Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Production of Protein Hydrolysates
2.3. Characterization of the Protein Hydrolysates
2.3.1. Oxygen Radical Absorbance Capacity (ORAC)
2.3.2. Enzymatic Inhibition
Enzymatic Inhibition of α-Glucosidase
Inhibition of α-Amylase
Inhibition of Lipase
Angiotensin-Converting Enzyme Inhibition Assay (ACE)
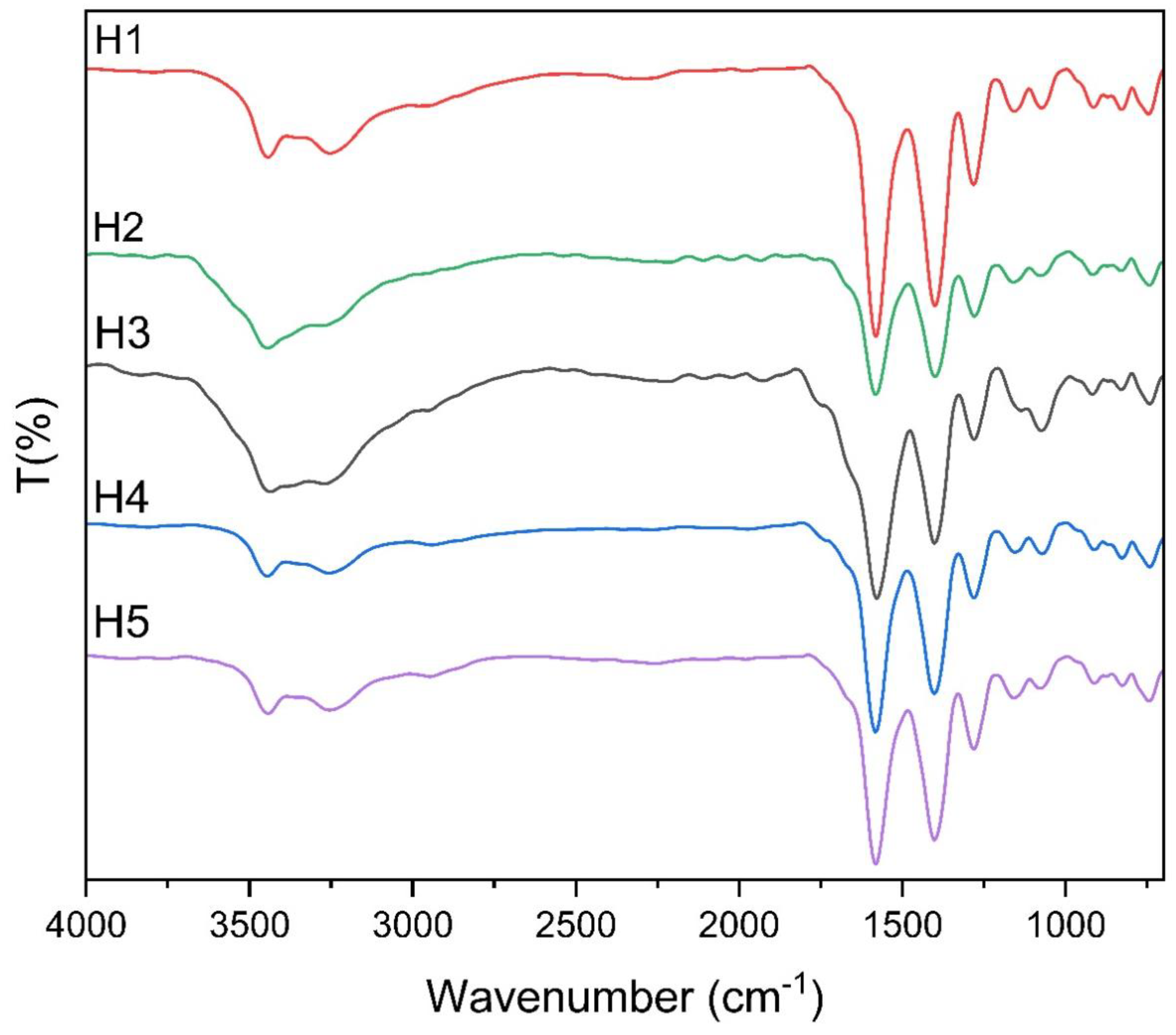
2.3.3. Infrared Spectroscopy
2.4. Development of the Giant Squid Sausage
2.5. Physicochemical Properties of the Sausage
2.5.1. Proximal Analysis and Microbiological Quality
2.5.2. Physicochemical Properties of Sausages During Storage
Determination of pH
Determination of Total Volatile Bases (TVB)
Determination of Water-Holding Capacity (WHC)
Color
Firmness Analysis
Microbiological Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Protein Hydrolysates
3.1.1. Antioxidant Capacity
3.1.2. Enzymatic Inhibition
3.1.3. Applications in Functional Foods
3.2. Infrared Spectroscopy
3.3. Physicochemical Properties of the Sausage
3.3.1. Proximal Analysis and Microbiological Quality
3.3.2. Physicochemical Properties of the Sausages During Storage
pH
Total Volatile Bases (TVB)
Water-Holding Capacity (WHC)
Color
Firmness Analysis
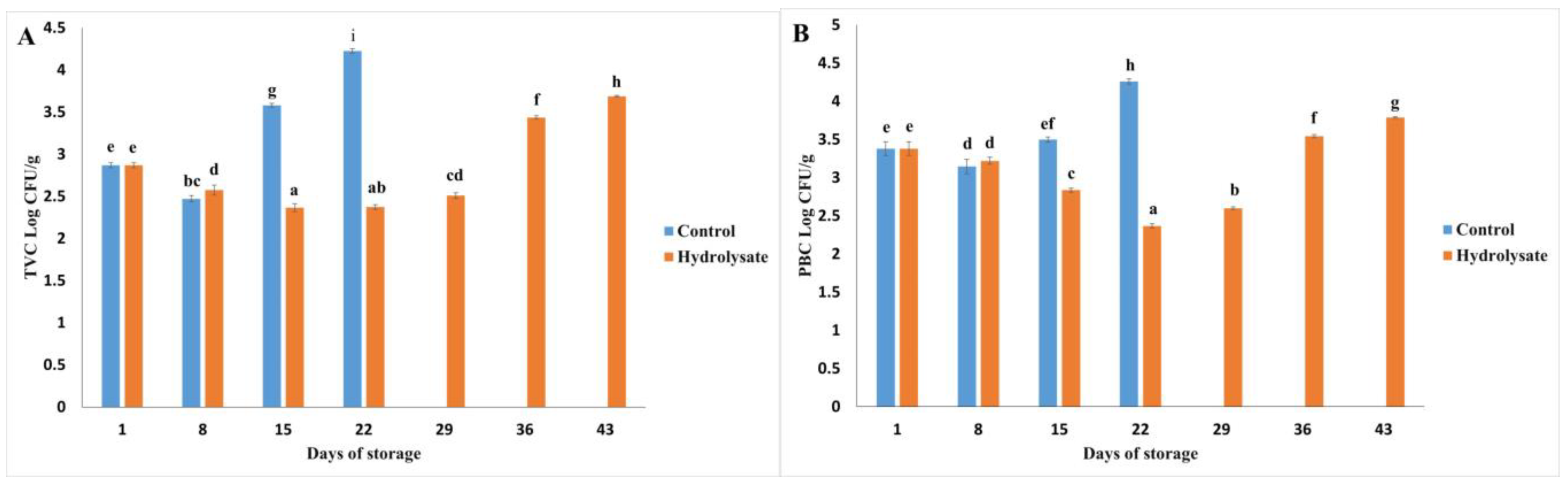
3.4. Microbiological Analysis
3.4.1. Microbial Growth Trends
3.4.2. End of Storage Period
3.4.3. Coliform Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| ACE | Angiotensin-converting enzyme inhibition assay |
| ACE-I | Angiotensin-converting enzyme |
| AOAC | Association of Official Analytical Chemists |
| FTIR | Fourier Transform Infrared |
| GRAS | Generally recognized as safe |
| H0 | A non-fermented control consisting of unprocessed muscle and collagen was included to assess baseline properties |
| H1 | Sample conditions: 4 h of fermentation, 25% collagen, 75% muscle |
| H2 | Sample conditions: 4 h of fermentation, 100% collagen |
| H3 | Sample conditions: 8 h of fermentation, 100% muscle |
| H4 | Sample conditions: 8 h of fermentation, 50% collagen, 50% muscle |
| H5 | Sample conditions: 8 h of fermentation, 100% collagen |
| ORAC | Oxygen Radical Absorbance Capacity |
| MPN | Most Probable Number |
| PBCs | Psychrotrophic bacterial counts |
| TVBs | Total volatile bases |
