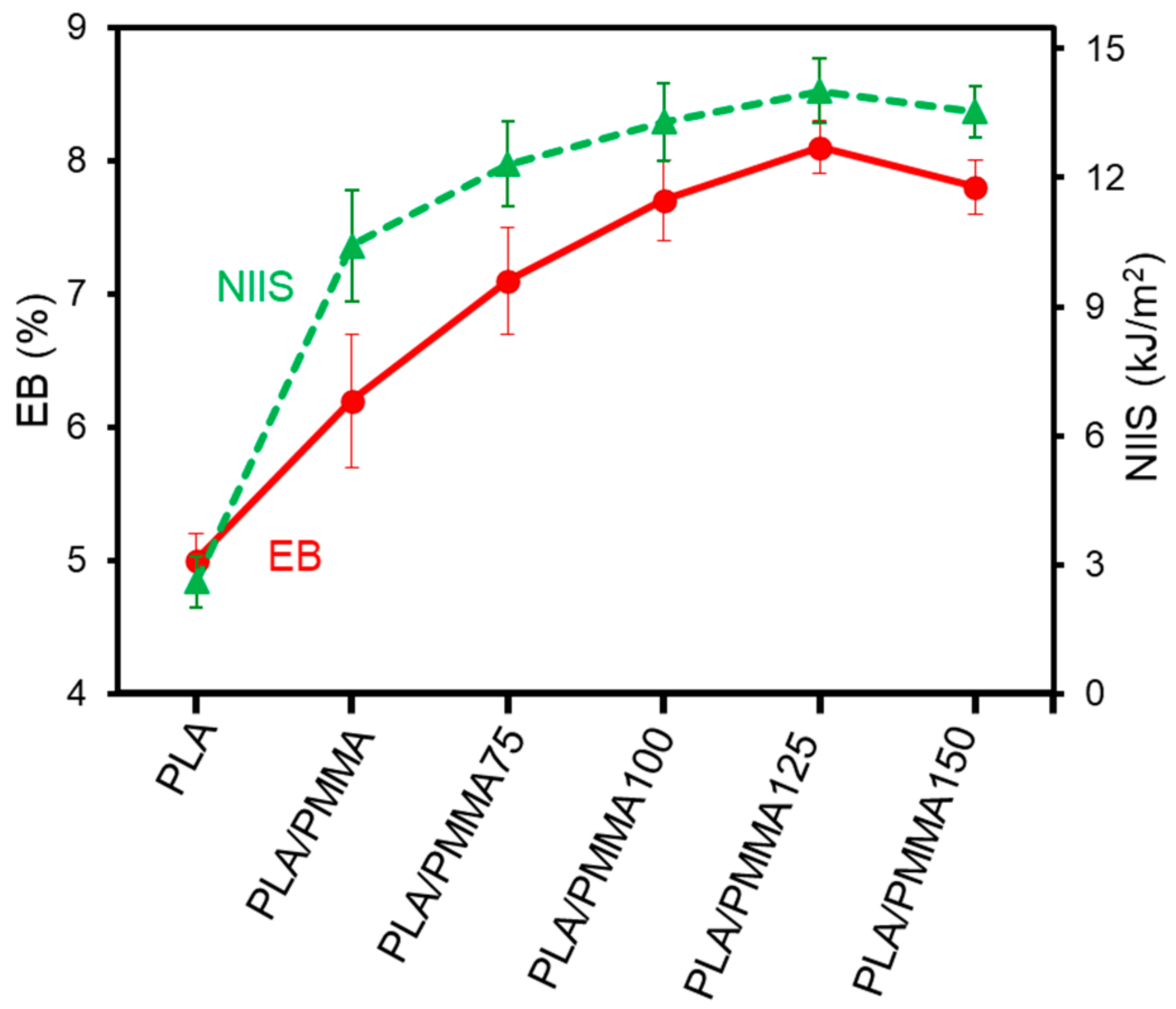
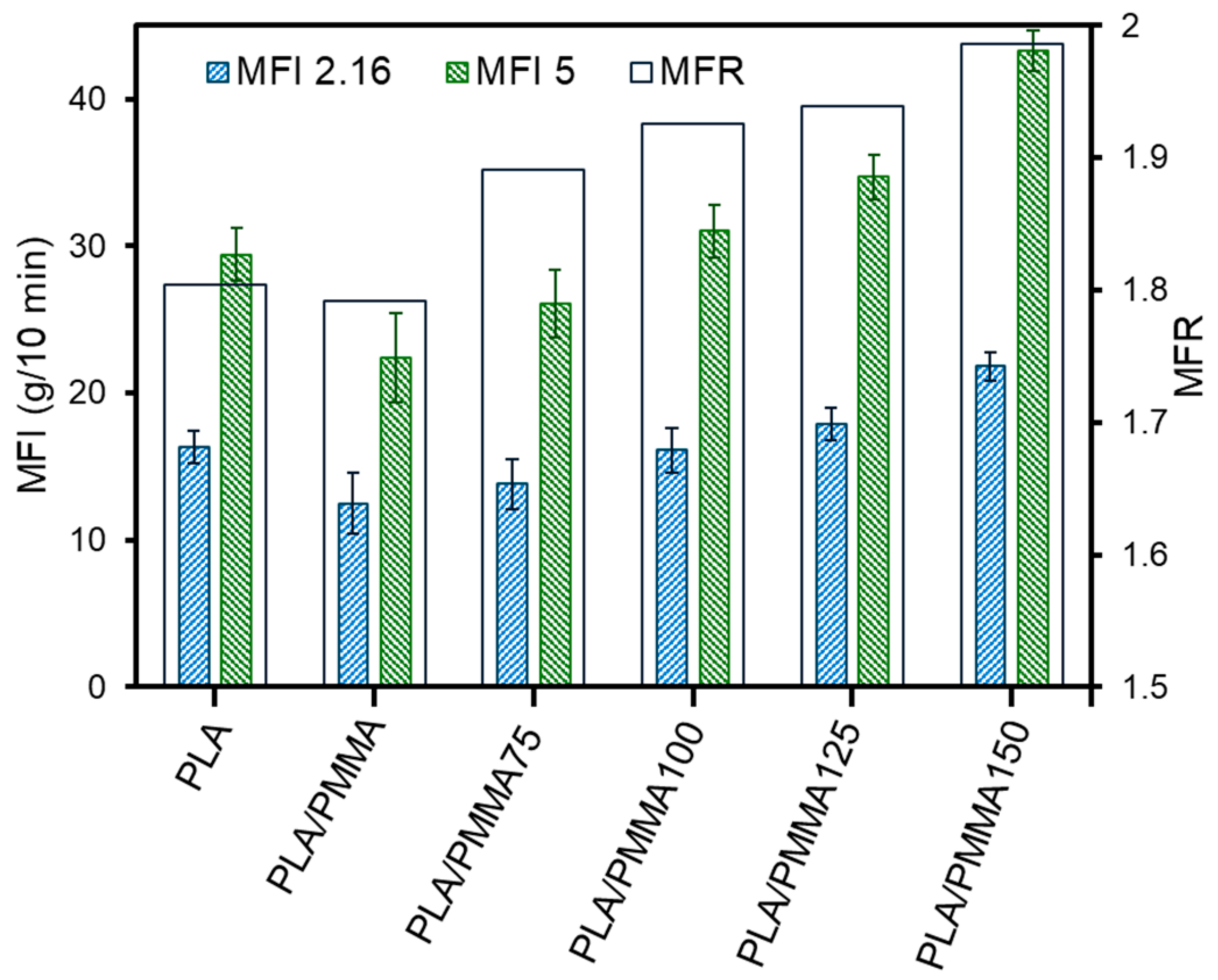
3.1. Mechanical Properties
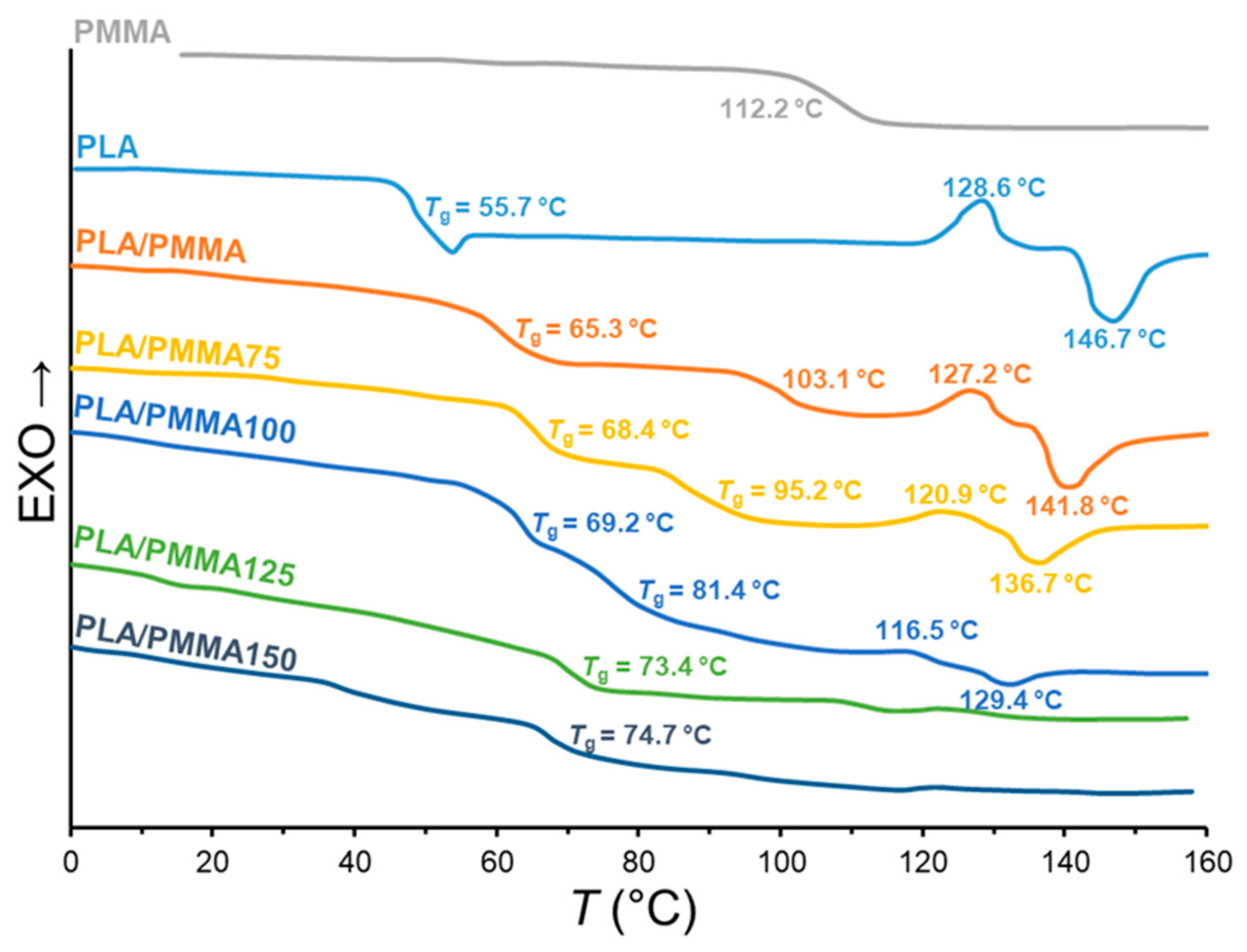
3.2. Thermal Behavior
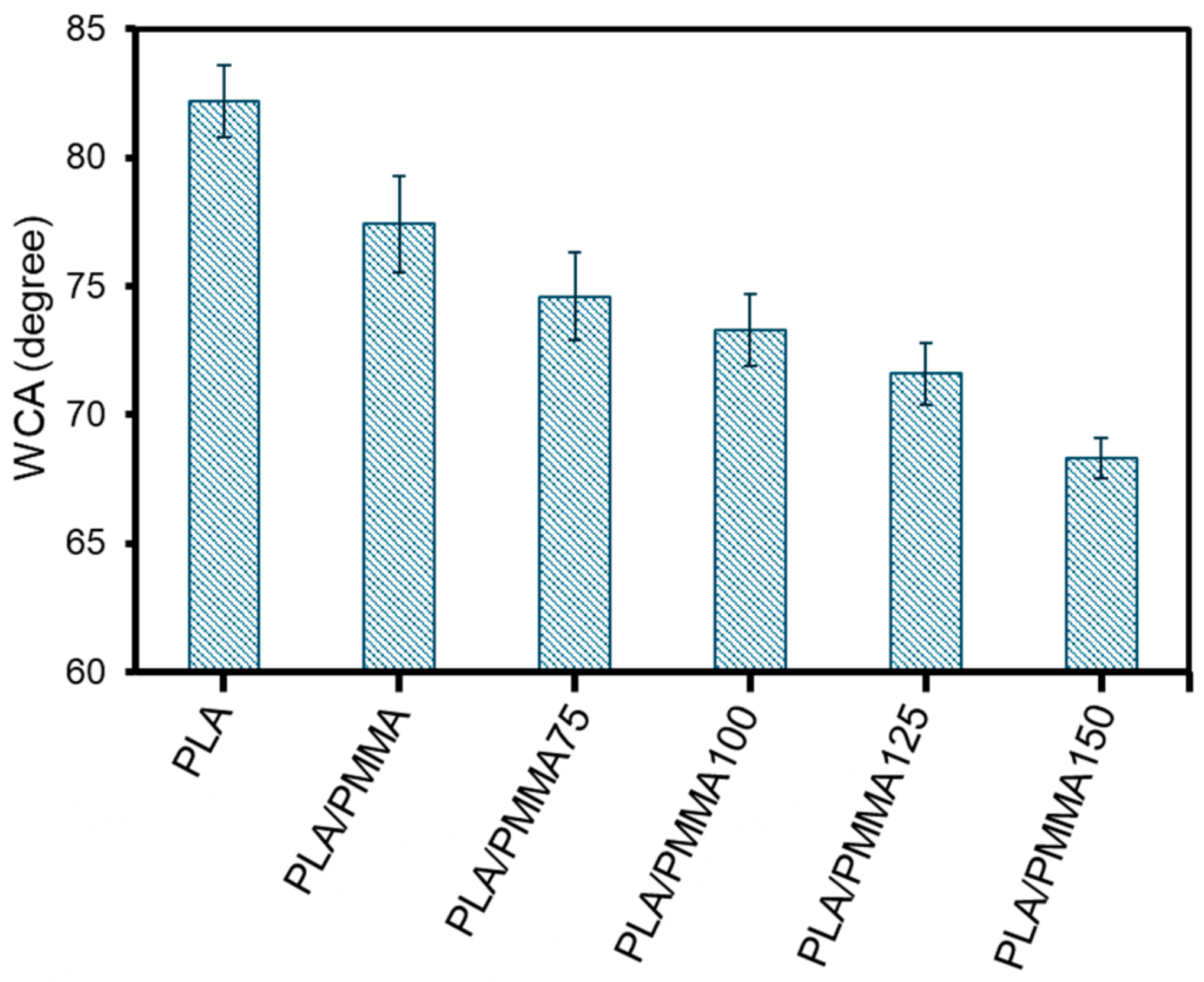
3.3. Wettability Assessment: Water Contact Angle (WCA)
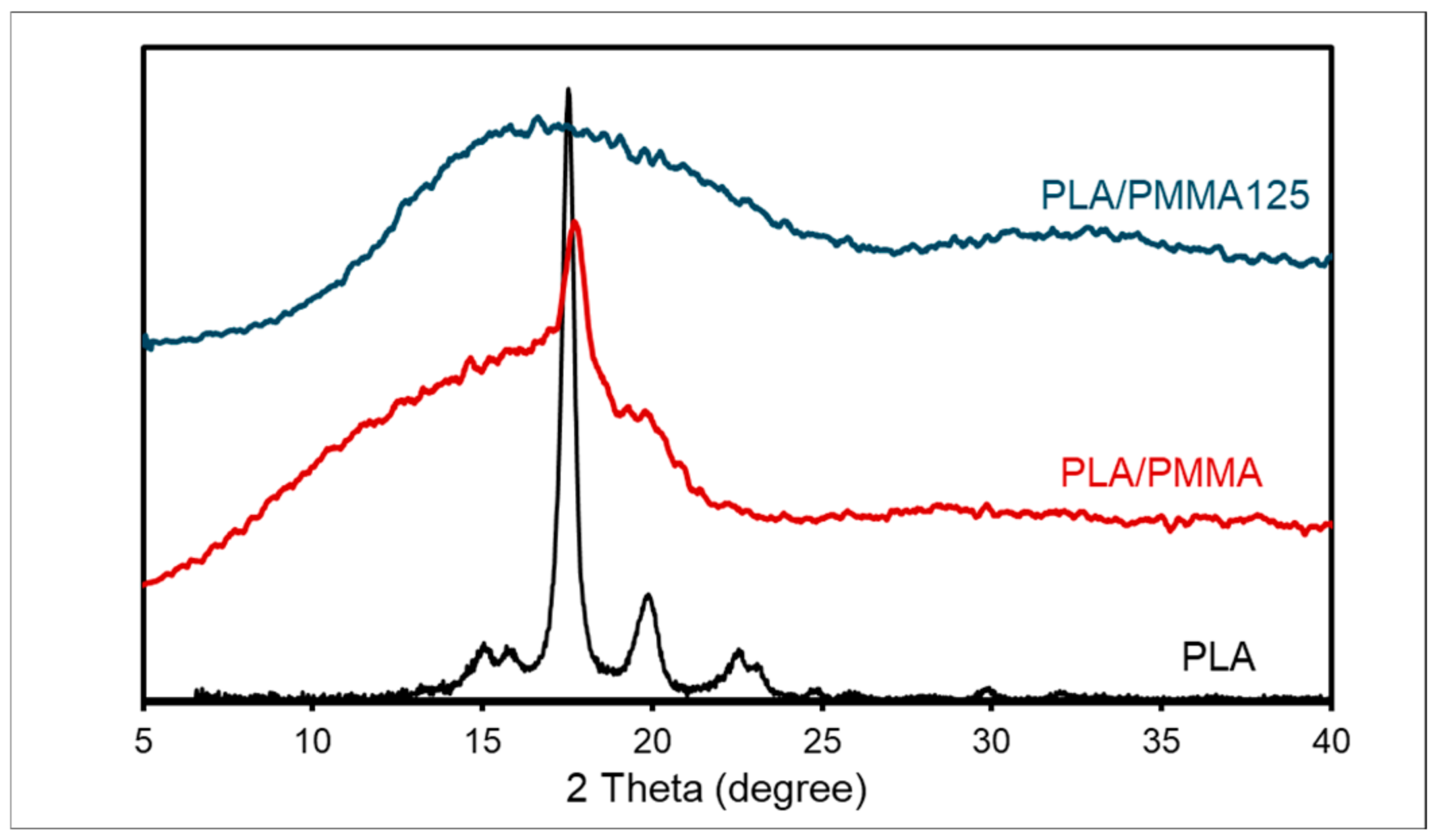
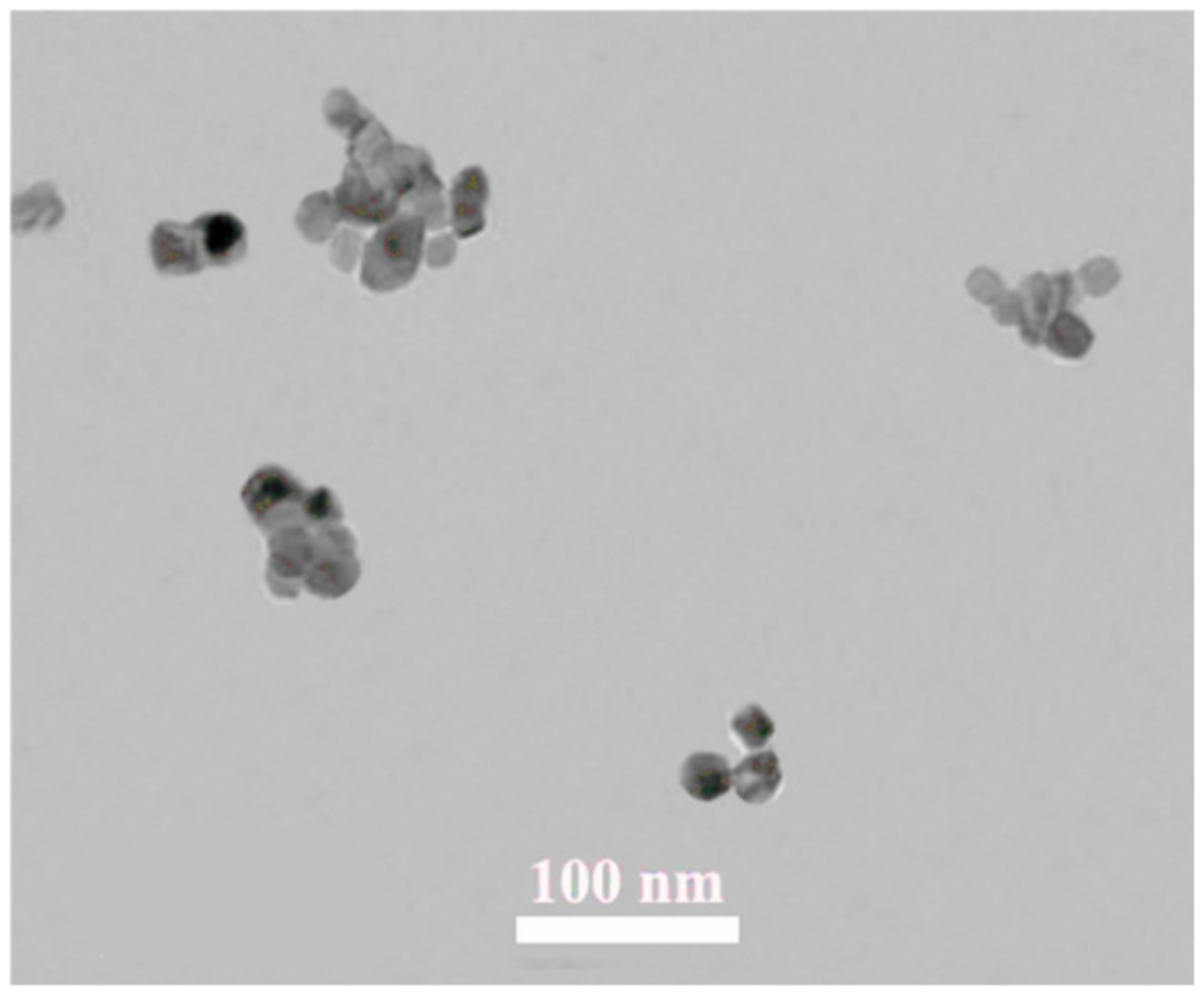
3.4. SEM, XRD, and TEM Analyses
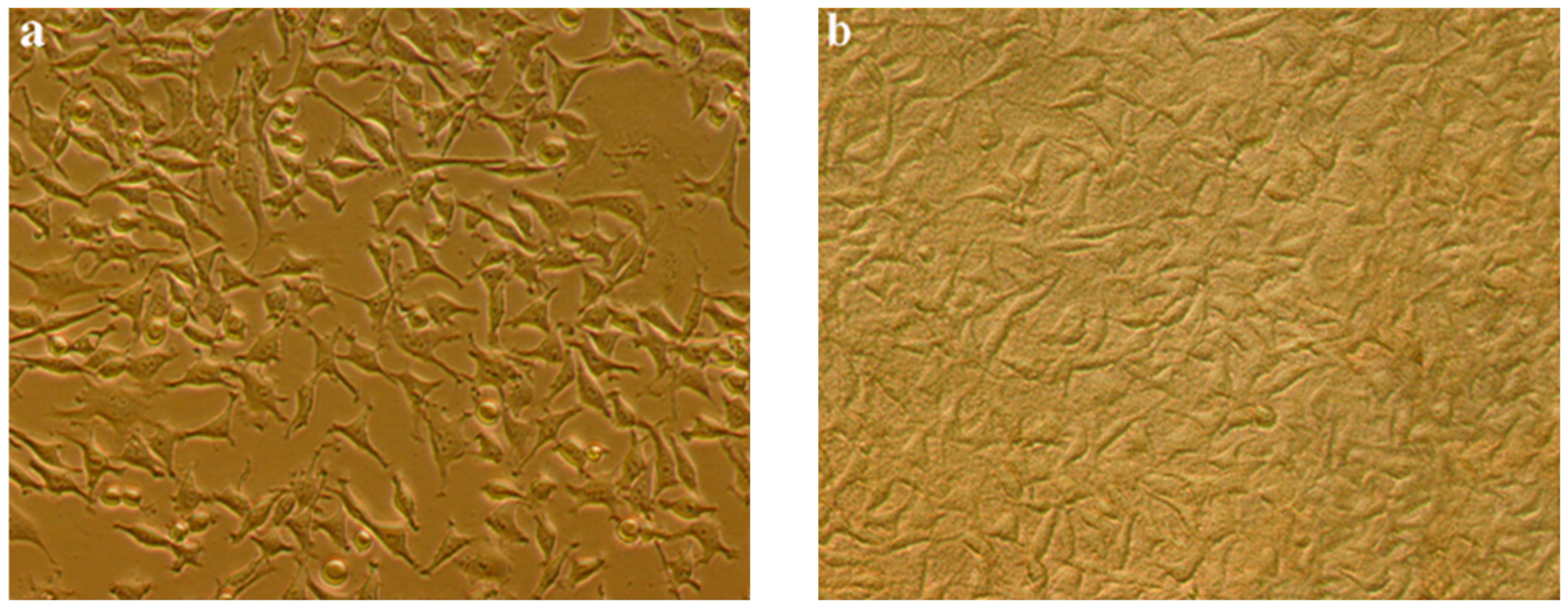
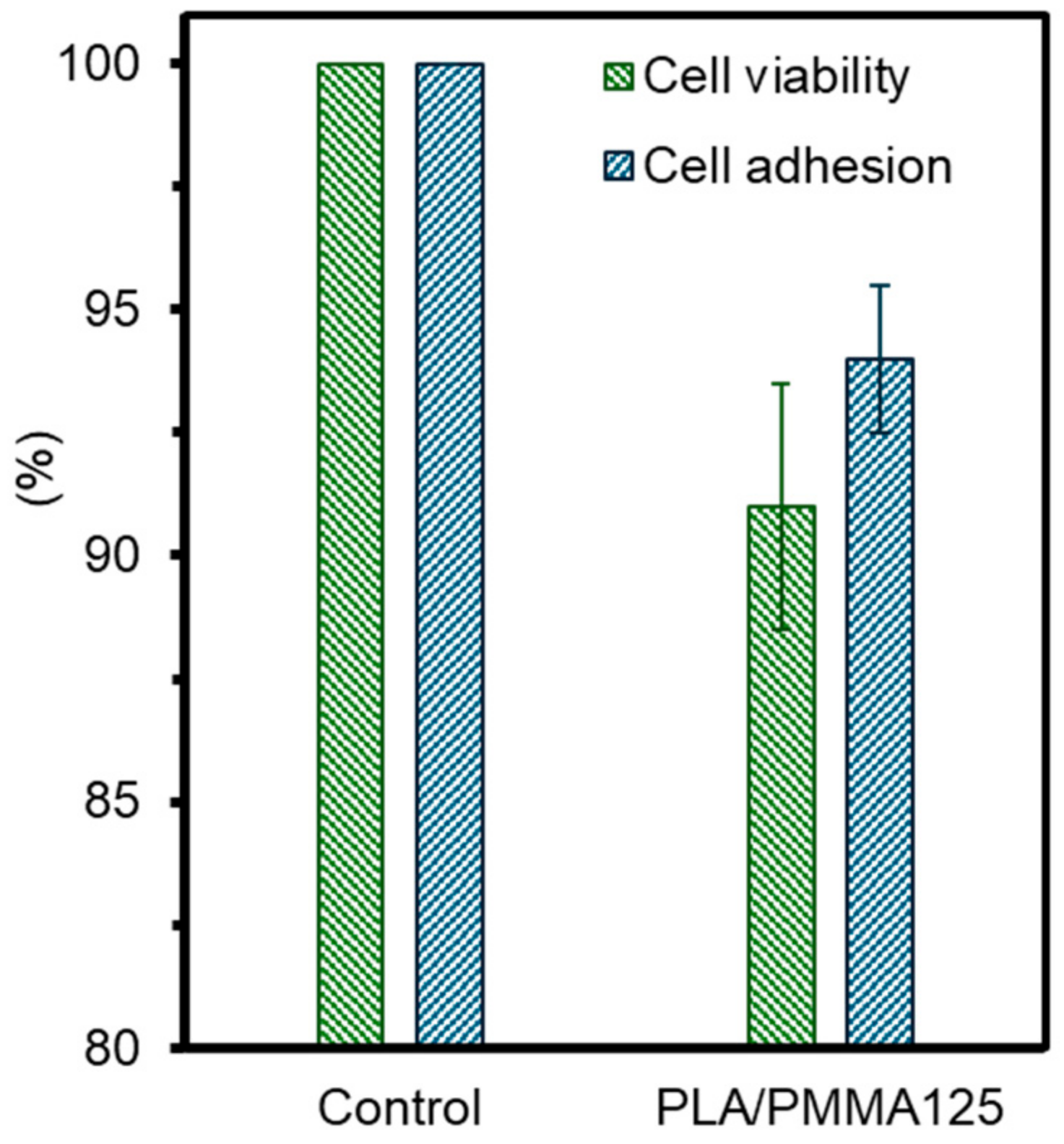
3.5. Cell Culture Assay
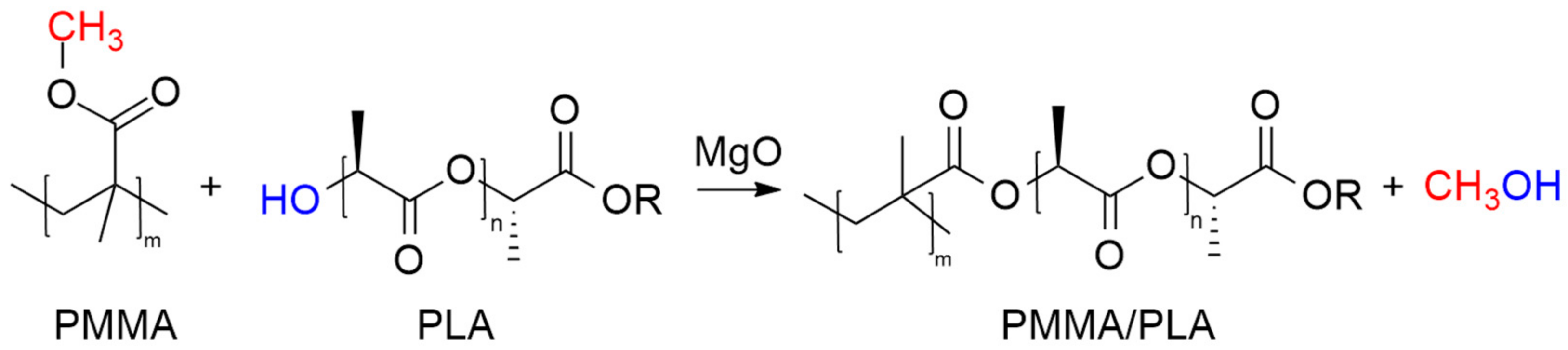
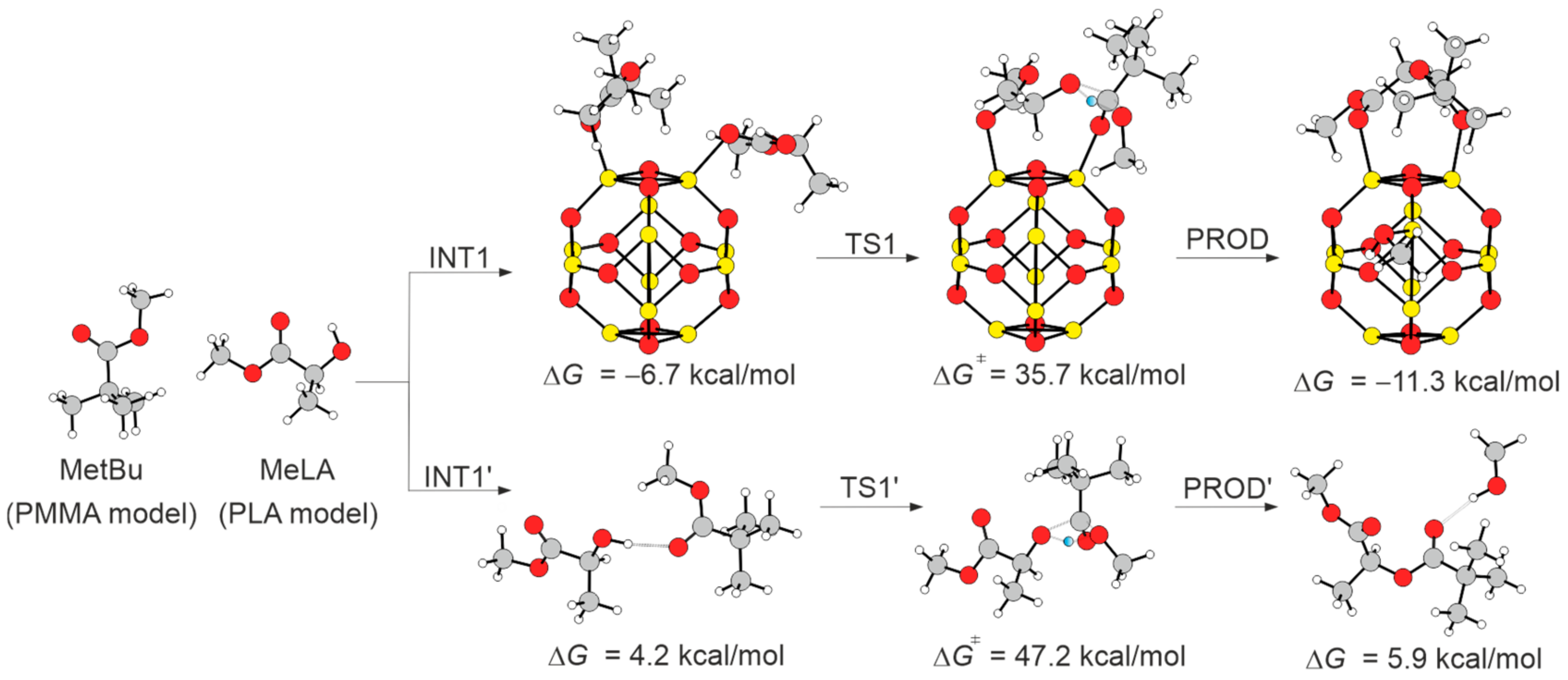
3.6. Mechanistic Insights and DFT Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Environmental impact, and resilience of renewable energy under a changing climate: A review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar]
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mudallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar]
- Luque-Urrutia, J.A.; Ortiz-García, T.; Solà, M.; Poater, A. Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics 2023, 11, 88. [Google Scholar] [CrossRef]
- Malashin, I.; Martysyuk, D.; Tynchenko, V.; Gantimurov, A.; Semikolenov, A.; Nelyub, V.; Borodulin, A. Machine Learning-Based Process Optimization in Biopolymer Manufacturing: A Review. Polymers 2024, 16, 3368. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef]
