Cl−, Na+ and Mg2+ Adsorption and Electronic Properties on 2-Octyl Acrylate and Isobornyl Acrylate Monomers: A Comprehensive DFT Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussions
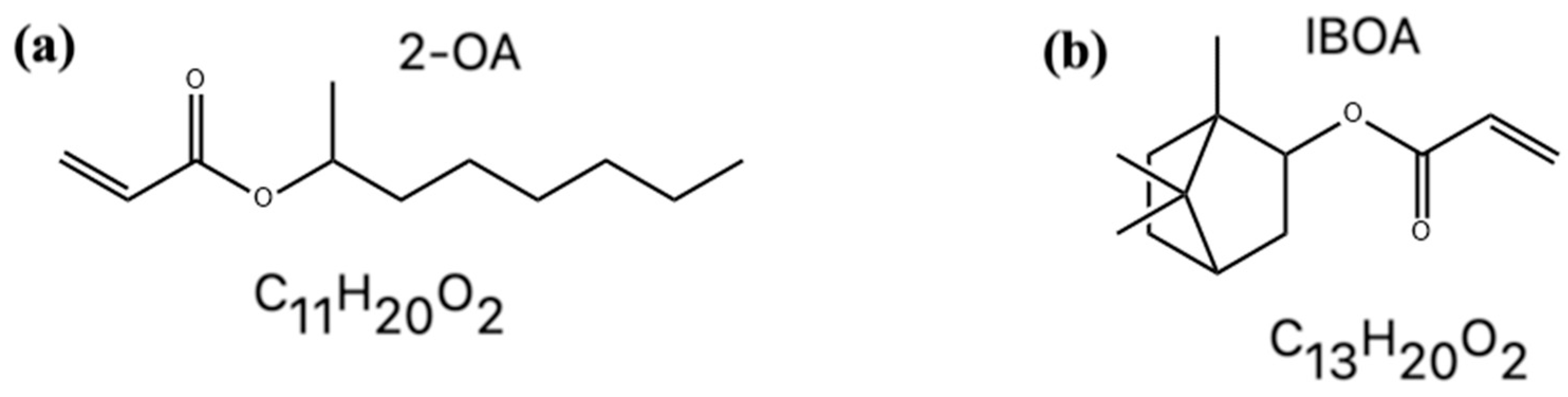
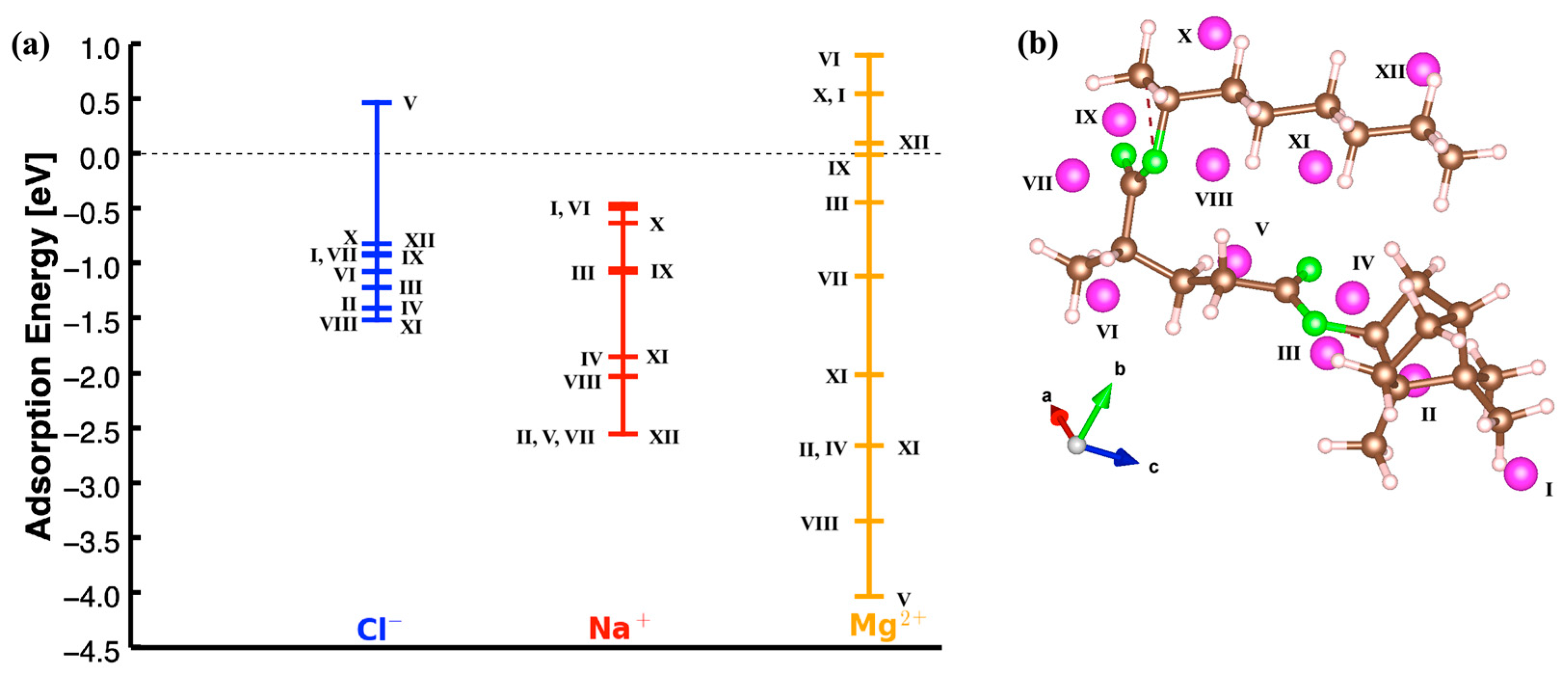
3.1. Adsorption Energy and Quantitative Analysis
3.1.1. Adsorption Energy Trends
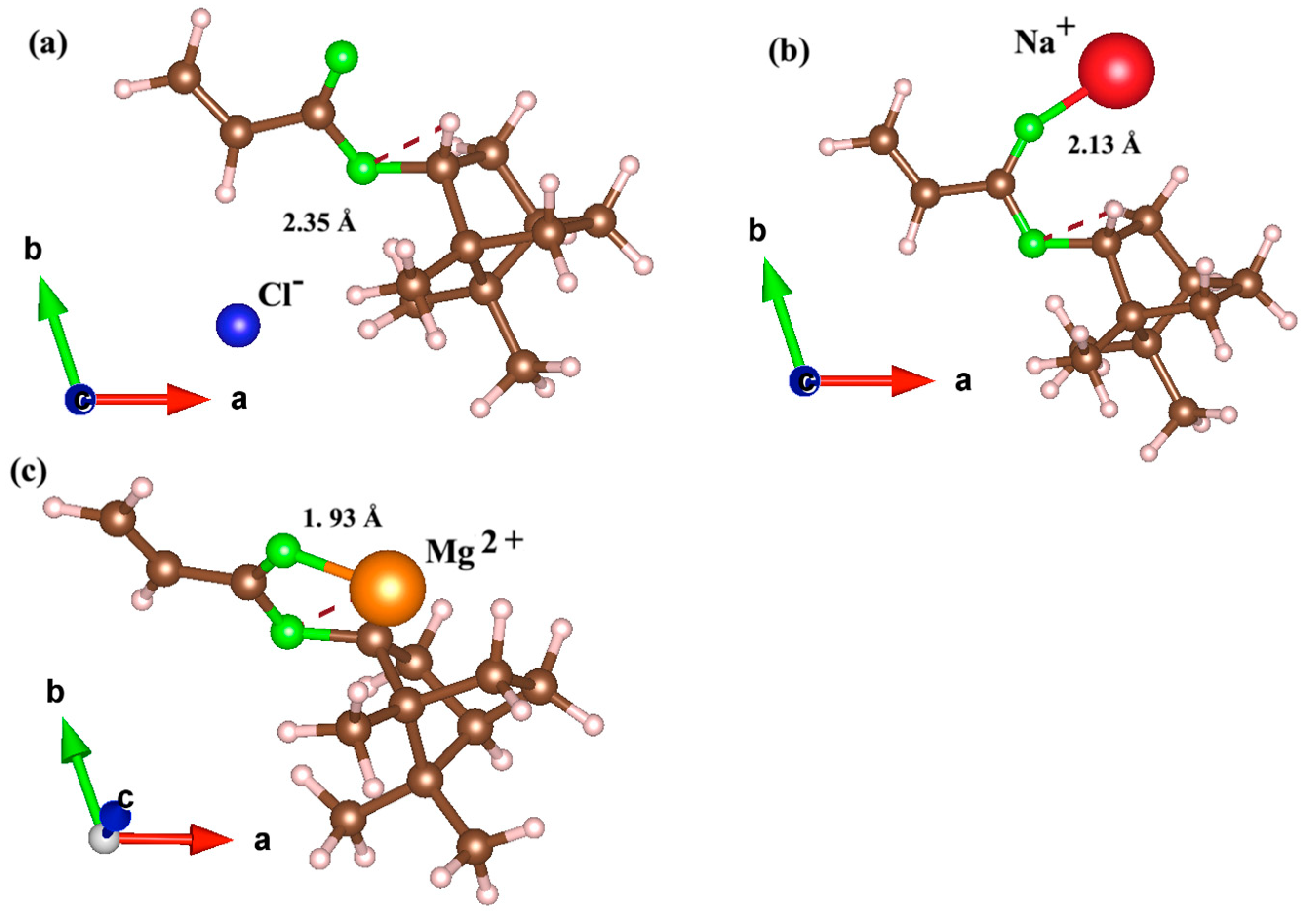
3.1.2. Structural Insights from Adsorption
3.1.3. Quantum Descriptors
3.2. Combining 2-OA and IBOA in Dimers
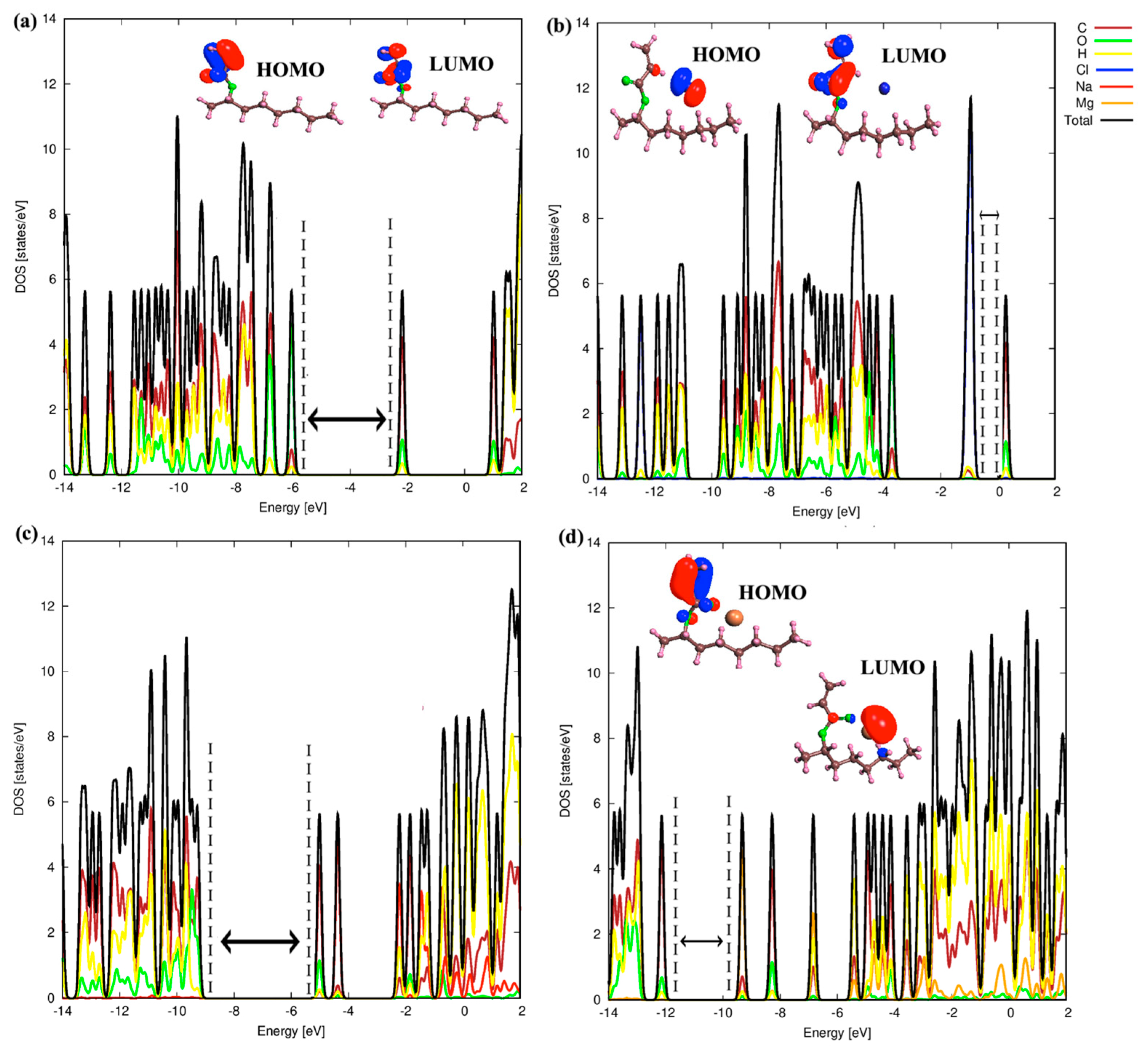
3.3. Electronic Properties
3.4. Electrostatic Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Y.; Sunnerscales, J.; Graham-Jones, J.; Meng, M.; Pemberton, R. Monomer selection for ın situ polymerization ınfusion manufacture of natural-fiber reinforced thermoplastic-matrix marine composites. Polymers 2020, 12, 2928. [Google Scholar] [CrossRef] [PubMed]
- Mutkumar, T.; Aravinthan, A.; Lakshmi, K.; Venkatesan, R. Fouling and stability of polymers and composites in marine enviroment. Int. Biodeterior. Biodegrad. 2011, 65, 276–284. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cumnis, E. Ranking of potential hazards from microplastics polymers in the marine enviroments. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Ni, C.; Yu, L.; Yuan, Z.; Li, C.; Yan, X. Research on the Marine Antifouling and Mechanism of Acrylate Copolymers and Marine Coatings Based on a Synergetic Effect. J. Ocean Univ. China 2023, 22, 717–727. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Tang, L.; Yang, H.; Lu, Z.; Wang, J.; Liu, L.; Takahashi, K. Synthesis of zinc-based acrylate copolymers and their marine antifoulding application. RSC. Adv. 2017, 7, 40020. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, M.; Luo, C.; Chen, X.; Kong, J.; Zhou, T. A novel composite paint (TiO2/fluorinated acrylic composite) for antifouling application in marine enviroments. J. Environ. Chem. Eng. 2016, 4, 2545–2555. [Google Scholar] [CrossRef]
- Ajekwene, K.K. Properties and applications of acrylates. In Acylate Polymers for Advanced Applications; Serrano-Aroca, A., Deb, S., Eds.; IntechOpen: London, UK, 2020; Chapter 3. [Google Scholar]
- Chalifoux, P.R. Acrylic and other resins: Provisional restorations. In Esthetic Dentistry; Aschheim, K.W., Ed.; Mosby: St. Louis, MO, USA, 2015; Volume 10, pp. 197–230. [Google Scholar]
- Riondel, A.; Graire, C.; Esch, M.; Linemann, R. Industrial Process for Manufacture of 2-Octyl Acrylate by Transesterification with High Purity and Output. WO 2013110876, 1 August 2013. p. A1. [Google Scholar]
- Aguirre, M.; Ballard, N.; Gonzalez, E.; Hamzehlou, S.; Sardon, H.; Calderon, M.; Paulis, M.; Tomovska, R.; Dupin, D.; Bean, R.H.; et al. Polymer Coolloids: Current Challenges, Emerging Applications; and New Developments. Macromolecules 2023, 56, 2579–2607. [Google Scholar] [CrossRef]