| TVC | Total viable count |
| WHC | Water-holding capacity |
References
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive compounds from pomegranate peels-Biological properties, structure–function relationships, health benefits and food applications—A comprehensive review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Minari, T.P.; Manzano, C.F.; Yugar, L.B.T.; Sedenho-Prado, L.G.; de Azevedo Rubio, T.; Tácito, L.H.B.; Pisani, L.P. De-mystifying Obesity: Understanding, Prevention, Treatment, and Stigmas. Nutr. Rev. 2024, nuae144. [Google Scholar] [CrossRef]
- Mittal, R.K.; Mishra, R.; Sharma, V.; Purohit, P. Bioactive Exploration in Functional Foods: Unlocking Nature’s Treas-ures. Curr. Pharm. Biotechnol. 2024, 25, 1419–1435. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Puniabangar, S.; Trif, M.; Samborska, K.; Barańska, A.; Aaliya, B.; Sunooj, K.V.; Tomas, M.; Capanoglu, E.; Rostamabadi, H. How do various encapsulation techniques improve the oral delivery of food protein hydrolysates? Food Front. 2024, 6, 40–64. [Google Scholar]
- López-Pedrouso, M.; Zaky, A.A.; Lorenzo, J.M.; Camiña, M.; Franco, D. A review on bioactive peptides derived from meat and by-products: Extraction methods, biological activities, applications and limitations. Meat Sci. 2023, 204, 109278. [Google Scholar]
- Rafieezadeh, D.; Esfandyari, G. Marine bioactive peptides with anticancer potential, a narrative review. Int. J. Biochem. Mol. Biol. 2024, 15, 118–126. [Google Scholar]
- Singh, A.; Mittal, A.; Benjakul, S. Full Utilization of Squid Meat and Its Processing By-products: Revisit. Food Rev. Int. 2020, 38, 455–479. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Yasemi, M. Protein Hydrolysates from Fishery Processing By-Products: Production, Characteristics, Food Applications, and Challenges. Foods 2023, 12, 4470. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and Bioactive Properties of Peptides Derived from Marine Side Streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Domingues, M.R.; Calado, R. Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability. Mar. Drugs 2024, 22, 73. [Google Scholar] [CrossRef]