- Cairone, S.; Hasan, S.W.; Choo, K.-H.; Lekkas, D.F.; Fortunato, L.; Zorpas, A.A.; Korshin, G.; Zarra, T.; Belgiorno, V.; Naddeo, V. Revolutionizing wastewater treatment toward circular economy and carbon neutrality goals: Pioneering sustainable and efficient solutions for automation and advanced process control with smart and cutting-edge technologies. J. Water Process Eng. 2024, 63, 105486. [Google Scholar]
- Das, T.K.; Poater, A. Review on Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar]
- Sanchez-Huerta, C.; Medina, J.S.; Wang, C.; Fortunato, L.; Hong, P.-Y. Understanding the role of sorption and biodegradation in the removal of organic micropollutants by membrane aerated biofilm reactor (MABR) with different biofilm thickness. Water Res. 2023, 236, 119935. [Google Scholar] [PubMed]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Han, Y.; Ning, N.; Wang, Z.; Zhang, L. A New Strategy for the Preparation of Fully Biobased and Biodegradable Polylactic Acid with Both High Rigidity and Flexibility. Macromolecules 2024, 57, 9216–9229. [Google Scholar]
- de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. [Google Scholar] [CrossRef]
- Rusconi, Y.; D’Alterio, M.C.; Grillo, A.; Poater, A.; De Rosa, C.; Talarico, G. The metal role on the activity and stereoselectivity of ring-opening polymerization of racemic lactide promoted by Salen catalysts. Polymer 2024, 292, 126639. [Google Scholar] [CrossRef]
