Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Setup
2.3. Preparation of Electrospun Films
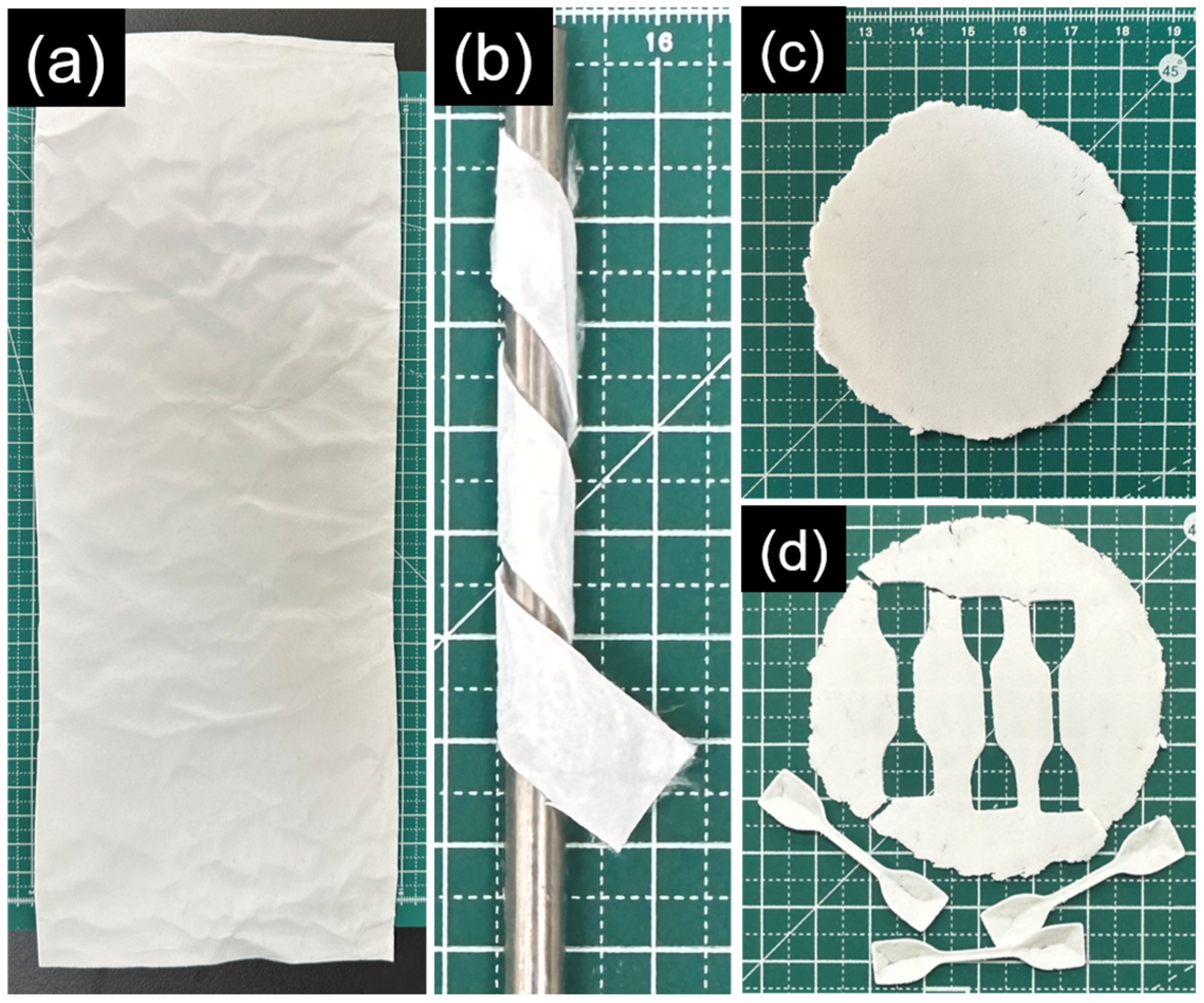
2.4. Preparation of Solvent-Cast Films
2.5. Characterization of the Films
2.5.1. Scanning Electron Microscopy
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. X-Ray Diffraction (XRD) Analysis
2.5.4. Differential Scanning Calorimetry (DSC)
2.5.5. Atomic Force Microscopy (AFM)
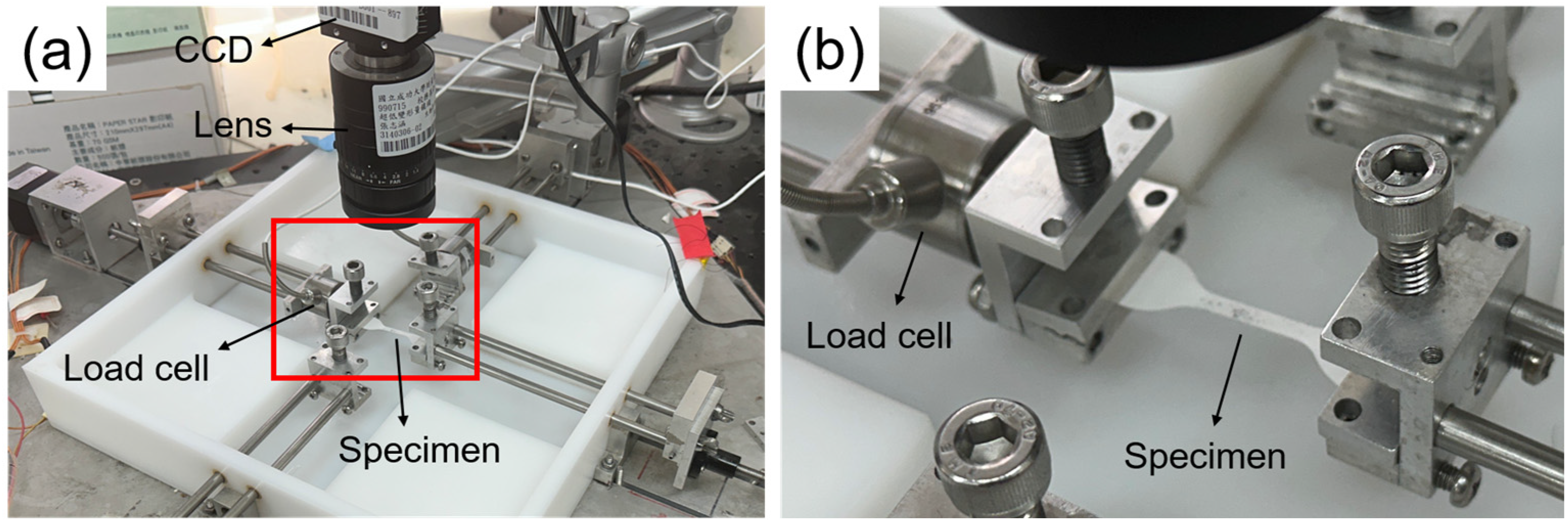
2.5.6. Uniaxial Tensile Testing
2.5.7. Water Contact Angle Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology of the Electrospun Films
3.2. Surface Morphology of the Solvent-Cast Films
3.3. FTIR Analysis of the Electrospun and Solvent-Cast Films
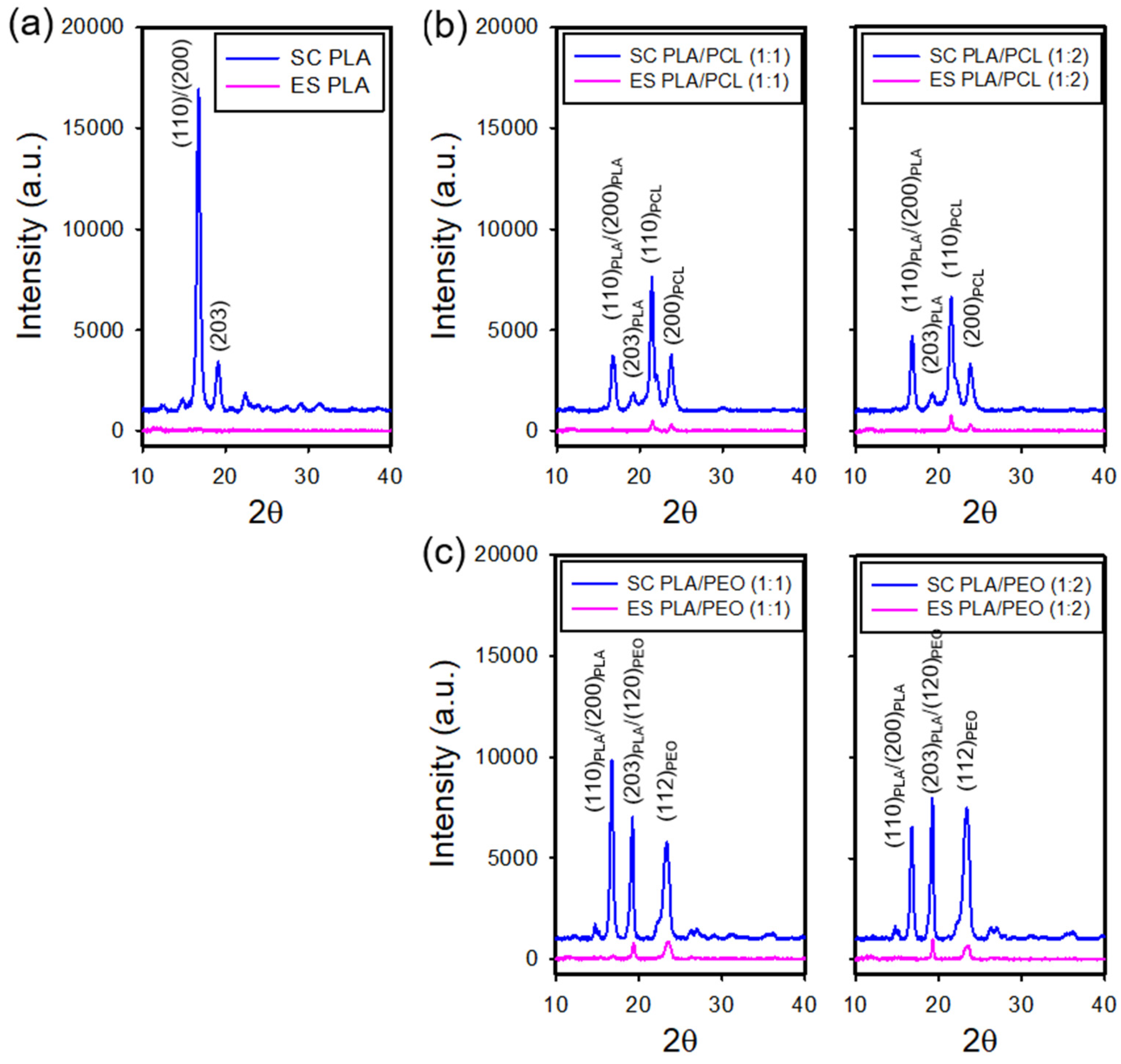
3.4. XRD Analysis of the Electrospun and Solvent-Cast Films
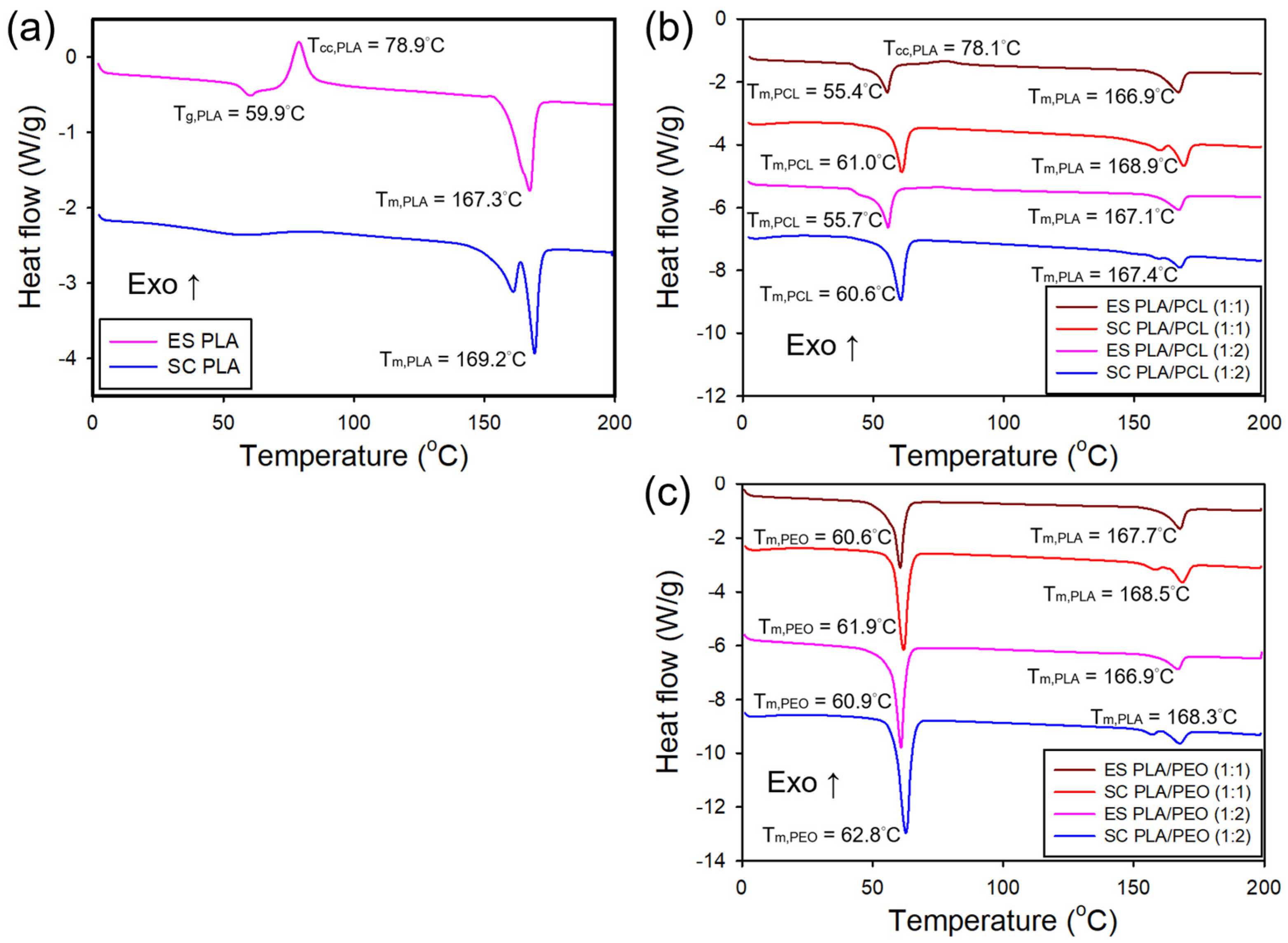
3.5. Thermal Properties of the Electrospun and Solvent-Cast Films
3.6. Mechanical Properties of the Electrospun and Solvent-Cast Films
3.7. SEM Images of the Fracture Surface
3.8. Selective Removal of PEO in PLA/PEO Blends
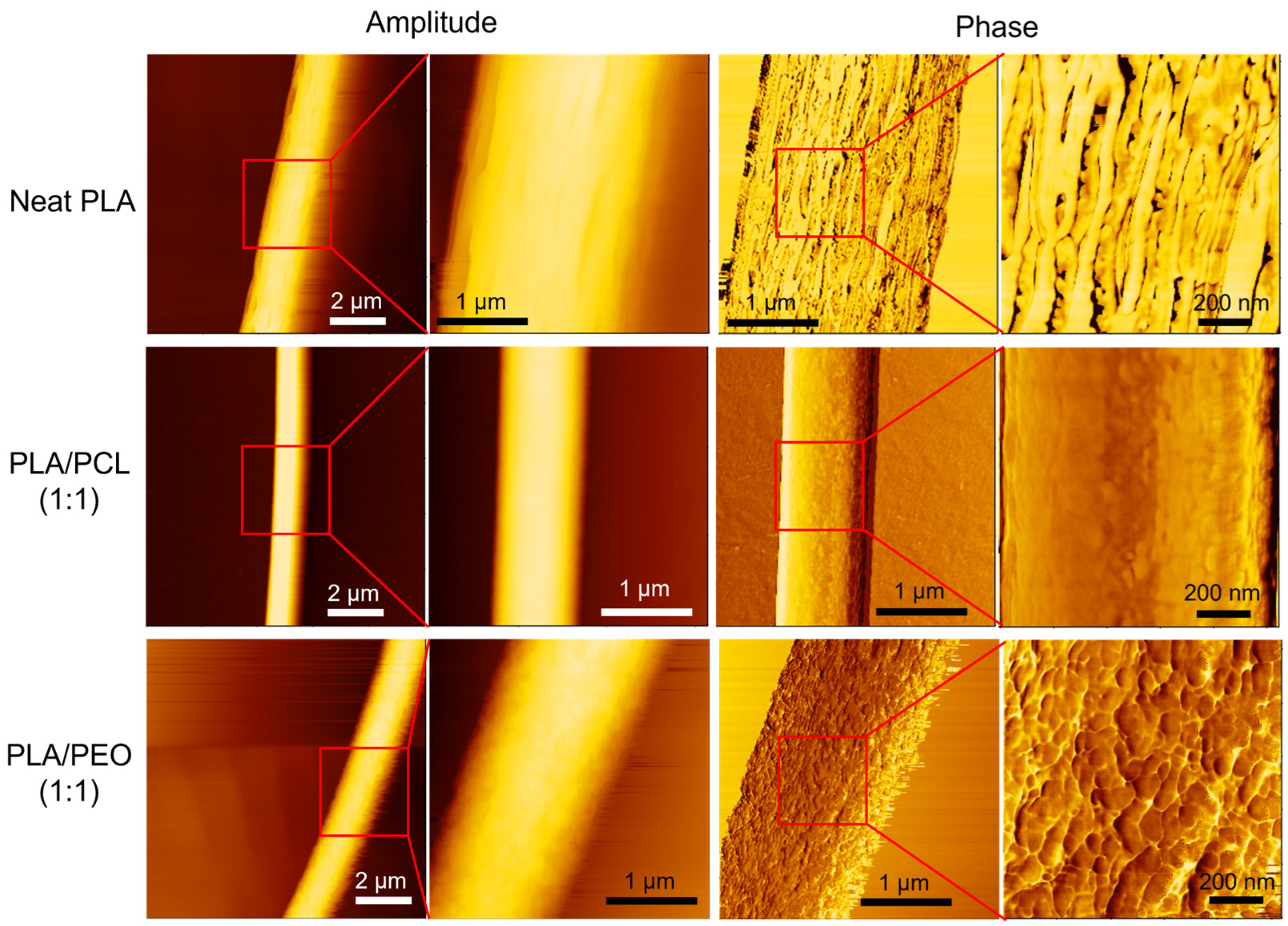
3.9. AFM Characterization of Single Fiber Morphology
3.10. Water Contact Angle Measurements of the PLA Films
3.11. Limitations and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solarski, S.; Mahjoubi, F.; Ferreira, M.; Devaux, E.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Ferreira, A.D.; et al. (Plasticized) polylactide/clay nanocomposite textile: Thermal, mechanical, shrinkage and fire properties. J. Mater. Sci. 2007, 42, 5105–5117. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Hernandez, A.; Rayon, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Hu, J.-J.; Chao, W.-C.; Lee, P.-Y.; Huang, C.-H. Construction and characterization of an electrospun tubular scaffold for small-diameter tissue-engineered vascular grafts: A scaffold membrane approach. J. Mech. Behav. Biomed. Mater. 2012, 13, 140–155. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Dawin, T.P.; Ahmadi, Z.; Taromi, F.A. Bio-based solution-cast blend films based on polylactic acid and polyhydroxybutyrate: Influence of pyromellitic dianhydride as chain extender on the morphology, dispersibility, and crystallinity. Prog. Org. Coat. 2018, 119, 23–30. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent Approaches to the Plasticization of Poly(lactic Acid) (PLA) (A Review). Polymers 2024, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, J.; Das, S.; Lipik, V.; Mohanty, A.K.; Misra, M.; You, X.Y.; Tan, L.P.; Chang, B.P. The Development of Poly(lactic acid) (PLA)-Based Blends and Modification Strategies: Methods of Improving Key Properties towards Technical Applications-Review. Materials 2024, 17, 4556. [Google Scholar] [CrossRef]
- Wake, M.C.; Gupta, P.K.; Mikos, A.G. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996, 5, 465–473. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, D.; Yang, R.; Wang, Y.; Zhang, J.; Li, X.; Wang, Y. Influence of Fused Deposition Molding Printing Process on the Toughness and Miscibility of Polylactic Acid/Polycaprolactone Blends. J. Mater. Eng. Perform. 2022, 31, 1338–1345. [Google Scholar] [CrossRef]
- Saha, D.; Samal, S.K.; Biswal, M.; Mohanty, S.; Nayak, S.K. Preparation and characterization of poly(lactic acid)/poly(ethylene oxide) blend film: Effects of poly(ethylene oxide) and poly(ethylene glycol) on the properties. Polym. Int. 2019, 68, 164–172. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Ercolani, E.; Nanni, F.; Bianco, A. Assessment of poly(ɛ-caprolactone)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends processed by solvent casting and electrospinning. Mater. Sci. Eng. A 2011, 528, 1764–1772. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef]
- Koide, Y.; Ikake, H.; Muroga, Y.; Shimizu, S. Effect of the cast-solvent on the morphology of cast films formed with a mixture of stereoisomeric poly(lactic acids). Polym. J. 2012, 45, 645–650. [Google Scholar] [CrossRef]