- López-Medina, F.A.; Dublán-García, O.; Morachis-Valdez, A.G.; López-Martínez, L.X.; Gómez-Oliván, L.M. In vitro bioactive properties of protein hydrolysates from giant squid (Dosidicus gigas) by Bacillus subtilis. Food Res. 2022, 7, 116–127. [Google Scholar]
- Ezquerra-Brauer, J.M.; Aubourg, S.P. Recent trends for the employment of jumbo squid (Dosidicus gigas) by-products as a source of bioactive compounds with nutritional, functional and preservative applications: A review. Int. J. Food Sci. Technol. 2019, 54, 987–998. [Google Scholar]
- Djellouli, M.; López-Caballero, M.E.; Arancibia, M.Y.; Karam, N.; Martínez-Alvarez, O. Antioxidant and antimicrobial enhancement by reaction of protein hydrolysates derived from shrimp by-products with glucosamine. Waste Biomass Valorization 2019, 11, 2491–2505. [Google Scholar]
- Obeidnejad, E.; Kavoosi, G.; Saharkhiz, M.J.; Shafiee, S.M. Chemical composition, functional properties, physico-chemical properties, and techno-functional characteristics of Satureja protein hydrolysate stabilized in a gelatin matrix. Food Sci. Nutr. 2024, 12, 8030–8042. [Google Scholar]
- Chalamaiah, M.; Ulug, S.K.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar]
- Choi, D.; Bedale, W.; Chetty, S.; Yu, J.H. Comprehensive review of clean-label antimicrobials used in dairy prod-ucts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13263. [Google Scholar]
- Fernando, I.P.S.; Jayawardena, T.U.; Wu, J. Marine proteins and peptides: Production, biological activities, and potential applications. Food Innov. Adv. 2022, 2, 69–84. [Google Scholar]
- González-Osuna, M.F.; Bernal-Mercado, A.T.; Wong-Corral, F.J.; Ezquerra-Brauer, J.M.; Soto-Valdez, H.; Castillo, A.; Rodríguez-Figueroa, J.C.; Del-Toro-Sánchez, C.L. Bioactive Peptides and Protein Hydrolysates Used in Meat and Meat Products’ Preservation—A Review. ACS Food Sci. Technol. 2024, 4, 1003–1016. [Google Scholar]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar]
- Rotter, A.; Varamogianni-Mamatsi, D.; Pobirk, A.Z.; Matjaž, M.G.; Cueto, M.; Díaz-Marrero, A.R.; Jónsdóttir, R.; Sveinsdóttir, K.; Catala, T.S.; Romano, G.; et al. Marine cosmetics and the blue bioeconomy: From sourcing to success stories. iScience 2024, 27, 111339. [Google Scholar]