- Aquino, G.; Viscusi, G.; D’Alterio, M.C.; Del Gaudio, P.; Rodriquez, M. A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications. Int. J. Mol. Sci. 2024, 25, 9608. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties. Polymers 2019, 11, 94. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Greene, J. Biodegradation of compostable plastics in green yard-waste compost environment. J. Polym. Environ. 2007, 15, 269–273. [Google Scholar]
- Balaguer, M.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [PubMed]
- D’alterio, M.C.; De Rosa, C.; Talarico, G. Stereoselective Lactide Polymerization: The Challenge of Chiral Catalyst Recognition. ACS Catal. 2020, 10, 2221–2225. [Google Scholar]
- Li, S.; Chen, T.; Liao, X.; Han, W.; Yan, Z.; Li, J.; Li, G. Effect of Macromolecular Chain Movement and the Interchain Interaction on Crystalline Nucleation and Spherulite Growth of Polylactic Acid under High-Pressure CO2. Macromolecules 2020, 53, 312–322. [Google Scholar]
- Azami, M.; Muarya, A.K.; Nagarajan, R.; Ameli, A. Comparative Study of Blending Approaches for Enhancing PLA/PBS Bioplastics: Pre-Process Melt Compounding vs. Direct Dry Blending. Annu. Tech. Conf. 2024, 2024, 204967. [Google Scholar]