- Byun, Y.; Whiteside, S.; Thomas, R.; Dharman, M.; Hughes, J.; Kim, Y.T. The effect of solvent mixture on the properties of solvent cast polylactic acid (PLA) film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Khil, M.S.; Ra, Y.M.; Lee, D.R. Characterization of nano-structured poly(ε-caprolactone) nonwoven mats via electrospinning. Polymer 2003, 44, 1287–1294. [Google Scholar] [CrossRef]
- You, Z.-R.; Hu, M.-H.; Tuan-Mu, H.-Y.; Hu, J.-J. Fabrication of poly(glycerol sebacate) fibrous membranes by coaxial electrospinning: Influence of shell and core solutions. J. Mech. Behav. Biomed. Mater. 2016, 63, 220–231. [Google Scholar] [CrossRef]
- Shibata, M.; Inoue, Y.; Miyoshi, M. Mechanical properties, morphology, and crystallization behavior of blends of poly(L-lactide) with poly(butylene succinate-co-L-lactate) and poly(butylene succinate). Polymer 2006, 47, 3557–3564. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Parsons, A.J.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Mechanical, crystallisation and moisture absorption properties of melt drawn polylactic acid fibres. Eur. Polym. J. 2014, 53, 270–281. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Jiménez-Pérez, J.L.; Sabino, M.A.; Cruz-Orea, A.; Loaiza, M. Photothermal and morphological characterization of PLA/PCL polymer blends. Appl. Phys. A-Mater. Sci. Process. 2015, 120, 1323–1329. [Google Scholar] [CrossRef]
- Balsamo, V.; Calzadilla, N.; Mora, G.; Müller, A.J. Thermal characterization of polycarbonate/polycaprolactone blends. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 771–785. [Google Scholar] [CrossRef]
- Li, X.; Hsu, S.L. An Analysis of the Crystallization Behavior of Poly(Ethylene Oxide) Poly(Methyl Methacrylate) Blends by Spectroscopic and Calorimetric Techniques. J. Polym. Sci. Part B Polym. Phys. 1984, 22, 1331–1342. [Google Scholar] [CrossRef]