- Palou, E.; López-Malo, A.; Barbosa-Canovas, G.V.; Swanson, B.G.; Rahman, M.S. High-pressure preservation of foods. In Handbook of Food Preservation, 3rd ed.; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 843–872. [Google Scholar]
- Rathod, N.B.; Smaoui, S.; Agrawal, R.; Bhagwat, P.; Amobonye, A.; Pillai, S.; Yilmaz, N.; Ozogul, F. Sustainable pro-cessing technologies (pulsed light, electrolysed water and ozonation) for microbial decontamination of muscle foods. Innov. Food Sci. Emer. Technol. 2024, 96, 103778. [Google Scholar]
- Yang, X.; Lin, J.; Shen, X.; Zhang, Z. Optimization of squid paste processing technology and quality characteristics. J. Fish. Res. 2024, 46, 468–479. [Google Scholar]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant activities and selected characteristics of gelatin hydrolysates from seabass (Lates calcarifer) skin as affected by production processes. J. Food Sci. Technol. 2016, 53, 197–208. [Google Scholar]
- Picos-Salas, M.A.; Gutiérrez-Grijalva, E.P.; Valdez-Torres, B.; Angulo-Escalante, M.A.; López-Martínez, L.X.; Delga-do-Vargas, F.; Heredia, J.B. Supercritical CO2 extraction of oregano (Lippia graveolens) phenolic compounds with antioxidant, α-amylase and α-glucosidase inhibitory capacity. J. Food Meas. Charact. 2021, 15, 3480–3490. [Google Scholar]
- Vo, C.-V.T.; Luu, N.V.H.; Nguyen, T.T.H.; Ho, B.Q.; Tran, T.-D.; Nguyen, Q.-T. Screening for pancreatic lipase inhibitors: Evaluating assay conditions using ρ-nitrophenyl palmitate as substrate. All Life 2022, 15, 13–22. [Google Scholar]
- Korczek, K.R.; Tkaczewska, J.; Duda, I.; Migdal, W. Effect of Heat Treatment on the Antioxidant and Antihy-pertensive Activity as Well as in vitro Digestion Stability of Mackerel (Scomber scombrus) Protein Hydrolysates. J. Aquat. Food Prod. Technol. 2020, 29, 73–89. [Google Scholar]
- López Medina, F.A.; López Martínez, L.X.; Gómez Oliván, L.M.; Dublán García, O.; Reyes García, A.; Cira Chávez, L.A. Bioactive Hydrolysates from Dosidicus Gigas: Functional Properties and Applications [Poster Presentation]. International Congress CUCCAL 12, Mexico City. Mexican Society of Food Safety and Quality for Consumers. 2023. Available online: https://someicca.com.mx/wp-content/uploads/Memorias-Congreso-Internacional-CUCCAL-12-FINALES-1.pdf (accessed on 12 March 2025).