- Karamishamloo, M.; Mirmohammadi, S.A.; Davachi, S.M. Polyethylene glycol/polyhedral oligomeric silsesquioxane as an in situ photocrosslinkable polymeric nanohybrid. Polym. Int. 2020, 69, 492–501. [Google Scholar]
- Sokhanvar, I.N.; Ghomi, N.S.K.; Mirmohammadi, S.A.; Alihosseini, A.; Nasirian, R. Enhancing poly(lactic acid)/maleated polypropylene blend with magnesium oxide catalyst: A reactive blending approach for improved mechanical properties. Int. J. Polym. Anal. Charact. 2024, 29, 253–268. [Google Scholar]
- Yang, R.; Cai, C.; Han, X.; Chen, Z.; Gu, G.; Zhang, C.; Zou, G.; Li, J. Supertough and Biodegradable Poly(Lactic Acid) Blends with “Hard–Soft” Core–Shell Unsaturated Poly(ether-ester) through Self-Vulcanization. Macromolecules 2023, 56, 7271–7285. [Google Scholar]
- Limírio, J.P.J.d.O.; Gomes, J.M.d.L.; Rezende, M.C.R.A.; Lemos, C.A.A.; Rosa, C.D.D.R.D.; Pellizzer, E.P. Pellizzer, Mechanical properties of polymethyl methacrylate as a denture base: Conventional versus CAD-CAM resin—A systematic review and meta-analysis of in vitro studies. J. Prost. Dent. 2022, 128, 1221–1229. [Google Scholar]
- Guan, J.; Ding, N.; Xu, P.; Yang, W.; Niu, D.; Zhang, X.; Liu, T.; Ma, P. Synchronous Toughening, Strengthening, and Ultraviolet Resistance of Immiscible Polylactic Acid/polypropylene Carbonate Blends Compatibilized by a Low Threshold of Reactive Janus Nanosheets. Macromolecules 2024, 57, 11407–11416. [Google Scholar]
