Enhanced Carbon Nanotube Ionogels for High-Performance Wireless Strain Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHA Ionogels
2.3. Preparation of PHAC Ionogels
2.4. Characterizations and Measurements
2.5. Mechanical Properties Measurements
2.6. Adhesion Properties
2.7. Electrochemical Testing of PHA Ionogels and PHAC Ionogels
3. Results and Discussion
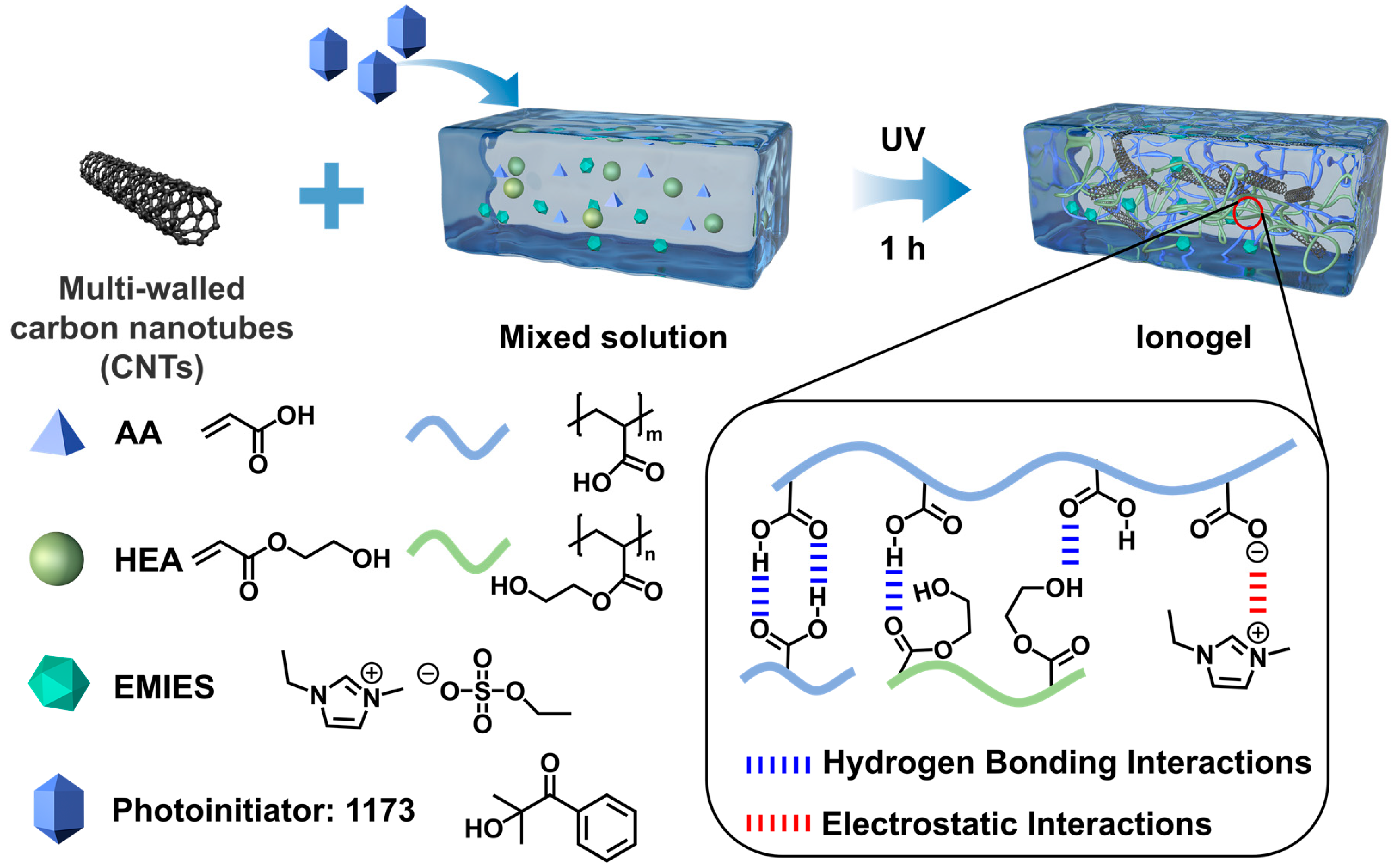
3.1. Formation of the Ionogels
3.2. Structure and Morphology of the Ionogels
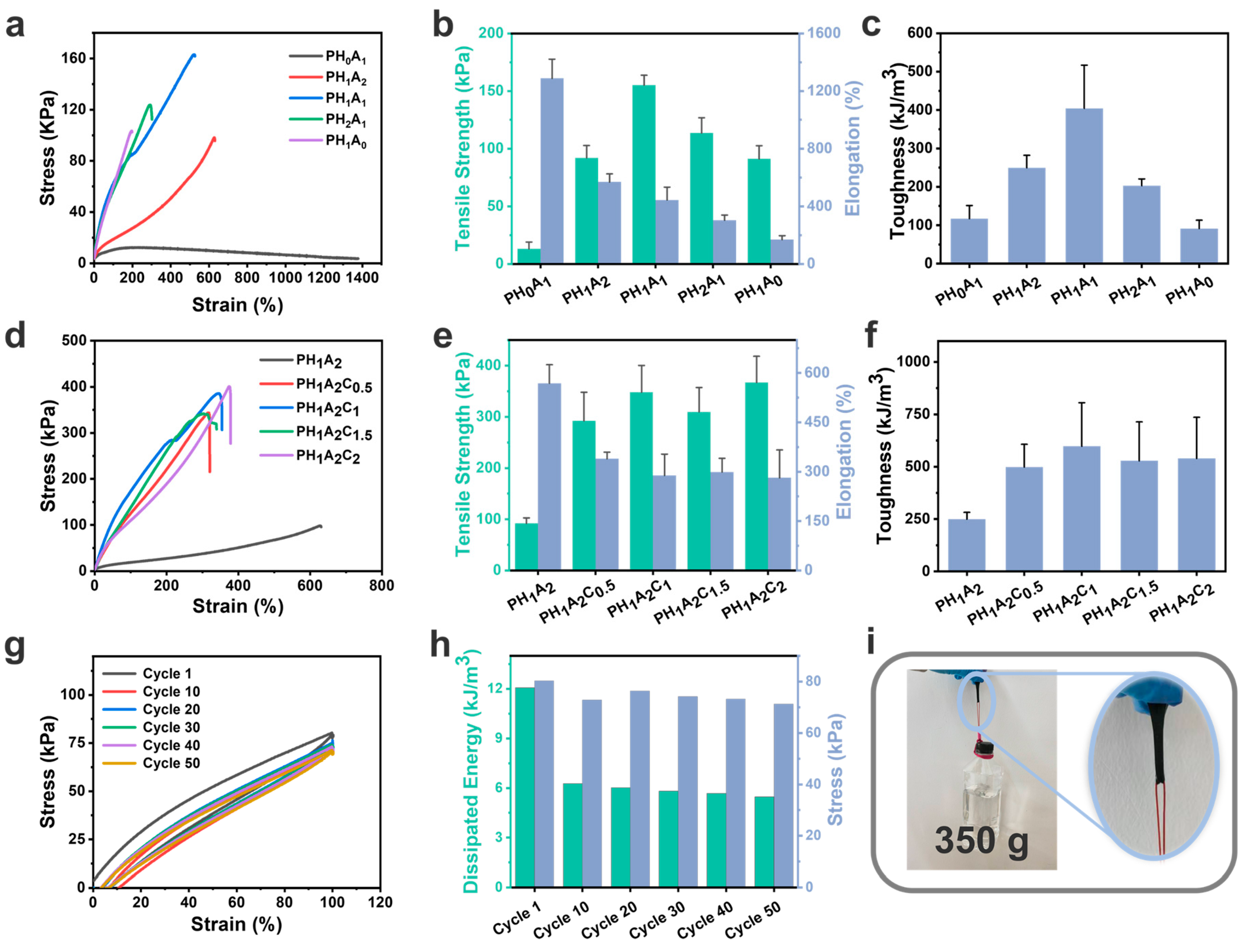
3.3. Mechanical Properties of the Ionogels
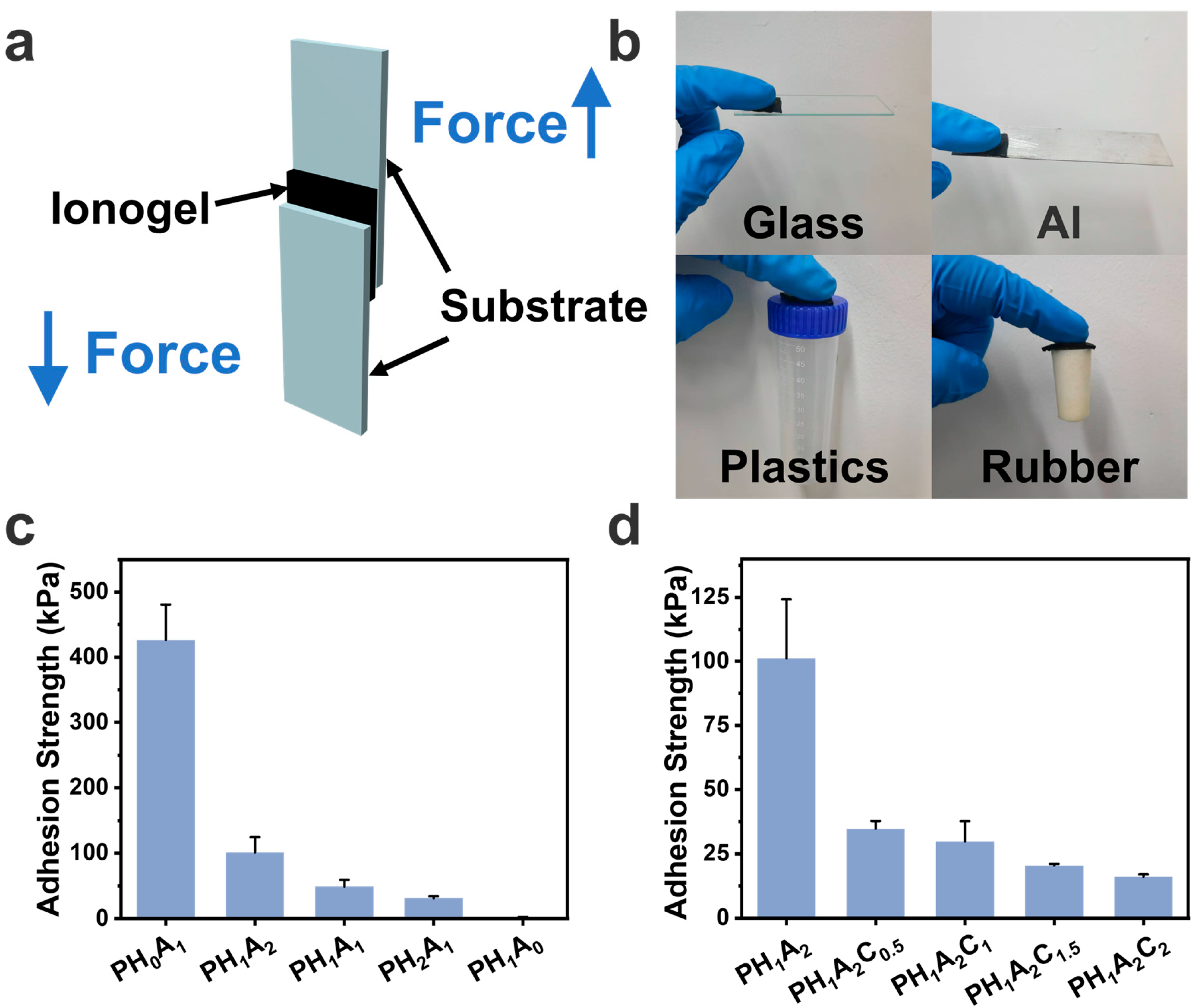
3.4. Adhesion Properties of the Ionogels
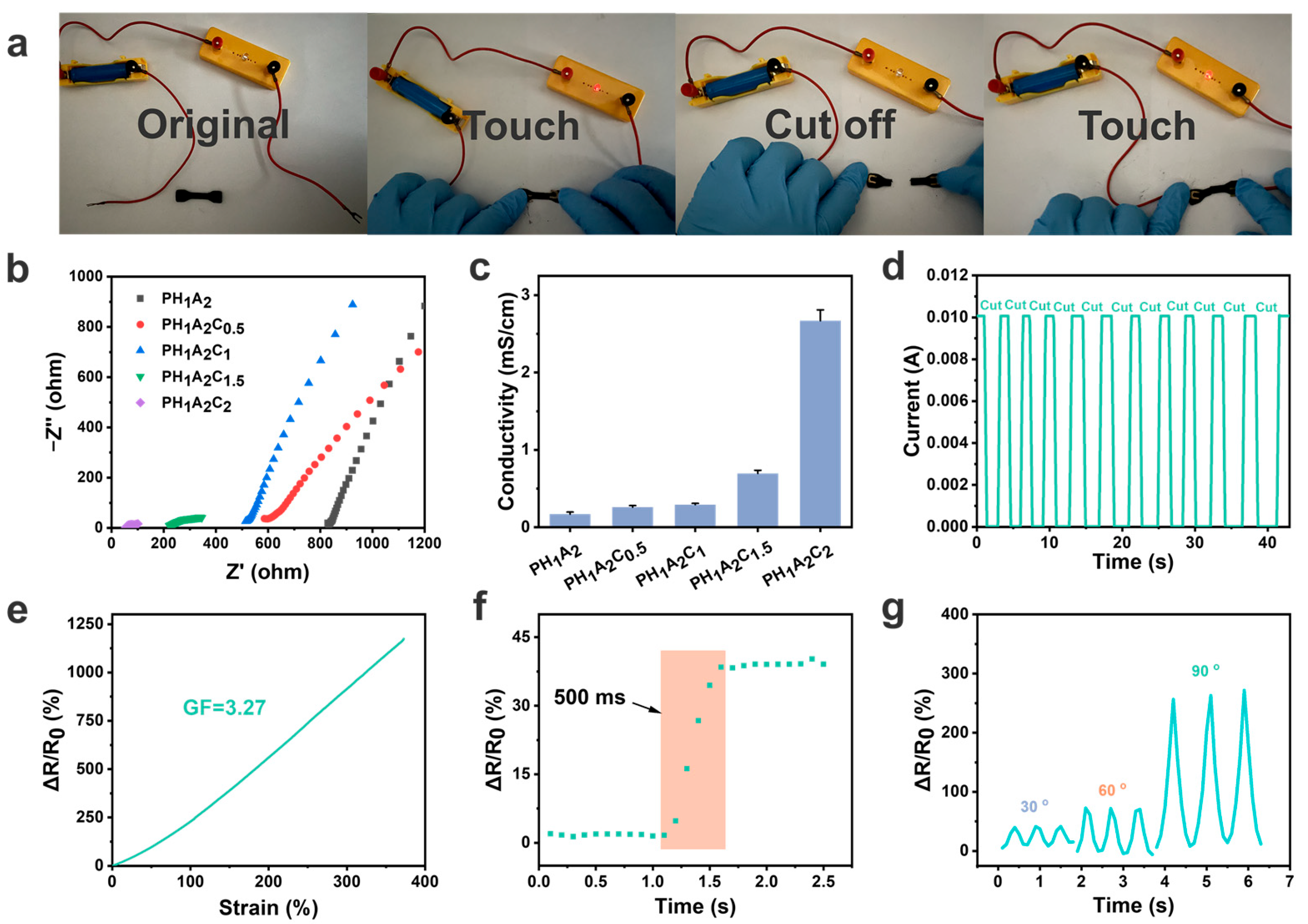
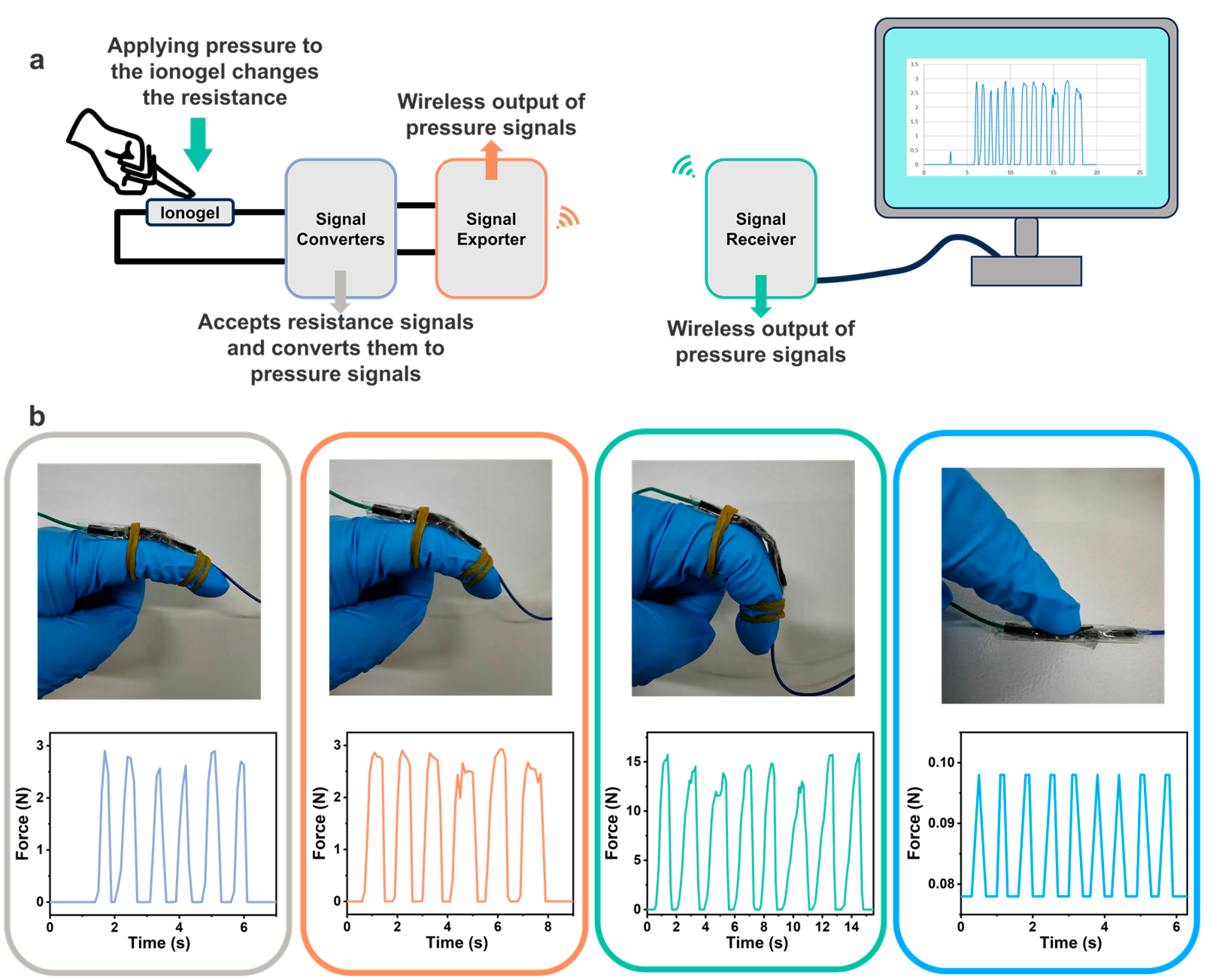
3.5. Electrical Properties of Ionogels and Wireless Physiological Signal Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, C.-C.; Li, W.; Liu, Z.; Zheng, S.; Hu, Y.; Zhou, Y.; Guo, J.; Ou, X.; Li, Q.; Yu, J.; et al. Ionogels: Preparation, Properties and Applications. Adv. Funct. Mater. 2024, 34, 2314408. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Yan, J.; Sun, J. Roles of Ionic Liquids in Adjusting Nature of Ionogels: A Mini Review. Adv. Funct. Mater. 2022, 32, 2203988. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Zhuang, M.; Bao, Y.; Chen, J.; Xu, H. Transparent, anti-freezing and highly stretchable solid-state ionic conductors. Polym. Chem. 2023, 14, 608–615. [Google Scholar] [CrossRef]
- Huang, C.; Jia, X.; Wang, D.; Sun, X.; Liang, Q.; Tian, R.; Guo, L.; Yang, J.; Song, H. Stretchable ionogels: Recent advances in design, toughening mechanisms, material properties and wearable devices applications. Chem. Eng. J. 2024, 490, 151850. [Google Scholar] [CrossRef]
- Ma, C.; Luo, H.; Liu, M.; Yang, H.; Liu, H.; Zhang, X.; Jiang, L. Preparation of intrinsic flexible conductive PEDOT:PSS@ ionogel composite film and its application for touch panel. Chem. Eng. J. 2021, 425, 131542. [Google Scholar] [CrossRef]