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Assessment of Physico-Chemical, Antioxidant and Antimicrobial Ac-tivity of Porcine Blood Protein Hydrolysate in Pork Emulsion Stored Under Aerobic Packaging Condition at 4 ± 1 °C. LWT 2018, 88, 71–79. [Google Scholar]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of Iced Storage of Bigeye Snapper (Priacanthus tayenus) on the Chemical Composition, Properties and Acceptability of Som-Fug, a Fermented Thai Fish Mince. Food Chem. 2007, 102, 270–280. [Google Scholar]
- Zhou, T.; Zhao, Y.; Fu, S.; Wang, W.; Liu, A. Effects of Pig Skin and Coconut Powder Mixture on Gelling and Rheological Properties of Composite Gel Prepared with Squid Myofibrillar Protein and Lard. Int. J. Food Eng. 2018, 14, 20170265. [Google Scholar]
- Hajfathalian, M.; Jorjani, S.; Ghelichi, S. Characterization of Fish Sausage Manufactured with Combination of Sunflower Oil and Fish Oil Stabilized with Fish Roe Protein Hydrolysates. J. Food Sci. Technol. 2019, 57, 1439–1448. [Google Scholar]
- ISO 21528-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO Standards: Geneva, Switzerland, 2007.
- Idowu, A.T.; Igiehon, O.O.; Idowu, S.; Olatunde, O.O.; Benjakul, S. Bioactivity Potentials and General Applications of Fish Protein Hydrolysates. Int. J. Pept. Res. Ther. 2020, 27, 109–118. [Google Scholar]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, Bioactive Properties, and Potential Applications of Fish Protein Hydrolysates: Developments and Challenges. Trends Food Sci. Technol. 2021, 110, 689–699. [Google Scholar]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the Preparation of Fish Protein Anti-Obesity Hydrolysates Using Response Surface Methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, A.; Vela-Gutiérrez, G.; Rather, I.A.; Fernández-Lafuente, R. Bioactive Peptides from Fisheries Residues: A Review of Use of Papain in Proteolysis Reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [PubMed]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the Bioactive Potential of Foods Fortified with Fish Protein Hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar]
- Walayat, N.; Liu, J.; Nawaz, A.; Aadil, R.M.; López-Pedrouso, M.; Lorenzo, J.M. Role of Food Hydrocolloids as Anti-oxidants with Modern Processing Techniques in Surimi Protein Gel Textural Properties, Developments, Limitations, and Future Perspectives. Antioxidants 2022, 11, 486. [Google Scholar] [CrossRef]
- Verma, R.; Sharma, S.; Kundu, L.M.; Pandey, L.M. Experimental Investigation of Molasses as a Sole Nutrient for the Production of an Alternative Metabolite Biosurfactant. J. Water Process Eng. 2020, 38, 101632. [Google Scholar]
- Ling, Z.; Ai, M.; Zhou, Q.; Guo, S.; Zhou, L.; Fan, H.; Cao, Y.; Jiang, A. Fabrication egg white gel hydrolysates-stabilized oil-in-water emulsion and characterization of its stability and digestibility. Food Hydrocoll. 2020, 102, 105621. [Google Scholar]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared Spectroscopy in Microbial Cell Biology and Environmental Microbiology: Advances, Challenges, and Future Perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar]