- Hu, J.J.; Chen, G.W.; Liu, Y.C.; Hsu, S.S. Influence of Specimen Geometry on the Estimation of the Planar Biaxial Mechanical Properties of Cruciform Specimens. Exp. Mech. 2014, 54, 615–631. [Google Scholar] [CrossRef]
- Liu, L.J.; Li, S.M.; Garreau, H.; Vert, M. Selective enzymatic degradations of poly(L-lactide) and poly(ε-caprolactone) blend films. Biomacromolecules 2000, 1, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Térol, A. Vibrational analysis of poly(L-lactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Aou, K.; Hsu, S.L. Trichroic Vibrational Analysis on the α-Form of Poly(lactic acid) Crystals Using Highly Oriented Fibers and Spherulites. Macromolecules 2006, 39, 3337–3344. [Google Scholar] [CrossRef]
- Coleman, M.M.; Zarian, J. Fourier-transform infrared studies of polymer blends. II. Poly(ɛ-caprolactone)–poly(vinyl chloride) system. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 837–850. [Google Scholar] [CrossRef]
- Rocco, A.M.; Pereira, R.P.; Felisberti, M.I. Miscibility, crystallinity and morphological behavior of binary blends of poly(ethylene oxide) and poly(methyl vinyl ether-maleic acid). Polymer 2001, 42, 5199–5205. [Google Scholar] [CrossRef]
- Vadas, D.; Nagy, Z.K.; Csontos, I.; Marosi, G.; Bocz, K. Effects of thermal annealing and solvent-induced crystallization on the structure and properties of poly(lactic acid) microfibres produced by high-speed electrospinning. J. Therm. Anal. Calorim. 2020, 142, 581–594. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of Poly(L-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Gorodzha, S.N.; Muslimov, A.R.; Syromotina, D.S.; Timin, A.S.; Tcvetkov, N.Y.; Lepik, K.V.; Petrova, A.V.; Surmeneva, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. A comparison study between electrospun polycaprolactone and piezoelectric poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloid Surf. B 2017, 160, 48–59. [Google Scholar] [CrossRef]