- Demarteau, J.; Fernandez de Añastro, A.; Shaplov, A.S.; Mecerreyes, D. Poly(diallyldimethylammonium) based poly(ionic liquid) di- and triblock copolymers by PISA as matrices for ionogel membranes. Polym. Chem. 2020, 11, 1481–1488. [Google Scholar] [CrossRef]
- Schorr, N.B.; Bhandarkar, A.; McBrayer, J.D.; Talin, A.A. Composite Ionogel Electrodes for Polymeric Solid-State Li-Ion Batteries. Polymers 2024, 16, 1763. [Google Scholar] [CrossRef]
- Li, W.; Lin, K.; Chen, L.; Yang, D.; Ge, Q.; Wang, Z. Self-Powered Wireless Flexible Ionogel Wearable Devices. ACS Appl. Mater. Interfaces 2023, 15, 14768–14776. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, H.; Gao, Z.; Song, H. Stretchable and thermo-mechanical stable ionogels with high thermoelectric properties for respiratory sensing and energy harvesting. Chem. Eng. J. 2024, 498, 155789. [Google Scholar] [CrossRef]
- Biria, S.; Pathreeker, S.; Genier, F.S.; Hosein, I.D. A Highly Conductive and Thermally Stable Ionic Liquid Gel Electrolyte for Calcium-Ion Batteries. ACS Appl. Polym. Mater. 2020, 2, 2111–2118. [Google Scholar] [CrossRef]
- Zhou, Q.; Men, Y. Thermoresponsive ionogels. Polym. Chem. 2024, 15, 2719–2739. [Google Scholar] [CrossRef]
- Fan, X.; Feng, W.; Wang, S.; Chen, Y.; Zheng, W.J.; Yan, J. Fluorine-Containing Ionogels with Stretchable, Solvent-Resistant, Wide Temperature Tolerance, and Transparent Properties for Ionic Conductors. Polymers 2024, 16, 1013. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Luo, Y.; Li, K.; Wong, Y.J.; Wang, C.; Yeo, J.C.C.; Yang, G.; Li, J.; Loh, X.J.; Li, Z.; et al. A Recyclable Ionogel with High Mechanical Robustness Based on Covalent Adaptable Networks. Adv. Mater. 2024, 36, 2407398. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Cui, T.; Zhang, M.; Yang, L.; Hu, Y.; Gui, Z. Transparent ionogel balancing rigidity and flexibility with prolonged stability for ultra-high sensitivity temperature sensing. Chem. Eng. J. 2024, 494, 152695. [Google Scholar] [CrossRef]