- Wang, X.; He, X.; Wang, X. FTIR Analysis of the Functional Group Composition of Coal Tar Residue Extracts and Ex-tractive Residues. Appl. Sci. 2023, 13, 5162. [Google Scholar] [CrossRef]
- Kang, S.-I.; Kim, J.-S.; Park, S.-Y.; Cho, H.-J.; Jang, M.-S.; Oh, J.-Y.; Choi, J.-S. Development and Quality Attributes of Paste Sausage Supplemented with Common Squid (Todarodes pacificus) Tailored for the Elderly. Appl. Sci. 2023, 13, 10735. [Google Scholar] [CrossRef]
- Nemati, M.; Shahosseini, S.R.; Ariaii, P. Review of Fish Protein Hydrolysates: Production Methods, Antioxidant and Antimicrobial Activity, and Nanoencapsulation. Food Sci. Biotechnol. 2024, 33, 1789–1803. [Google Scholar]
- Tang, T.; Wu, N.; Tang, S.; Xiao, N.; Jiang, Y.; Tu, Y.; Xu, M. Industrial Application of Protein Hydrolysates in Food. J. Agric. Food Chem. 2023, 71, 1788–1801. [Google Scholar]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Antimicrobial and Antioxidant Potential of Papain Liver Hydrolysate in Meat Emulsion Model at Chilling Storage under Aerobic Packaging Condition. Waste Biomass Valorization 2022, 13, 417–429. [Google Scholar]
- Yesiltas, B.; Caindec, A.M.S.; García-Moreno, P.J.; Echers, S.G.; Olsen, T.H.; Jones, N.C.; Jacobsen, C. Physical and Ox-idative Stability of Fish Oil-in-Water Emulsions Stabilized with Emulsifier Peptides Derived from Seaweed, Methanotrophic Bacteria, and Potato Proteins. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131069. [Google Scholar]
- Zhong, H.; Jin, Y.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Recent Advances of Hepatoprotective Peptides: Production, Structure, Mechanisms, and Interactions with Intestinal Microbiota. Food Biosci. 2024, 58, 103744. [Google Scholar]
- Ochiai, A.; Itoh, T.; Kawamata, A.; Hashimoto, W.; Murata, K. Plant cell wall degradation by saprophytic Bacillus subtilis strains: Gene clusters responsible for rhamnogalacturonan depolymerization. Appl. Environ. Microbiol. 2007, 73, 3803–3813. [Google Scholar] [CrossRef]
- Cruz Ramos, H.; Hoffmann, T.; Marino, M.; Nedjari, H.; Presecan-Siedel, E.; Dreesen, O.; Glaser, P.; Jahn, D. Fermentative me-tabolism of Bacillus subtilis: Physiology and regulation of gene expression. J. Bacteriol. 2000, 182, 3072–3080. [Google Scholar] [CrossRef]
- Wu, Y.; Tao, Y.; Jin, J.; Tong, S.; Li, S.; Zhang, L. Multi-omics analyses of the mechanism for the formation of soy sauce-like and soybean flavor in Bacillus subtilis BJ3-2. BMC Microbiol. 2022, 22, 142. [Google Scholar] [CrossRef]
- El-Diehy, M.A.; Farghal, I.I.; Amin, M.A.; Ghobashy, M.M.; Nowwar, A.I.; Gayed, H.M. Radiation Synthesis of Sodium Alginate/Gelatin-Based Ultra-Absorbent Hydrogel for Efficient Water and Nitrogen Management in Wheat under Drought Stress. Sci. Rep. 2024, 14, 19463. [Google Scholar] [CrossRef]
- Al-Matarneh, C.M.; Pinteala, M.; Nicolescu, A.; Silion, M.; Mocci, F.; Puf, R.; Gratteri, P. Synthetic Approaches to Novel Human Carbonic Anhydrase Isoform Inhibitors Based on Pyrrol-2-One Moiety. J. Med. Chem. 2024, 67, 3018–3038. [Google Scholar] [CrossRef] [PubMed]