- Xu, X.; Chen, I.; Guo, J.; Cao, X.; Wang, S. Synthesis and characteristics of tung oil-based acrylated-alkyd resin modified by isorborn acyrlated. Polym. Sci. C 2015, 57, 120–127. [Google Scholar]
- Dong, F.; Qian, Y.; Xu, X.; Sihaghaleh, H.; Guo, L.; Liu, H. Preparation and characterization of UV-curable waterborne polyurethane using isobornyl acrylate modified via copolymerization. Polym. Degrad. Stab. 2021, 184, 109474. [Google Scholar] [CrossRef]
- Peng, C.W.; Chang, K.C.; Weng, C.J.; Lai, M.C.; Hsu, C.H.; Hsu, S.C.; Li, S.-Y.; Wei, Y.; Yeh, J.-M. UV-curable nanocasting technique to prepare bio-mimetic super-hydrophobic non-fluorinated polymeric surfaces for advanced anticorrosive coatings. Polym. Chem. 2013, 4, 926–932. [Google Scholar] [CrossRef]
- Lo, C.W.; Zhu, D.; Jiang, H. An infrared-light responsive graphene-oxide incorporated poly(N-isopropylacrylamide) hydrogel nanocomposite. Soft Matter 2011, 7, 5604–5609. [Google Scholar] [CrossRef]
- Shin, M.; Lee, Y.; Rahman, M.; Kim, H. Synthesis and properties of waterborne fluorinated polyurethane-acrylate using a solvent-/emulsifier-free method. Polymer 2013, 54, 4873–4882. [Google Scholar] [CrossRef]
- Khandelwal, D.; Hooda, S.; Brar, A.S.; Shankar, R. 1D and 2D NMR studies of isobornyl acrylate—Methyl methacrylate copolymers. J. Mol. Struct. 2011, 1004, 121–130. [Google Scholar] [CrossRef]
