Self-Made Cyclodextrin Inclusion Complexes for Enhanced Mechanical Properties of Polycaprolactone Composites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Fabrication
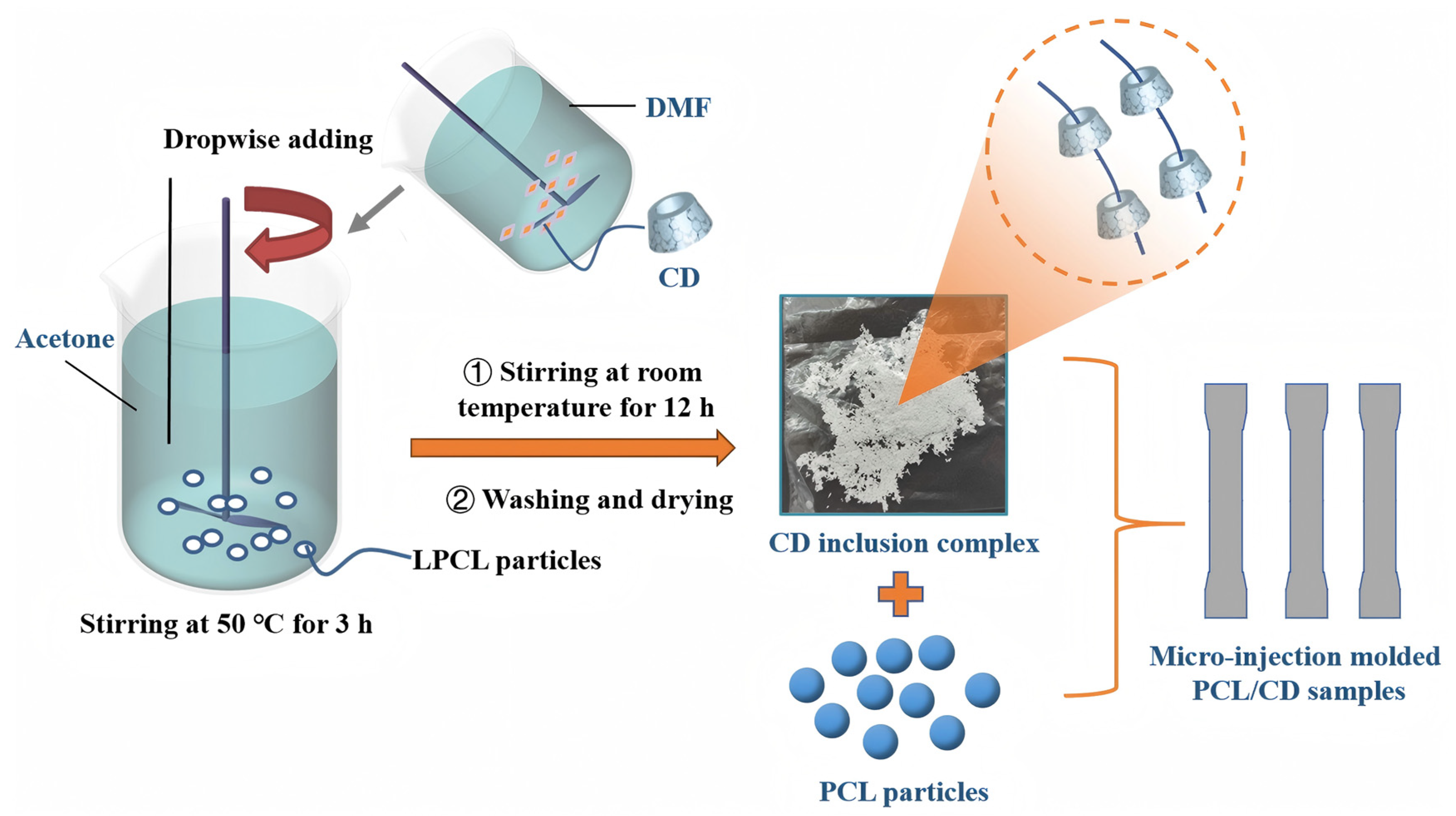
2.2.1. Preparation of CD Inclusion Complexes Using Low-Molecular-Weight PCL
2.2.2. Preparation of the Resulting PCL Composites
2.3. Characterization
3. Results and Discussion
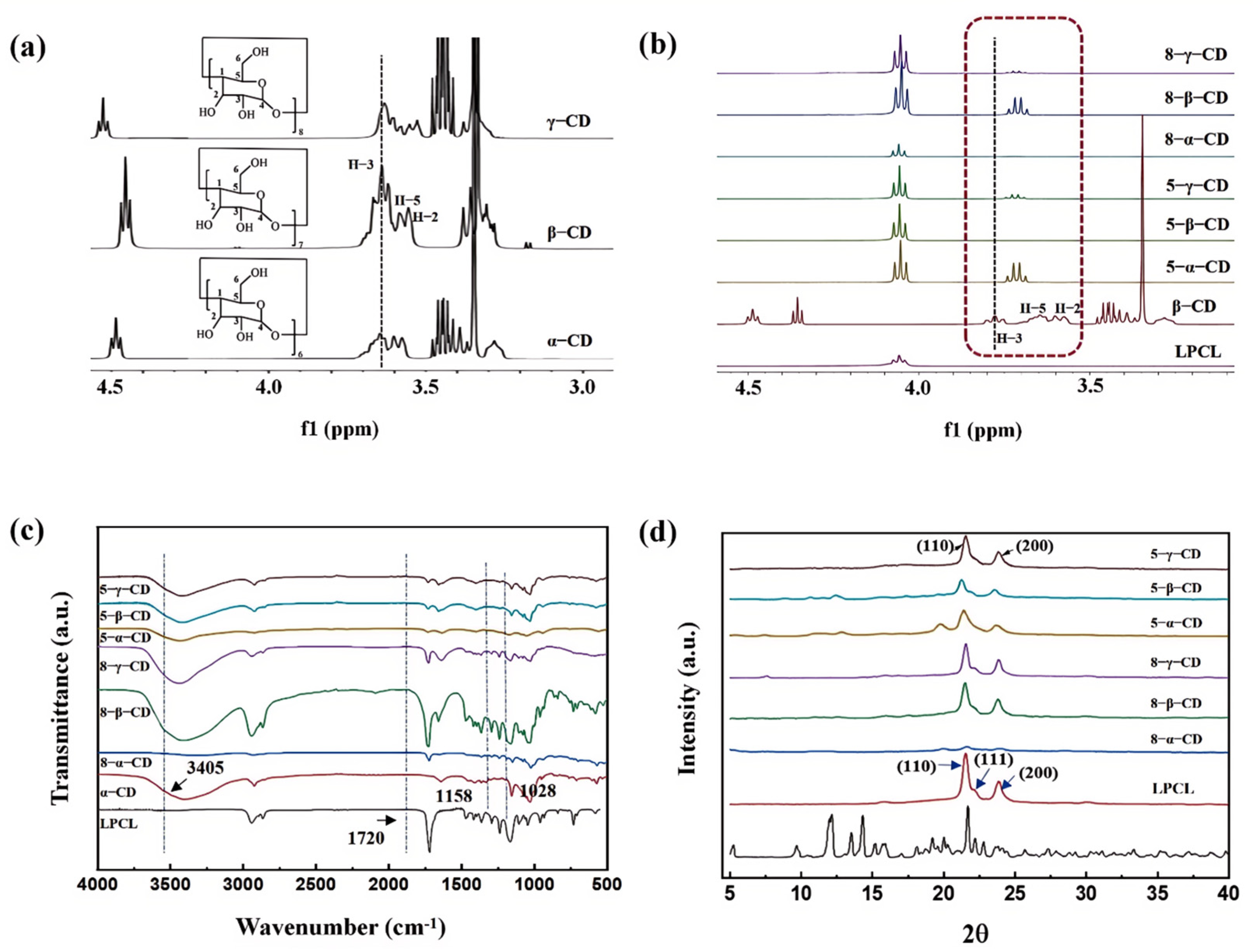
3.1. Structure and Crystal Analysis of CD Inclusion Complexes
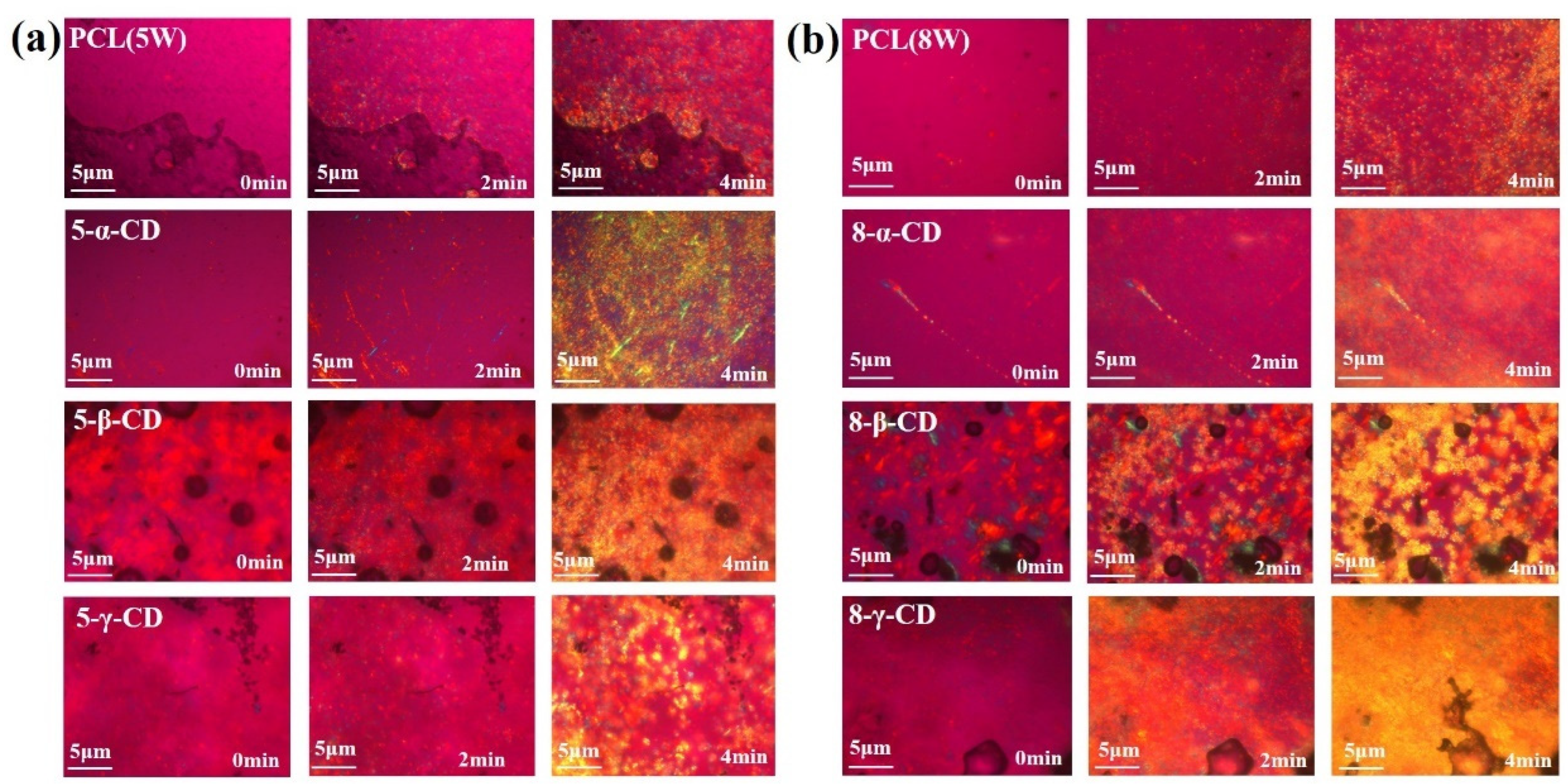
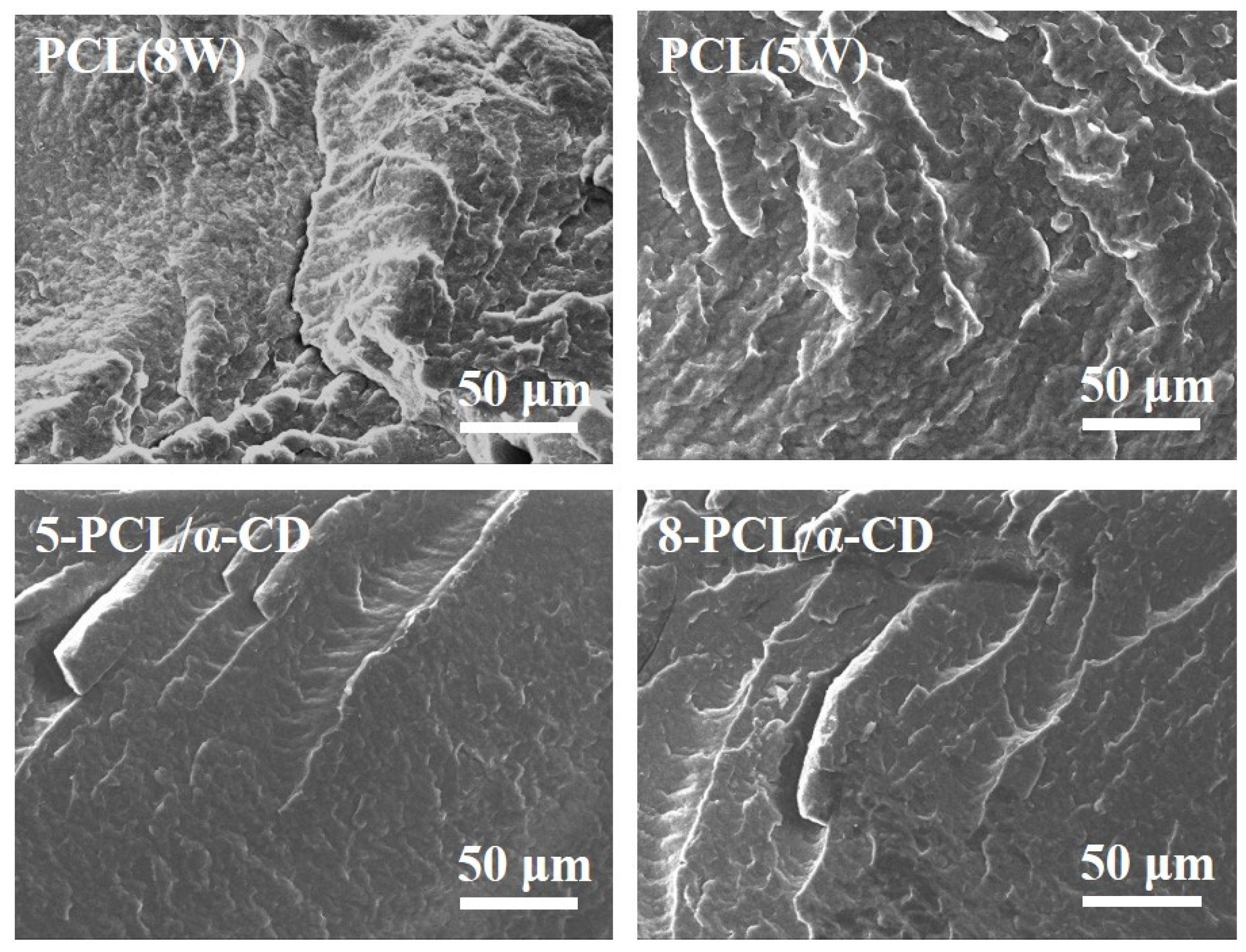
3.2. Structure and Crystal Analysis of PCL/α-CD Composites
3.3. Rheological Behavior Analysis of PCL/α-CD Composites
3.4. Mechanical Property of PCL/α-CD Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachan, R.; Purwar, R. Effect of PCL Chain Length on Rheological and Mechanical Properties of PCL-PDMS-PCLTriblock Copolymer Films. J. Appl. Polym. Sci. 2024, 141, e55542. [Google Scholar] [CrossRef]