- Cortez, P.M. Espectroscopia FTIR-ATR aplicada al análisis de alimentos y bebidas. In En Principios y Aplicaciones de la Espectroscopia de Infrarrojo en el Análisis de Alimentos y Bebidas; Cortez, P.M., Ed.; CIATEJ: Guadalajara, Mexico, 2020; pp. 83–99. [Google Scholar]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A Comprehensive Review on Polarity, Partitioning, and Interactions of Phenolic Antioxidants at Oil–Water Interface of Food Emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Rao, A. Clean-Label Alternatives for Food Preservation: An Emerging Trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Calvo, M.; Carranco, M.E.; Salinas, C.A.; Carrillo, S. Chemical Composition of Giant Squid Dosidicus gigas meal. Arch. Latinoam. Nutr. 2016, 66, 74–81. [Google Scholar]
- Hao, S.; Qian, M.; Wang, Y.; Zhang, K.; Tian, J.; Wang, X. Research Progress on the Gel Properties of Fermented Sausage. Food Mater. Res. 2024, 4, e007. [Google Scholar] [CrossRef]
- Feng, C.-H.; Arai, H. Estimating Moisture Content of Sausages with Different Types of Casings via Hyperspectral Imaging in Tandem with Multivariate Analysis. Appl. Sci. 2023, 13, 5300. [Google Scholar] [CrossRef]
- Salama, Y.; Chennaoui, M. Microbial Spoilage Organisms in Seafood Products: Pathogens and Quality Control. Eur. J. Microbiol. Infect. Dis. 2024, 1, 66–89. [Google Scholar] [CrossRef]
- Santos, J.F.; Lima, D.G.; Oliveira, S.M. Microbiological quality of fresh meat and seafood-based sausages in Brazil: In-dicator microorganisms and their implications for food safety. Food Res. Int. 2023, 175, 106823. [Google Scholar]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein Hydrolysate from Salmon Frames: Production, Characteristics, and Antioxidative Activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on color and texture of food products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Ocaño-Higuera, V.M.; Márquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Graciano-Verdugo, A.Z. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.V.; Keyata, E.O. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 2022, 9901018. [Google Scholar]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood Spoilage Microbiota and Associated Volatile Organic Compounds at Different Storage Temperatures and Packaging Conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [PubMed]
- Sharaf Eddin, A.; Adegoke, S.; Issa, A.T.; Wilson, C.; Tahergorabi, R. Physicochemical Changes of Surimi Gels with Addition of Different Particle Sizes of Citrus Peel Fiber. J. Aquat. Food Prod. Technol. 2020, 29, 1029–1040. [Google Scholar]
- Jin, S.-K.; Choi, J.-S.; Kim, G. Effect of porcine plasma hydrolysate on physicochemical, antioxidant, and antimicrobial properties of emulsion-type pork sausage during cold storage. Meat Sci. 2021, 171, 108293. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Liu, Q.; Nan, F.; Chen, C.Y.O.; Xie, S. Arthrospira (Spirulina) platensis Extracts Improve Oxidative Stability and Product Quality of Chinese-Style Pork Sausage. J. Appl. Phycol. 2018, 30, 1667–1677. [Google Scholar]
- Starowicz, M.; Zieliński, H. How Maillard Reactions Influence Sensorial Properties (Color, Flavor, and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar]