- Kamann, S.; Oppel, E. Hydrocollod blister plaster decreases allergic contact dermatititis caused by Freestyle Libre and isorbornyl acrylate. Contact Dermat. 2019, 81, 380–381. [Google Scholar] [CrossRef]
- Ng, K.L.; Nixon, R.L.; Grillis, C.; Tam, M.M. Solution using stomahasive wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Aust. J. Dermatol. 2022, 63, 56–59. [Google Scholar] [CrossRef]
- Brogden, E.M.; Bon, S.A.F. Water-based polymer colloids with a branched chain architcture as low-gel pressure-sensitive adhesives. Polym. Chem. 2024, 15, 2489. [Google Scholar] [CrossRef]
- Stouten, J.; Cao, H.; Pich, A.; Bernaerts, K.V. Renewable and functional latexes synthesized by polymerization-induced self-assmeblyy for UV-curable films. ACS Appl. Mater. Interfaces 2023, 15, 52939–52952. [Google Scholar]
- Badia, A.; Agirre, A.; Barandiaran, M.J.; Leiza, J.R. Easy removable and UV tunable biobased waterborne pressure sensitive adhesives. Int. J. Adhes. Adhes. 2021, 108, 102860. [Google Scholar] [CrossRef]
- Montemore, M.M.; Medlin, J.W. A Unified Picture of Adsorption on Transition Metals Through Diffrent Atoms. J. Am. Chem. Soc. 2014, 136, 9272–9275. [Google Scholar] [CrossRef]