- Lu, X.; Zou, H.; Liao, X.; Xiong, Y.; Hu, X.; Cao, J.; Pan, J.; Li, C.; Zheng, Y. Construction of PCL-Collagen@PCL@PCL-Gelatin Three-Layer Small Diameter Artificial Vascular Grafts by Electrospinning. Biomed. Mater. 2023, 18, 015008. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Processing of PCL and PCL-PEG Blends to Produce Improved PCL-Based Porous Scaffolds. J. Supercrit. Fluids 2022, 186, 105611. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and Properties of Polycaprolactone/Tricalcium Phosphate Scaffolds Containing Polyethylene Glycol Fabricated by 3D Printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- Lim, H.; Shin, J.-Y.; Lee, D.Y.; Kim, B.-Y.; Song, Y.-S. Drug Delivery Behavior of PCL and PCL/PEG Microcapsules Prepared by High-Speed Agitator and Syringe Pump. Polymer Korea 2020, 44, 487–494. [Google Scholar] [CrossRef]
- Pethile, S.; Chen, X.-J.; Hou, D.; Wang, L. Effect of Changing Coating Process Parameters in the Preparation of Antimicrobial-Coated Silk Sutures: An in Vitro Study. Fibers Polym. 2014, 15, 1589–1595. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P.; Yin, X. Degradation and Drug-Release Behavior of Polylactic Acid (PLA) Medical Suture Coating with Tea Polyphenol (TP)-Polycaprolactone (PCL)/Polyglycolide (PGA). Fibers Polym. 2019, 20, 229–235. [Google Scholar] [CrossRef]
- Gao, X.; Kan, B.; Gou, M.; Zhang, J.; Guo, G.; Huang, N.; Zhao, X.; Qian, Z. Preparation of Anti-CD40 Antibody Modified Magnetic PCL-PEG-PCL Microspheres. J. Biomed. Nanotechnol. 2011, 7, 285–291. [Google Scholar] [CrossRef]
- Sakurai, T.; Nojima, S. Significant Increase in the Melting Temperature of Poly(ɛ-Caprolactone) Blocks Confined in the Crystallized Lamellar Morphology of Poly(ɛ-Caprolactone)-Block-Polyethylene Copolymers. Polym. J. 2011, 43, 370–377. [Google Scholar] [CrossRef]
- Gu, L.; Li, Y.; Yang, Y.; Wang, Z.; Jin, Y. Preparation and Adsorption Performance of Cellulose-Graft-Polycaprolactone/Polycaprolactone Porous Material. Bioresources 2017, 12, 5539–5549. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, F.; Qiu, Z. Miscibility and Crystallization Behavior of Biodegradable Poly(Ε-caprolactone)/Tannic Acid Blends. J. Appl. Polym. Sci. 2012, 124, 4409–4415. [Google Scholar] [CrossRef]
- Sun, T.; Li, J.; Hao, A. Cyclodextrin-Graphene Supramolecular System. Chin. J. Org. Chem. 2012, 32, 2054. [Google Scholar] [CrossRef]
- Xu, P.; Song, L. Thermal Decomposition Kinetics of Survived β-Cyclodextrin from an Inclusion Complex of β-Cyclodextrin with Clove Oil. Acta Phys.-Chim. Sin. 2008, 24, 2214–2220. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.-R.; Rong, R.-X.; Liu, M.-H.; Wang, Q.; Li, X.-L. Host-Guest Controlled Assembly and Recognition of Perylene Bisimide-Based Glycocluster with Cyclodextrin. Dyes Pigments 2020, 174, 108043. [Google Scholar] [CrossRef]
- Nie, C.; Liu, C.; Sun, S.; Wu, S. Visible-Light-Controlled Azobenzene-Cyclodextrin Host-Guest Interactions for Biomedical Applications and Surface Functionalization. ChemPhotoChem 2021, 5, 893–901. [Google Scholar] [CrossRef]