- Fleming, S.; Ulijn, R.V. Design of Nanostructures Based on Aromatic Peptide Amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar]
- Roy, P.K.; Roy, A.; Jeon, E.B.; DeWitt, C.A.M.; Park, J.W.; Park, S.Y. Comprehensive Analysis of Predominant Patho-genic Bacteria and Viruses in Seafood Products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13410. [Google Scholar]
- Shaltout, F.A. Impact of Preservatives on Food Preservation and Their Effect on Bacteria. Univ. Libr. Innov. Res. Stud. 2024, 12, 1152. [Google Scholar] [CrossRef]
- Lueangsakulthai, J.; Jangpromma, N.; Temsiripong, T.; McKendrick, J.E.; Khunkitti, W.; Maddocks, S.E.; Klaynongs-ruang, S. A Novel Antibacterial Peptide Derived from Crocodylus siamensis Hemoglobin Hydrolysate Induces Membrane Permeabilization Causing Iron Dysregulation, Oxidative Stress, and Bacterial Death. J. Appl. Microbiol. 2017, 123, 819–831. [Google Scholar] [CrossRef] [PubMed]


| µmol Trolox Eq/g | Inhibition (%) | Inhibition (%) | Inhibition (%) | Inhibition (%) | |
|---|---|---|---|---|---|
| Hydrolysate | ORAC | α-Amylase | α-Glucosidase | Lypase | Angiotensin-Converting Enzyme (ACE-I) |
| H1 | 337.70 c | 12.69 b | 78.52 c | 25.46 c | 62.2 c |
| H2 | 383.59 d | 16.17 c | 80.76 c | 34.68 d | 71.85 d |
| H3 | 215.44 a | 9.7 a | 44.84 a | 11.16 a | 22.11 a |
| H4 | 742.17 e | 20.87 d | 93.29 d | 35.44 d | 88.96 e |
| H5 | 266.88 b | 13.83 b | 62.42 b | 19.38 b | 48.73 b |
| Sample | pH | TVB (mg N/100 g) | WHC (%) | L* | a* | b* |
|---|---|---|---|---|---|---|
| B | 6.53 ± 0.05 ab | 2.387 ± 0.06 a | 86.08 ± 0.89 a | 74.32 ± 0.04 d | 7.13 ± 0.02 a | 34.51 ± 0.01 a |
| C22 | 6.47 ± 0.05 a | 52.217 ± 0.65 c | 87.15 ± 0.96 a | 72.80 ± 0.02 a | 7.70 ± 0.02 c | 35.12 ± 0.02 b |
| H22 | 6.51 ± 0.03 a | 33.578 ± 1.11 b | 87.46 ± 1.46 a | 73.17 ± 0.02 c | 7.50 ± 0.01 b | 34.59 ± 0.06 a |
| H43 | 6.63 ± 0.07 b | 57.687 ± 2.4 d | 88.21 ± 0.36 a | 72.98 ± 0.05 b | 7.9 ± 0.03 d | 35.27 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Medina, F.A.; Dublán-García, O.; Morachis-Valdez, A.G.; Saucedo-Vence, K.; López-García, G.; Díaz-Bandera, D.; Gómez-Espinoza, R.M. Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages. Polymers 2025, 17, 839. https://doi.org/10.3390/polym17070839
López-Medina FA, Dublán-García O, Morachis-Valdez AG, Saucedo-Vence K, López-García G, Díaz-Bandera D, Gómez-Espinoza RM. Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages. Polymers. 2025; 17(7):839. https://doi.org/10.3390/polym17070839
Chicago/Turabian StyleLópez-Medina, Francisco Antonio, Octavio Dublán-García, Ana Gabriela Morachis-Valdez, Karinne Saucedo-Vence, Guadalupe López-García, Daniel Díaz-Bandera, and Rosa María Gómez-Espinoza. 2025. "Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages" Polymers 17, no. 7: 839. https://doi.org/10.3390/polym17070839
APA StyleLópez-Medina, F. A., Dublán-García, O., Morachis-Valdez, A. G., Saucedo-Vence, K., López-García, G., Díaz-Bandera, D., & Gómez-Espinoza, R. M. (2025). Biopolymeric Hydrolysates from Dosidicus gigas: Functional Applications and Shelf-Life Extension in Squid Sausages. Polymers, 17(7), 839. https://doi.org/10.3390/polym17070839