- Tie, J.; Mao, Z.; Zhang, L.; Zhong, Y.; Sui, X.; Xu, H. Highly transparent, self-healing and adhesive wearable ionogel as strain and temperature sensor. Polym. Chem. 2022, 13, 4064–4075. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, K.; Tang, L.; Chen, Z.; Hu, Q.; Gao, J.; Zhang, Y.; Zhang, J.; Zhang, S. A Strong and Highly Transparent Ionogel Electrolyte Enabled by In Situ Polymerization-Induced Microphase Separation for High-Performance Electrochromic Devices. Macromol. Rapid Commun. 2024, 45, 2300736. [Google Scholar] [CrossRef]
- Hopson, C.; Villar-Chavero, M.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Cellulose ionogels, a perspective of the last decade: A review. Carbohydr. Polym. 2021, 274, 118663. [Google Scholar] [CrossRef]
- Wan, X.; He, Y.; Xu, Z.; Li, C.; Yang, C. Progress of Hydrophobic Ionogels: A Review. Macromol. Rapid Commun. 2023, 44, 2200957. [Google Scholar] [CrossRef]
- Fan, X.; Liu, S.; Jia, Z.; Koh, J.J.; Yeo, J.C.C.; Wang, C.-G.; Surat’man, N.E.; Loh, X.J.; Le Bideau, J.; He, C.; et al. Ionogels: Recent advances in design, material properties and emerging biomedical applications. Chem. Soc. Rev. 2023, 52, 2497–2527. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, A.S.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogels derived from polycationic polysilsesquioxanes for lithium ion batteries. Polymer 2017, 117, 160–166. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Fojud, Z.; Robakowska, M.; Skrzypczak, A.; Voelkel, A.; Marcinkowska, A. Thiol–ene ionogels based on polymerizable imidazolium ionic liquids. Polym. Chem. 2022, 13, 3154–3170. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, D.; Dong, T.; Das, R.; Pan, D.; Sun, C.; Wu, Z.; Zhang, Q.; Liu, C.; Guo, Z. Overview of Ionogels in Flexible Electronics. Chem. Rec. 2020, 20, 948–967. [Google Scholar] [CrossRef]