- Yaminsky, V.V.; Ninham, B.W.; Christenson, H.K.; Pashlev, R.M. Adsorption Forces between Hydrophobic Monolayers. Langmuir 1996, 12, 1936–1943. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Ren, B.; Min, F.; Liu, L.; Chen, J.; Liu, C.; Lv, K. Adsorption of different PAM structural units on kaolinite (0 0 1) surface: Density functional theory study. Appl. Surf. Sci. 2020, 504, 144324. [Google Scholar] [CrossRef]
- Lai, W.; Zhang, K.; Shao, P.; Yang, L.; Ding, L.; Pavlostathis, S.G.; Shi, H.; Zou, L.; Liang, D.; Luo, X. Optimization of adsorption configuration by DFT calculation for design of adsorbent: A case study of palladium ion-imprinted polymers. J. Hazard. Mater. 2019, 379, 120791. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Yang, Z.; Zhuo, Q.; Zhang, H.; Geng, P. DFT study of the adsorption of 2,3-epoxypropyltrimethylammonium chloride on montmorillonite surfaces. J. Mol. Liq. 2021, 334, 116145. [Google Scholar] [CrossRef]
- Zhang, L.; Min, F.; Chen, J.; Liu, C.; Wang, T. New insights into the interaction between monomers from acrylamide-based polymeric flocculants and montmorillonite: A DFT study. J. Mol. Liq. 2022, 365, 120171. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garcia, A.; Junquera, J.; Sanchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Condens. Matter Phys. 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Ordejon, P.; Artacho, E.; Soler, J.M. Self-consistent order-N density functional calculations for very large systems. Phys. Rev. B 1996, 53, 10441–10444. [Google Scholar] [CrossRef]