- Yan, H.; Wang, T.; Liu, L.J.; Song, T.L.; Li, C.L.; Wu, L.J.; Zheng, J.C.; Dai, Y. High voltage stable cycling of all-solid-state lithium metal batteries enabled by top-down direct fluorinated poly (ethylene oxide)-based electrolytes. J. Power Sources 2023, 557, 232559. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.W. The Effects of LiTDI Salt and POSS-PEG (=4) Hybrid Nanoparticles on Crystallinity and Ionic Conductivity of PEO Based Solid Polymer Electrolytes. Sci. Adv. Mater. 2016, 8, 931–940. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PTA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of Conformationally Disordered Crystals (α′-Phase) of Poly(L-lactic acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schick, C. Solid-state reorganization, melting and melt-recrystallization of conformationally disordered crystals (α′-phase) of poly (L-lactic acid). Polymer 2014, 55, 4932–4941. [Google Scholar] [CrossRef]
- Simões, C.L.; Viana, J.C.; Cunha, A.M. Mechanical properties of poly(ε-caprolactone) and poly(lactic acid) blends. J. Appl. Polym. Sci. 2009, 112, 345–352. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(D,L-lactic acid)/poly(epsilon-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Yeh, J.-T.; Wu, C.-J.; Tsou, C.-H.; Chai, W.-L.; Chow, J.-D.; Huang, C.-Y.; Chen, K.-N.; Wu, C.-S. Study on the Crystallization, Miscibility, Morphology, Properties of Poly(lactic acid)/Poly(ε-caprolactone) Blends. Polym.-Plast. Technol. Eng. 2009, 48, 571–578. [Google Scholar] [CrossRef]
- Murphy, S.H.; Marsh, J.J.; Kelly, C.A.; Leeke, G.A.; Jenkins, M.J. CO2 assisted blending of poly(lactic acid) and poly(epsilon-caprolactone). Eur. Polym. J. 2017, 88, 34–43. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, D.; Zhang, K.; Wang, Y.; Guo, Y.; Wang, Y. Research on the miscibility, mechanical properties and printability of polylactic acid/poly (ε-caprolactone) blends: Insights from molecular dynamics simulation and experiments. J. Mater. Sci. 2021, 56, 9754–9768. [Google Scholar] [CrossRef]