- Jin, Q.; Pan, L.; Wang, Y.; Zhou, Z.; Zhu, M. Self-Adhesive, Antifreezing, and High Resilience Biobased Ionogel as a Flexible Strain–Temperature Bimodal Sensor. ACS Appl. Polym. Mater. 2024, 6, 4798–4807. [Google Scholar] [CrossRef]
- Xia, X.; Cao, X.; Zhang, B.; Zhang, L.; Dong, J.; Qin, J.; Xuan, P.; Liu, L.; Sun, Y.; Fan, W.; et al. Human Skin-Mimicking Ionogel-Based Electronic Skin for Intelligent Robotic Sorting. Macromol. Rapid Commun. 2024, 2024, 2400379. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Pham-Truong, T.-N. Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications. Polymers 2024, 16, 2506. [Google Scholar] [CrossRef]
- Patel, V.; Das, E.; Bhargava, A.; Deshmukh, S.; Modi, A.; Srivastava, R. Ionogels for flexible conductive substrates and their application in biosensing. Int. J. Biol. Macromol. 2024, 254, 127736. [Google Scholar] [CrossRef]
- Gao, J.; Zeb, A.; Li, H.; Xie, Y.; Li, Z.; Zhang, J.; Zhang, Y.; Zhang, S. Poly(ionic liquid)s-Based Ionogels for Sensor Applications. ACS Appl. Polym. Mater. 2024, 6, 14260–14272. [Google Scholar] [CrossRef]
- Cui, X.; Xi, Y.; Tu, S.; Zhu, Y. An overview of flexible sensors from ionic liquid-based gels. TrAC Trends Anal. Chem. 2024, 174, 117662. [Google Scholar] [CrossRef]
- Ma, C.; Wei, J.; Zhang, Y.; Chen, X.; Liu, C.; Diao, S.; Gao, Y.; Matyjaszewski, K.; Liu, H. Highly Processable Ionogels with Mechanical Robustness. Adv. Funct. Mater. 2023, 33, 2211771. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Luo, S.; Zhang, W.; Bae, C.-J.; Wei, Z.; Zhang, H. A gradient “Ceramic-in-Ionogel” electrolyte with tidal ion flow for ultra-stable lithium metal batteries. Nano Energy 2023, 113, 108571. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, R.; Tao, X.; He, Y.; Li, X.; Tian, F.; Chen, X.; Huang, W. Mechanically Robust and Highly Conductive Ionogels for Soft Ionotronics. Adv. Funct. Mater. 2023, 33, 2208083. [Google Scholar] [CrossRef]