- Zhang, G.; Zhang, J.; Wang, S.; Shen, D. Miscibility and phase structure of binary blends of polylactide and poly(methyl methacrylate). J. Polym. Sci. B Polym. Phys. 2003, 41, 23–30. [Google Scholar]
- Nouri-Ahangarani, F.; Nekoomanesh, M.; Mirmohammadi, S.A.; Bahri-Laleh, N. Effects of FeCl3 doping on the performance of MgCl2/TiCl4/DNPB catalyst in 1-hexene polymerization. Polyolefins. J. 2017, 4, 253–262. [Google Scholar]
- Mirmohammadi, S.A.; Nekoomanesh-Haghighi, M.; Gezaz, S.M.; Bahri-Laleh, N. Polybutadiene/polyhedral oligomeric silsesquioxane nanohybrid: Investigation of various reactants in polyesterification reaction. Polym. Int. 2016, 65, 516–525. [Google Scholar]
- Mirmohammadi, S.A.; Nekoomanesh-Haghighi, M.; Gezaz, S.M.; Bahri-Laleh, N.; Atai, M. In-situ photocrosslinkable nanohybrid elastomer based on polybutadiene/polyhedral oligomeric silsesquioxane. Mater. Sci. Eng. C 2016, 68, 530–539. [Google Scholar]
- Wen-Dong, T.; Guang-Jian, H.; Wei-Tao, H.; Xin-Liang, Z.; Xian-Wu, C.; Xiao-Chun, Y. The reactive compatibilization of PLA/PP blends and improvement of PLA crystallization properties induced by in situ UV irradiation. CrystEngComm 2021, 23, 864–875. [Google Scholar]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened Renewable PLA Reactive Multiphase Blends System: Phase Morphology and Performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar]