- Junquera, J.; Paz, O.; Sanchez-Portal, D.; Artacho, E. Numerical atomic orbitals for linear-scaling calculations. Phy. Rev. B 2001, 64, 235111. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1994, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Hestenes, M.R.; Stiefel, E. Methods of Conjugate Gradients for Solving Linear Systems. J. Res. Natl. Inst. Stand. Technol. 1952, 49, 2379. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Samadizadeh, M.; Rategar, S.F.; Petghan, A.A. F−, Cl− and Na+ adsorption on AIN nanotubes: A DFT study. Phys. E 2015, 69, 75–80. [Google Scholar] [CrossRef]
- Koopmans, T. Ordering of Wave Functions and Eigenenergies to the Individual Electrons of an Atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Philips, J.C. Generalized Koopmans’ Theorem. Phys. Rev. 1961, 123, 420–424. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Majidi, R.; Ghanbari, M. Density Functional Theory and Voltammetric Study of Niclosamide Adsorption Behaviour on Carbon Nnaotubes. J. Electron. Mater. 2021, 50, 1260–1266. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Majidi, R.; Sohouli, E. Experimental and theoretical evaluation of clonazepam adsorption onto carbon nantotubes. Chem. Phys. 2022, 558, 111505. [Google Scholar] [CrossRef]
- Available online: http://www.arguslab.com/arguslab.com/ArgusLab.html (accessed on 16 December 2024).