- Pankaj. Cyclodextrin Modified Block Polymer for Oral Chemotherapy. J. Pharm. Res. Int. 2021, 32, 21–29. [Google Scholar] [CrossRef]
- Prasannan, A.; Bich-Tram, T.-L.; Hsu, D.-Y.; Hong, P.-D.; Pan, G.-R. Nucleation Effects of α-Cyclodextrin Inclusion Complexes on the Crystallization Behavior of Biodegradable Poly(1,4-Butylene Adipate). CrystEngComm 2013, 15, 5119. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Gharechahi, M.; Alibolandi, M.; Tekie, F.M.; Khashyarmanesh, B.; Hadizadeh, F. Self-Assembled Supramolecular Hydrogel Based on PCL-PEG-PCL Triblock Copolymer and γ-Cyclodextrin Inclusion Complex for Sustained Delivery of Dexamethasone. Int. J. Pharm. Investig. 2016, 6, 78. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant Activity and Photostability of α-Tocopherol/β-Cyclodextrin Inclusion Complex Encapsulated Electrospun Polycaprolactone Nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Aboumaashzadeh, M.; Tekie, F.S.M.; Hadizadeh, F.; Tabassi, S.A.S.; Mohajeri, S.A.; Khashyarmanesh, Z.; Haghighi, H.M. Sustained Drug Release Using Supramolecular Hydrogels Composed of Cyclodextrin Inclusion Complexes with PCL/PEG Multiple Block Copolymers. Iran. Polym. J. 2014, 23, 707–716. [Google Scholar] [CrossRef]
- Ma, S.J.; Wang, S.W.; Zhang, Q.; Li, Q. Improved Layer Mechanical Properties of Micro Injection Molded PP. Int. Polym. Process. 2017, 32, 138–142. [Google Scholar] [CrossRef]
- Shen, Y.K.; Wu, W.Y. An Analysis of the Three-Dimensional Micro-Injection Molding. Int. Commun. Heat Mass Transf. 2002, 29, 423–431. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Zhao, N.; Jiang, J.; Li, Q. A Novel Morphology Development of Micro-Injection Molded Isotactic Polypropylene. RSC Adv. 2015, 5, 41608–41610. [Google Scholar] [CrossRef]
- GB/T1040.2-2006; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Standardization Administration of the Peopleʹs Republic of China: Beijing, China, 2006.