- Rahimipour, S.; Bahri-Laleh, N.; Ehsani, M.; Moghaddam, A.H.; Aliabad, J.M.; Tabatabaei, S.S.; Mirmohammadi, S.J. Preparation and Properties of Enhanced Bio-Based PLA/PA6/Graphene Nanocomposites in the Presence of an Ester–Amide Exchange Catalyst. J. Polym. Environ. 2021, 29, 2302–2309. [Google Scholar]
- Irani-Kolash, E.; Moshiri-Gomchi, N.; Talebi-Liasi, A.; Sabahi, S.; Bahri-Laleh, N.; Mehdipour-Ataei, S.; Mokhtari-Aliabad, J.; Mirmohammadi, S.A. Preparation of an enhanced nanohybrid alloy based on polylactic acid/polycarbonate/nanosilica. Plast. Rubber. Compos. 2020, 49, 263–270. [Google Scholar]
- Gonzalez-Garzon, M.; Shahbikian, S.; Huneault, M.A. Properties and phase structure of melt-processed PLA/PMMA blends. J. Polym. Res. 2018, 25, 1–13. [Google Scholar]
- Lin, G.-P.; Lin, L.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. PBT/PC blends compatibilized and toughened via copolymers in situ formed by MgO-catalyzed transesterification. Ind. Eng. Chem. Res. 2015, 54, 1282–1291. [Google Scholar]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Boccia, A.C.; Tritto, I. Structure and Mechanical Properties of Ethylene/1-Octene Multiblock Copolymers from Chain Shuttling Technology. Macromolecules 2019, 52, 2669–2680. [Google Scholar]