- He, X.; Zhang, B.; Liu, Q.; Chen, H.; Cheng, J.; Jian, B.; Yin, H.; Li, H.; Duan, K.; Zhang, J.; et al. Highly conductive and stretchable nanostructured ionogels for 3D printing capacitive sensors with superior performance. Nat. Commun. 2024, 15, 6431. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Oguzlu, H.; Zhu, J.; Zhu, P.; Yang, P.; Zhu, Y.; Wan, Z.; Rojas, O.J.; Jiang, F. Ultrastretchable Ionogel with Extreme Environmental Resilience through Controlled Hydration Interactions. Adv. Funct. Mater. 2023, 33, 2209787. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Wan, X.; Xu, H.; Zhang, C.; Lu, X.; Jing, W.; Guo, C.; Wei, X. Microbubble-based fabrication of resilient porous ionogels for high-sensitivity pressure sensors. Microsyst. Nanoeng. 2024, 10, 177. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Z.; Deng, Z.; Du, Z.; Sun, T.L.; Guo, Z.-H.; Yue, K. A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv. Mater. 2021, 33, 2105306. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, H.; Jiang, L. Transparent, mechanically robust, and ultrastable ionogels enabled by hydrogen bonding between elastomers and ionic liquids. Mater. Horiz. 2020, 7, 912–918. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, J.; Long, F.; Qiu, W.; Meng, G.; Zeng, Z.; Huang, H.; Wang, H.; Lin, N.; Liu, X.-Y. Bioinspired Conductive Enhanced Polyurethane Ionic Skin as Reliable Multifunctional Sensors. Adv. Sci. 2023, 10, 2300857. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, J.; Liu, M.; Lu, J.; Zhang, Q.; Chen, F. Advanced Polymer Electrolyte with Enhanced Electrochemical Performance for Lithium-Ion Batteries: Effect of Nitrile-Functionalized Ionic Liquid. ACS Appl. Energy Mater. 