- Dong, T.; Mori, T.; Pan, P.; Kai, W.; Zhu, B.; Inoue, Y. Crystallization Behavior and Mechanical Properties of Poly(Ε-caprolactone)/Cyclodextrin Biodegradable Composites. J. Appl. Polym. Sci. 2009, 112, 2351–2357. [Google Scholar] [CrossRef]
- Jiang, M.-W.; Guo, C.-G.; Wang, L.; Li, Y.-K.; Wang, C.-Q. Studies on the Orthogonal Assembly of β-Cyclodextrin-Poly (ε-Caprolactone) and Ferrocene-Poly (Ethylene Oxide). Carbohydr. Polym. 2013, 92, 1566–1572. [Google Scholar] [CrossRef]
- Luo, H.; Fan, M.; Yu, Z.; Meng, X.; Li, B.; Zhang, S. Preparation and Properties of Degradable Shape Memory Material Based on Partial α-Cyclodextrin–Poly(ε-caprolactone) Inclusion Complex. Macromol. Chem. Phys. 2009, 210, 669–676. [Google Scholar] [CrossRef]
- Iguchi, H.; Uchida, S.; Koyama, Y.; Takata, T. Polyester-Containing α-Cyclodextrin-Based Polyrotaxane: Synthesis by Living Ring-Opening Polymerization, Polypseudorotaxanation, and End Capping Using Nitrile N-Oxide. ACS Macro Lett. 2013, 2, 527–530. [Google Scholar] [CrossRef]
- Moyers-Montoya, E.; García-Casillas, P.; Vargas-Requena, C.; Escobedo-González, R.; Martel-Estrada, S.-A.; Martínez-Pérez, C. Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique. Polymers 2016, 8, 395. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Dong, C. Supramolecular Inclusion Complexes of Star-shaped Poly(Ε-caprolactone) with A-cyclodextrin. J Polym. Sci. A Polym. Chem. 2005, 43, 4721–4730. [Google Scholar] [CrossRef]
- Serag, E.; Eltawila, A.M.; Salem, E.M.; El-Maghraby, A.; Abd El-Aziz, A.M. Development of an Innovative Cylindrical Carbon Nanofiber/Gelatin-Polycaprolactone Hydrogel Scaffold for Enhanced Bone Regeneration. Int. J. Biol. Macromol. 2025, 306, 141250. [Google Scholar] [CrossRef]
- Khan, S.H.; Dhakal, H.N.; Saifullah, A.; Zhang, Z. Improved Mechanical and Thermal Properties of Date Palm Microfiber-Reinforced PCL Biocomposites for Rigid Packaging. Molecules 2025, 30, 857. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Wang, Z.; Hu, G.; Yang, W.; Hu, Y. Chitosan/PCL Fibrous Membranes Loaded Potassium Cinnamate/β-CD Clathrate Compounds Developed by ELS for Fruit Preservation. Food Hydrocoll. 2024, 156, 110341. [Google Scholar] [CrossRef]
- Abbasi, E.; Ghamkhari, A.; Ghorbani, M.; Salati, M.A.; Abbasi, F. Facile Construction of PH-Responsive Nanocarrier Based on Host–Guest Interactions Chemistry for Triggered Drug Delivery. J. Polym. Res. 2024, 31, 182. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of Porous HPCL/LPCL/HA Scaffolds with Supercritical CO 2 Gas Foaming Method. J. Mech. Behav. Biomed. Mater. 2017, 69, 115–127. [Google Scholar] [CrossRef]
- Rehman, R.; Rafiq, M.; Shafi, H.; Rather, A.H.; Khan, R.S.; Raza, S.N.; Rather, S.; Majeed, S.; Khan, N.A.; Sheikh, F.A. Designing Sustained Release from Nanofiber Patch for Paclitaxel as Prospective Localized Nanotherapeutic Delivery in Breast Cancer. Int. J. Pharm. 2025, 671, 125158. [Google Scholar] [CrossRef]
- Hoviezi, E.; Mojezi-badil, S.; Ansari-Asl, Z. Fabrication, Characterization, and Biocompatibility Assessment of Polycaprolactone/Polyacrylonitrile/Casein Nanofibers Scaffold for Tissue Engineering Applications. Fibers Polym. 2025, 26, 1075–1089. [Google Scholar] [CrossRef]