- Puglisi, C.; Samperi, F. Exchange Reaction Mechanisms in the Reactive Extrusion of Condensation Polymers. In Reactive Extrusion: Principles and Applications; Beyer, G., Hopmann, C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fiorini, M.; Pilati, F.; Berti, C.; Toselli, M.; Ignatov, V. Reactive blending of poly(ethylene terephthalate) and bisphenol-A polycarbonate: Effect of various catalysts and mixing time on the extent of exchange reactions. Polymer 1997, 38, 413–419. [Google Scholar]
- Raquez, J.-M.; Ramy-Ratiarison, R.; Murariu, M.; Dubois, P. Processing, Properties, Additives and Applications. In Poly(lactic acid) Science and Technology; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 99–123. [Google Scholar]
- Zhang, Z.; Huo, S.; Yu, L.; Ye, G.; Wang, C.; Zhang, Q.; Liu, Z. A generalizable reactive blending strategy to construct flame-retardant, mechanically-strong and toughened poly(L-lactic acid) bioplastics. Int. J. Biol. Macromol. 2024, 265, 130806. [Google Scholar]
- Charasseangpaisarn, T.; Wiwatwarrapan, C.; Thunyakitpisal, P.; Srimaneepong, V. Development of poly(methyl methacrylate)/poly(lactic acid) blend as sustainable biomaterial for dental applications. Sci. Rep. 2023, 13, 16904. [Google Scholar]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D256; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2021, 9, 63–84. [Google Scholar]
- ASTM D1238; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2023.