- Tipduangta, P.; Belton, P.; Fábián, L.; Wang, L.Y.; Tang, H.R.; Eddleston, M.; Qi, S. Electrospun Polymer Blend Nanofibers for Tunable Drug Delivery: The Role of Transformative Phase Separation on Controlling the Release Rate. Mol. Pharm. 2016, 13, 25–39. [Google Scholar] [CrossRef]
- Maccaferri, E.; Mazzocchetti, L.; Benelli, T.; Brugo, T.M.; Zucchelli, A.; Giorgini, L. Rubbery nanofibers by co-electrospinning of almost immiscible NBR and PCL blends. Mater. Des. 2020, 186, 108210. [Google Scholar] [CrossRef]
- Wang, B.; Yao, J.Y.; Wang, H.Y.; Wang, M.Q. Construction of a ternary system: A strategy for the rapid formation of porous poly(lactic acid) fibers. RSC Adv. 2022, 12, 6476–6483. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.Y.; Liu, Y.A.; Yu, D.G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Bianco, A.; Calderone, M.; Cacciotti, I. Electrospun PHBV/PEO co-solution blends: Microstructure, thermal and mechanical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1067–1077. [Google Scholar] [CrossRef]
- Lim, C.T.; Tan, E.P.S.; Ng, S.Y. Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl. Phys. Lett. 2008, 92, 141908. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Rodriguez, I.A.; Wesner, D.; Schonherr, H.; Bowlin, G.L.; Jhurry, D. Poly(ester-ether)s: III. assessment of cell behaviour on nanofibrous scaffolds of PCL, PLLA and PDX blended with amorphous PMeDX. J. Mater. Chem. B 2015, 3, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Liu, Y.; Deng, L.; Zhang, C.; Chen, K.; Feng, F.; Zhang, H. Comparison of ethyl cellulose-gelatin composite films fabricated by electrospinning versus solvent casting. J. Appl. Polym. Sci. 2018, 135, 46824. [Google Scholar] [CrossRef]
- Dong, F.Y.; Zhang, M.; Tang, W.W.; Wang, Y. Formation and Mechanism of Superhydrophobic/Hydrophobic Surfaces Made from Amphiphiles through Droplet-Mediated Evaporation-Induced Self-Assembly. J. Phys. Chem. B 2015, 119, 5321–5327. [Google Scholar] [CrossRef]