- Ridley, J.; Zerner, M. An Intermediate Neglect of Differential Overlap Technique for Spectroscopy: Pyrrole and the Azines. Theoret. Chim. Acta 1973, 32, 111–134. [Google Scholar] [CrossRef]
- Zerner, M.C.; Loew, G.H.; Kirschner, R.F.; Mueller-Westerhoff, U.T. An Intermediate Neglect of Differential Overlap Technique for Spectroscopy of Transition-Metal Complexes. Ferrocene. J. Am. Chem. Soc. 1980, 102, 589–599. [Google Scholar] [CrossRef]
- Zerner, M.C. Semiempirical Molecular Orbital Methods. In Reviews in Computational Chemistry II; Lipkowitz, K.B., Boyd, D.B., Eds.; VCH Publishers Inc.: Hoboken, NJ, USA, 1991; Chapter 8; pp. 313–366. [Google Scholar]
- Setianto, S.; Men, L.K.; Panatarani, C.; Joni, I.M. Visualization the electrostatic potential energy map of graphene quantum dots. AIP Conf. Proc. 2020, 2219, 060001. [Google Scholar]
- Bartyzel, A.; Kaczor, A.A.; Mahmoudi, G.; Masoudiasl, A.; Wrobel, T.M.; Pitucha, M.; Matosiuk, D. Experimental and Computational Structural Studies of 2,3,5-Trisubstitted and 1,2,3,5-Tetrasubstituted Indoles as Non-Competitive Antagonists of GluK1/GluK2 Receptors. Molecules 2022, 27, 2479. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, J.; Dhas, D.A.; Joe, I.H.; Balachandran, S. Spectroscopic (FT-IR, FT-Raman) investigation, topological (QTAIM, RDG, ELF) analysis, drug-likeness and anti-inflammatory activity study on 2-methylaminobenzoic acid alkaloid. J. Mol. Struct. 2023, 1273, 13461. [Google Scholar] [CrossRef]
- Baweja, S.; Lochab, A.; Baxi, S.; Saxena, R. Computational investigation of thallium interactions with functionalized multi-walled carbon nanotubes for electrochemical sensing applications. Pure Appl. Chem. 2024, 96, 421–428. [Google Scholar] [CrossRef]




| 2-OA | I | A | |||
|---|---|---|---|---|---|
| Cl− | 0.92 | −0.28 | −0.32 | 0.60 | 0.09 |
| Na+ | 7.26 | 6.13 | −6.70 | 0.57 | 39.40 |
| Mg2+ | 12.10 | 9.32 | −10.70 | 1.40 | 40.90 |
| IBOA | I | A | |||
|---|---|---|---|---|---|
| Cl− | 1.05 | −0.2 | −0.43 | 0.63 | 0.15 |
| Na+ | 8.65 | 4.85 | −6.75 | 1.9 | 12.00 |
| Mg2+ | 11.98 | 9.05 | −10.52 | 1.47 | 37.65 |
| Dimer | I | A | |||
|---|---|---|---|---|---|
| Cl− | 1.61 | −1.25 | −0.18 | 1.43 | 0.01 |
| Na+ | 8.30 | 3.80 | −6.05 | 2.25 | 8.13 |
| Mg2+ | 9.76 | 7.23 | −8.50 | 1.27 | 28.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolen, E.; Dolado, J.S.; Ayuela, A. Cl−, Na+ and Mg2+ Adsorption and Electronic Properties on 2-Octyl Acrylate and Isobornyl Acrylate Monomers: A Comprehensive DFT Study. Polymers 2025, 17, 799. https://doi.org/10.3390/polym17060799
Bolen E, Dolado JS, Ayuela A. Cl−, Na+ and Mg2+ Adsorption and Electronic Properties on 2-Octyl Acrylate and Isobornyl Acrylate Monomers: A Comprehensive DFT Study. Polymers. 2025; 17(6):799. https://doi.org/10.3390/polym17060799
Chicago/Turabian StyleBolen, Emre, Jorge S. Dolado, and Andrés Ayuela. 2025. "Cl−, Na+ and Mg2+ Adsorption and Electronic Properties on 2-Octyl Acrylate and Isobornyl Acrylate Monomers: A Comprehensive DFT Study" Polymers 17, no. 6: 799. https://doi.org/10.3390/polym17060799
APA StyleBolen, E., Dolado, J. S., & Ayuela, A. (2025). Cl−, Na+ and Mg2+ Adsorption and Electronic Properties on 2-Octyl Acrylate and Isobornyl Acrylate Monomers: A Comprehensive DFT Study. Polymers, 17(6), 799. https://doi.org/10.3390/polym17060799