2019, 2, 1685–1694. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Barrau, S.; Demont, P.; Peigney, A.; Laurent, C.; Lacabanne, C. DC and AC Conductivity of Carbon Nanotubes–Polyepoxy Composites. Macromolecules 2003, 36, 5187–5194. [Google Scholar] [CrossRef]
- Ok, J.G.; Lee, J.Y.; Baac, H.W.; Tawfick, S.H.; Guo, L.J.; Hart, A.J. Rapid Anisotropic Photoconductive Response of ZnO-Coated Aligned Carbon Nanotube Sheets. ACS Appl. Mater. Interfaces 2014, 6, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Aghdam, M.K.; Mahmoodi, M.J.; Ansari, R. Creep performance of CNT polymer nanocomposites—An emphasis on viscoelastic interphase and CNT agglomeration. Compos. Part B Eng. 2019, 168, 274–281. [Google Scholar] [CrossRef]
- Killi, K.; Madmon, Y.; Verker, R.; Yerushalmi, R. Combined Atomic and Molecular Layer Deposition Surface Modification of Carbon Nanotubes for CNT–Epoxy Polymer Composites. ACS Appl. Nano Mater. 2022, 5, 15429–15440. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Sladkevich, S.; Medvedev, A.G.; Prikhodchenko, P.V.; Gun, J.; Sakharov, K.A.; Xu, Z.J.; Kulish, V.; Nikolaev, V.A.; Lev, O. Enhanced Thermal Buffering of Phase Change Materials by the Intramicrocapsule Sub per Mille CNT Dopant. ACS Appl. Mater. Interfaces 2020, 12, 16227–16235. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Li, B.; Liu, J.; Shen, Y.; Zhang, M.; Ji, K.; Mao, X.; Sun, R.; Zhou, F. Advances in Carbon-Based Resistance Strain Sensors. ACS Appl. Electron. Mater. 2023, 5, 674–689. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes—A review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef]
- Jo, S.; Lee, K.; Jung, Y.; Woo, D.; Kim, T.; Kim, P.J. Direct-spun carbon nanotube sheet: A flexible, ultralight, stackable three-dimensional current collector for high-performance lithium-ion batteries. Carbon 2024, 219, 118786. [Google Scholar] [CrossRef]