- Kali, G.; Mayer, A.H.; To, D.; Truszkowska, M.; Seybold, A.; Braun, D.E.; Plangger, R.; Gallei, M.; Bernkop-Schnürch, A. Polycaprolactone/α-Cyclodextrin Polyrotaxanes with Cellular Uptake Enhancing Properties. J. Mater. Chem. B 2025, 13, 3471–3482. [Google Scholar] [CrossRef]
- Lee, L.-T.; Tsai, C.-Y.; Wu, T.-Y. Discussions on Crystallization Behaviors and Physical Properties of Biodegradable Poly(Ethylene Succinate) Composites Containing PCL-CD-IC. J. Polym. Res. 2023, 30, 75. [Google Scholar] [CrossRef]
- Nie, Y.-J.; Zhao, X.-P.; Li, S.-T. Influence of Trap Characteristics on DC Surface Flashover Performance of Low Density Polyethylene in Vacuum. Acta Phys. Sin. 2019, 68, 227201. [Google Scholar] [CrossRef]
- Geng, Y. Crystalline Cooperativity in Double-Cable Conjugated Polymers. Acta Physico-Chimica Sinica 2019, 35, 1311–1312. [Google Scholar] [CrossRef]
- Gao, X.; Su, Y.; Xie, B.; Fu, D.; Wang, D. Progress in Studies of Confined Crystallzaiton of Long-Chain n-Alkanes. Acta Polym. Sin. 2014, 14, 22–30. [Google Scholar] [CrossRef]
- Yang, J.-S.; Zhu, Z.-L.; Cao, Q.-L. Effect of Pre-Orientation on Formation of Microstructure of Lamella Crystal and the Stress Response of Semicrystalline Polymers: Molecular Dynamics Simulations. Acta Phys. Sin. 2020, 69, 038101. [Google Scholar] [CrossRef]




| Sample | LPCL/α, β, γ-CD (mol/mol) | LPCL/Acetone (g/mL) | A, β, γ-CD/DMF (g/mL) |
|---|---|---|---|
| 5-α-CD | 1/40 | 0.250/60 | 0.1946/10 |
| 5-β-CD | 1/40 | 0.250/60 | 0.227/10 |
| 5-γ-CD | 1/40 | 0.250/60 | 0.2594/10 |
| 8-α-CD | 1/40 | 0.400/60 | 0.1946/10 |
| 8-β-CD | 1/40 | 0.400/60 | 0.227/10 |
| 8-γ-CD | 1/40 | 0.400/60 | 0.2594/10 |
| Sample | Tc/°C | Tm/°C | Xc% | Heat Flow (w/g) |
|---|---|---|---|---|
| PCL | 32.55 | 55.74 | 39.3 | 49.45 |
| PCL/5-α-CD | 34.44 | 56.18 | 44.6 | 55.452 |
| PCL/8-α-CD | 34.12 | 56.01 | 45.1 | 50.214 |
| Sample | Glass Transition Temperature Tg (°C) |
|---|---|
| PCL | −43.2 |
| PCL/5-α-CD | −41.9 |
| PCL/8-α-CD | −39.9 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PCL | 15.2 | 15.2 |
| PCL/8-α-CD | 24.8 | 19.9 |
| PCL/5-α-CD | 22.3 | 16.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wang, X.; Zhang, J.; Wang, W.; Han, R. Self-Made Cyclodextrin Inclusion Complexes for Enhanced Mechanical Properties of Polycaprolactone Composites. Polymers 2025, 17, 834. https://doi.org/10.3390/polym17070834
Yin Y, Wang X, Zhang J, Wang W, Han R. Self-Made Cyclodextrin Inclusion Complexes for Enhanced Mechanical Properties of Polycaprolactone Composites. Polymers. 2025; 17(7):834. https://doi.org/10.3390/polym17070834
Chicago/Turabian StyleYin, Yanji, Xiaotong Wang, Jiayan Zhang, Wenyan Wang, and Rui Han. 2025. "Self-Made Cyclodextrin Inclusion Complexes for Enhanced Mechanical Properties of Polycaprolactone Composites" Polymers 17, no. 7: 834. https://doi.org/10.3390/polym17070834
APA StyleYin, Y., Wang, X., Zhang, J., Wang, W., & Han, R. (2025). Self-Made Cyclodextrin Inclusion Complexes for Enhanced Mechanical Properties of Polycaprolactone Composites. Polymers, 17(7), 834. https://doi.org/10.3390/polym17070834