- Farrugia, C.A.; Groves, M.J. Gelatin behaviour in dilute aqueous solution: Designing a nanoparticulate formulation. J. Pharm. Pharmacol. 1999, 51, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Natu, M.V.; de Sousa, H.C.; Gil, M.H. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int. J. Pharm. 2010, 397, 50–58. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
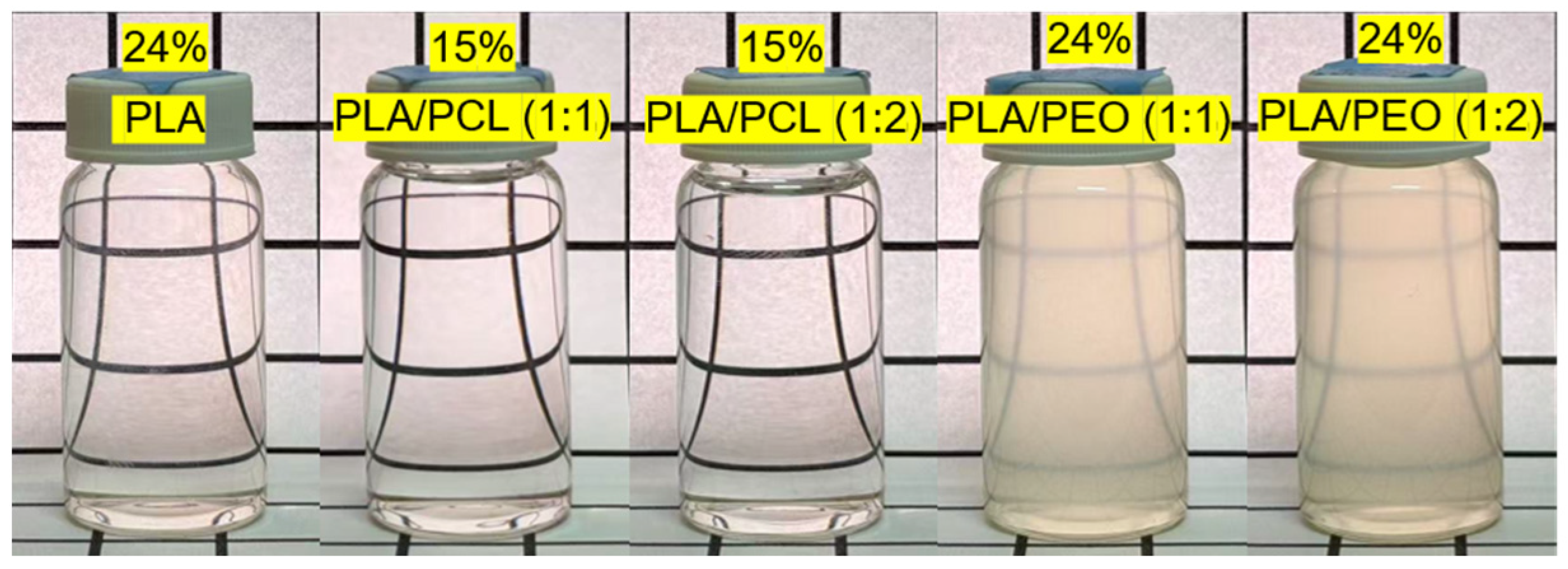







| DCM | DMF | DCM/DMF (7:3 v/v) | ||||
|---|---|---|---|---|---|---|
| g solute/100 g solvent | w/v % | g solute/100 g solvent | w/v % | g solute/100 g solvent | w/v % | |
| PLA | 55.10 | 73.28 | 3.01 | 2.84 | 27.74 | 33.68 |
| PCL | 40.10 | 53.33 | 24.83 | 23.44 | 35.52 | 43.09 |
| PEO | negligible | negligible | negligible | |||
| (°C) | (J/g) | (J/g) | (%) | (°C) | (J/g) | (%) | (°C) | (J/g) | (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | ES | 167.3 | 24.93 | 52.84 | 30.0 | - | - | - | - | - | |
| SC | 169.2 | 0 | 67.33 | 72.4 | - | - | - | - | - | - | |
| PLA/PCL (1:1) | ES | 166.9 | 17.16 | 54.64 | 40.3 | 55.4 | 60.91 | 43.7 | - | - | - |
| SC | 168.9 | 0 | 64.38 | 69.2 | 61.0 | 94.96 | 68.1 | - | - | - | |
| PLA/PCL (1:2) | ES | 167.1 | 17.97 | 59.64 | 44.8 | 55.7 | 63.93 | 45.8 | - | - | - |
| SC | 167.4 | 0 | 61.92 | 66.6 | 60.6 | 94.65 | 67.9 | - | - | - | |
| PCL | ES | - | - | - | - | 55.9 | 71.49 | 51.3 | - | - | - |
| SC | - | - | - | - | 59.8 | 98.71 | 70.8 | - | - | - | |
| PLA/PEO (1:1) | ES | 167.7 | 4.50 | 57.26 | 56.7 | - | - | 60.6 | 156.44 | 73.3 | |
| SC | 168.5 | 0 | 64.68 | 69.6 | - | - | 61.9 | 200.6 | 94.0 | ||
| PLA/PEO (1:2) | ES | 166.9 | 4.89 | 57.75 | 56.8 | - | - | 60.9 | 170.55 | 79.9 | |
| SC | 168.3 | 0 | 64.17 | 69.0 | - | - | 62.8 | 201.4 | 94.4 | ||
| PEO | pristine | - | - | - | - | - | - | - | 62.2 | 205.9 | 96.5 |
| Young’s Modulus | Ultimate Strength | Elongation at Break | Key Observations | ||