- Liao, J.; Hu, Q.; Du, Y.; Li, J.; Duan, L.; Bao, J.; Zhou, X. Robust carbon nanotube-interwoven KFeSO4F microspheres as reliable potassium cathodes. Sci. Bull. 2022, 67, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.T.; Wu, P.Y. Spectral insights into microdynamics of thermoresponsive polymers from the perspective of two-dimensional correlation spectroscopy. Chin. J. Polym. Sci. 2017, 35, 700–712. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, B.; Sun, S.; Wu, P. Skin-like mechanoresponsive self-healing ionic elastomer from supramolecular zwitterionic network. Nat. Commun. 2021, 12, 4082. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and Near-Infrared Spectroscopic Evidence for the Coexistence of Various Hydrogen-Bond Forms in Poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Zhu, X.; Han, K.; Li, C.; Wang, J.; Yuan, J.; Pan, Z.; Pan, M. Tough, Photoluminescent, Self-Healing Waterborne Polyurethane Elastomers Resulting from Synergistic Action of Multiple Dynamic Bonds. ACS Appl. Mater. Interfaces 2023, 15, 19414–19426. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, G.; Chen, J.; Chang, X.; Huang, J.; Zhu, Y. Lightweight and highly compressible/stretchable ionogel foams for designing pressure and strain sensors. Polymer 2024, 293, 126616. [Google Scholar] [CrossRef]
- Wei, C.; Bai, G.; Yu, S.; Ding, X.; Yang, W.; Zhu, S.; Wang, S.; Lu, H. Ultra-stretchable, toughened composite ionogel via synergetic hydration and hydrogen bonding interaction. Compos. Commun. 2024, 48, 101900. [Google Scholar] [CrossRef]
- Peng, H.; Yang, F.; Tang, Y.; Wang, X.; Li, Y.; Xie, P.; Ma, G.; Lei, Z. Highly Stretchable, Transparent, Solvent-Resistant Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Wearable Strain Sensors and Barrier-Free Information Transfer. ACS Appl. Mater. Interfaces 2024, 16, 54673–54684. [Google Scholar] [CrossRef]