- Mirmohammadi, S.A.; Imani, M.; Atai, M. Hybrid Organic-Inorganic Nanocomposites Based on Poly(E-Caprolactone)/Polyhedral Oligomeric Silsesquioxane: Synthesis and In Vitro Evaluations. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 624–631. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [PubMed]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar]
- Zhang, M.C.; Guo, B.H.; Xu, J. A Review on Polymer Crystallization Theories. Crystals 2017, 7, 4. [Google Scholar]
- Bahri-Laleh, N.; Abbas-Abadi, M.S.; Haghighi, M.N.; Akbari, Z.; Tavasoli, M.R.; Mirjahanmardi, S.H. Effect of halocarbon promoters on polyethylene properties using MgCl2 (ethoxide type)/TiCl4/AlEt3/H2 catalyst system. J. Appl. Polym. Sci. 2010, 117, 1780–1786. [Google Scholar]
- Chen, T.; Li, L.; Xu, G.; Wang, X.; Wang, J.; Chen, Y.; Jiang, W.; Yang, Z.; Lin, G. Cytotoxicity of InP/ZnS quantum dots with different surface functional groups toward two lung-derived cell lines. Front. Pharmacol. 2018, 9, 763. [Google Scholar]
- Jia, X.; Ma, X.; Zhao, L.; Xin, M.; Hao, Y.; Sun, P.; Wang, C.; Chao, D.; Liu, F.; Wang, C.; et al. A biocompatible and fully erodible conducting polymer enables implanted rechargeable Zn batteries. Chem. Sci. 2023, 14, 2123–2130. [Google Scholar]
- Cossement, D.; Gouttebaron, R.; Cornet, V.; Viville, P.; Hecq, M.; Lazzaroni, R. PLA-PMMA blends: A study by XPS and ToF-SIMS. Appl. Surf. Sci. 2006, 252, 6636–6639. [Google Scholar]
- Rentero, C.; Damián, J.; Medel, A.; Fernández-Millán, M.; Rusconi, Y.; Talarico, G.; Cuenca, T.; Sessini, V.; Mosquera, M.E.G. Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies. Polymers 2022, 14, 2982. [Google Scholar] [CrossRef]
- Rusconi, Y.; D’alterio, M.C.; De Rosa, C.; Lu, Y.; Severson, S.M.; Coates, G.W.; Talarico, G. Mechanism of Alternating Poly(lactic-co-glycolic acid) Formation by Polymerization of (S)- and (R)-3-Methyl Glycolide Using an Enantiopure Aluminum Complex. ACS Catal. 2024, 14, 318–323. [Google Scholar]
- Allangawi, A.; Kosar, N.; Ayub, K.; Gilani, M.A.; Arfan, N.H.B.Z.; Hamid, M.H.S.A.; Imran, M.; Sheikh, N.S.; Mahmood, T. Decorating Mg12O12 Nanocage with Late First-Row Transition Metals to Act as Single-Atom Catalysts for the Hydrogen Evolution Reaction. ACS Omega 2023, 8, 37820–37829. [Google Scholar]
- Hirschmann, M.; Zunino, R.; Meninno, S.; Falivene, L.; Fuoco, T. Bi-functional and mono-component organocatalysts for the ring-opening alternating co-polymerisation of anhydride and epoxide. Catal. Sci. Technol. 2023, 13, 7011–7021. [Google Scholar]
- Zhang, J.; Lui, K.H.; Zunino, R.; Jia, Y.; Morodo, R.; Warlin, N.; Hedrick, J.L.; Talarico, G.; Waymouth, R.M. Waymouth, Highly Selective O-Phenylene Bisurea Catalysts for ROP: Stabilization of Oxyanion Transition State by a Semiflexible Hydrogen Bond Pocke. J. Am. Chem. Soc. 2024, 146, 22295–22305. [Google Scholar]
| Sample Code | PLA (g) | PMMA (g) | PMMA (wt%) | MgO (g) | MgO (wt%) |
|---|---|---|---|---|---|
| PLA | 60 | - | - | - | - |
| PLA/PMMA | 42 | 18 | 30 | - | - |
| PLA/PMMA75 | 42 | 18 | 30 | 0.045 | 0.075 |
| PLA/PMMA100 | 42 | 18 | 30 | 0.060 | 0.100 |
| PLA/PMMA125 | 42 | 18 | 30 | 0.075 | 0.125 |
| PLA/PMMA150 | 42 | 18 | 30 | 0.090 | 0.150 |
| Sample Code | Tg (°C) 1 | Tcc (°C) 2 | Tm (°C) 3 | ∆Hcc (J/g) 4 | ∆Hm (J/g) 5 | Xc (%) 6 |
|---|---|---|---|---|---|---|
| PLA | 55.7 | 128.6 | 146.8 | 15.1 | 24.8 | 10.4 |
| PMMA | 112.2 | - | - | - | - | |
| PLA/PMMA | 65.3 & 103.1 | 127.2 | 141.8 | 12.9 | 23.1 | 10.9 |
| PLA/PMMA75 | 68.4 & 95.2 | 120.9 | 136.7 | 8.1 | 15.0 | 7.4 |
| PLA/PMMA100 | 69.2 & 81.4 | 116.5 | 129.4 | 2.4 | 6.2 | 4.1 |
| PLA/PMMA125 | 73.4 | - | - | - | - | |
| PLA/PMMA150 | 74.7 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komeijani, M.; Bahri-Laleh, N.; Mirjafary, Z.; D’Alterio, M.C.; Rouhani, M.; Sakhaeinia, H.; Moghaddam, A.H.; Mirmohammadi, S.A.; Poater, A. PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst. Polymers 2025, 17, 845. https://doi.org/10.3390/polym17070845
Komeijani M, Bahri-Laleh N, Mirjafary Z, D’Alterio MC, Rouhani M, Sakhaeinia H, Moghaddam AH, Mirmohammadi SA, Poater A. PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst. Polymers. 2025; 17(7):845. https://doi.org/10.3390/polym17070845
Chicago/Turabian StyleKomeijani, Masoud, Naeimeh Bahri-Laleh, Zohreh Mirjafary, Massimo Christian D’Alterio, Morteza Rouhani, Hossein Sakhaeinia, Amin Hedayati Moghaddam, Seyed Amin Mirmohammadi, and Albert Poater. 2025. "PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst" Polymers 17, no. 7: 845. https://doi.org/10.3390/polym17070845
APA StyleKomeijani, M., Bahri-Laleh, N., Mirjafary, Z., D’Alterio, M. C., Rouhani, M., Sakhaeinia, H., Moghaddam, A. H., Mirmohammadi, S. A., & Poater, A. (2025). PLA/PMMA Reactive Blending in the Presence of MgO as an Exchange Reaction Catalyst. Polymers, 17(7), 845. https://doi.org/10.3390/polym17070845