|---|---|---|---|---|---|
| PLA | ES | Lower | Higher | Greater | Flexible, lower crystallinity; aligned nanostructure enhances elongation. |
| SC | Higher | Lower | Brittle | High crystallinity leads to stiffness but causes brittleness. | |
| PLA/PCL (1:1) | ES | Comparable to PLA | Could not be determined | >80% (estimated) | PCL increases flexibility; phase separation is suppressed, maintaining ductility. |
| SC | Lower than PLA | Reduced | Moderate | Large phase separation leads to weak interfacial adhesion, reducing strength. | |
| PLA/PCL (1:2) | ES | Comparable to PLA | Could not be determined | >80% (estimated) | Higher PCL content further enhances flexibility and fiber uniformity. |
| SC | Further reduced | Moderate | Increased | Heterogeneous phase morphology lowers stiffness but improves elongation. | |
| PLA/PEO (1:1) | ES | Lower than PLA | Reduced | Lower | PEO increases hydrophilicity but weakens mechanical strength. |
| SC | Fragile | Not measurable | Not measurable | Large-scale phase separation. | |
| PLA/PEO (1:2) | ES | Lower than PLA | Higher than 1:1 | Lower | Higher PEO content further reduces mechanical integrity. |
| SC | Fragile | Not measurable | Not measurable | Excessive phase separation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Q.-H.; Hu, M.-H.; Wang, J.-F.; Hu, J.-J. Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers 2025, 17, 800. https://doi.org/10.3390/polym17060800
Weng Q-H, Hu M-H, Wang J-F, Hu J-J. Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers. 2025; 17(6):800. https://doi.org/10.3390/polym17060800
Chicago/Turabian StyleWeng, Qi-Hong, Ming-Hsien Hu, Ji-Feng Wang, and Jin-Jia Hu. 2025. "Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting" Polymers 17, no. 6: 800. https://doi.org/10.3390/polym17060800
APA StyleWeng, Q.-H., Hu, M.-H., Wang, J.-F., & Hu, J.-J. (2025). Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers, 17(6), 800. https://doi.org/10.3390/polym17060800