- Qu, M.; Zhu, M.; Lv, Y.; Liu, Q.; Li, J.; Gao, Y.; Sun, C.-L.; He, J. Hydrophobic TPU/CNTs-ILs Ionogel as a Reliable Multimode and Flexible Wearable Sensor for Motion Monitoring, Information Transfer, and Underwater Sensing. ACS Appl. Mater. Interfaces 2024, 16, 35626–35638. [Google Scholar] [CrossRef]
- Peng, H.; Yang, F.; Wang, X.; Feng, E.; Sun, K.; Hao, L.; Zhang, X.; Ma, G. Rapid Radiation Synthesis of a Flexible, Self-Healing, and Adhesive Ionogel with Environmental Tolerance for Multifunctional Strain Sensors. ACS Appl. Mater. Interfaces 2023, 15, 51763–51773. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Zhu, T.; Lian, M.; Nguyen, D.H.; Zhang, C. Highly stretchable, self-healable and wide temperature-tolerant deep eutectic solvent-based composite ionogels for skin-inspired strain sensors. Compos. Commun. 2023, 41, 101658. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tian, M.; Wan, J.; Mei, S.; Pan, M.; Pan, Z. Enhanced Carbon Nanotube Ionogels for High-Performance Wireless Strain Sensing. Polymers 2025, 17, 817. https://doi.org/10.3390/polym17060817
Wang X, Tian M, Wan J, Mei S, Pan M, Pan Z. Enhanced Carbon Nanotube Ionogels for High-Performance Wireless Strain Sensing. Polymers. 2025; 17(6):817. https://doi.org/10.3390/polym17060817
Chicago/Turabian StyleWang, Xiao, Menglin Tian, Jiajia Wan, Shuxing Mei, Mingwang Pan, and Zhicheng Pan. 2025. "Enhanced Carbon Nanotube Ionogels for High-Performance Wireless Strain Sensing" Polymers 17, no. 6: 817. https://doi.org/10.3390/polym17060817
APA StyleWang, X., Tian, M., Wan, J., Mei, S., Pan, M., & Pan, Z. (2025). Enhanced Carbon Nanotube Ionogels for High-Performance Wireless Strain Sensing. Polymers, 17(6), 817. https://doi.org/10.3390/polym17060817






