Experimental Optimization of Tannic Acid-Crosslinked Hydrogels for Neomycin Delivery in Infected Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Optimization of Hydrogels
2.3. Preparation of Hydrogels
2.4. Mechanical Properties
2.5. Water Content
2.6. Water Absorption
2.7. Erosion
2.8. Water Vapor Transmission Rate
2.9. Morphology
2.10. Drug Loading
2.11. Drug Release
2.12. Time-Kill Assay
2.13. Statistical Analysis
3. Results
3.1. The Effect of PVA and PVP on Each Response
3.2. Mechanical Properties of Hydrogels
3.3. Water Content (%)
3.4. Water Absorption
3.5. Erosion
3.6. Water Vapor Transmission Rate
3.7. Optimization of Hydrogel
3.8. Drug Content
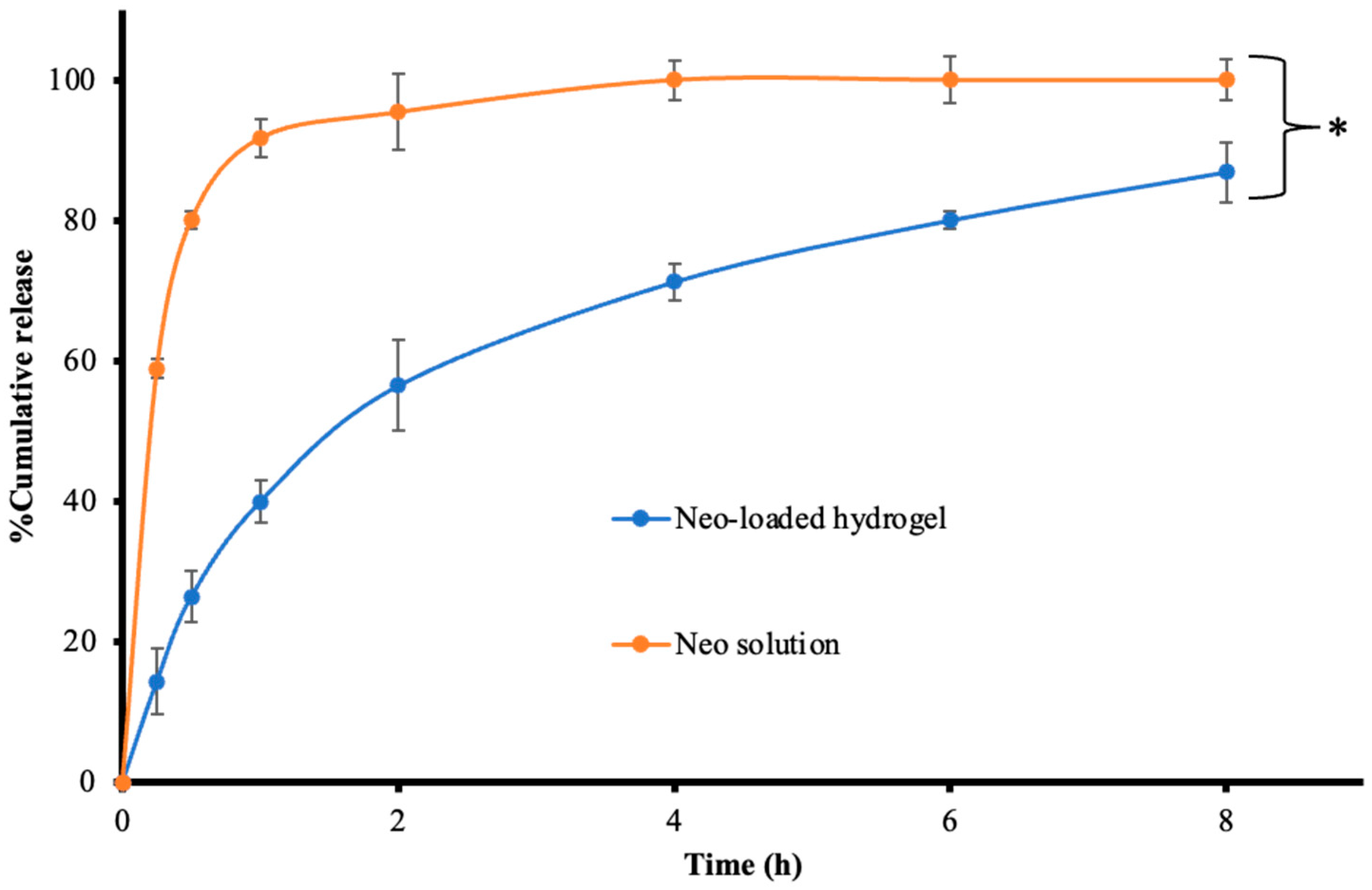
3.9. Drug Release
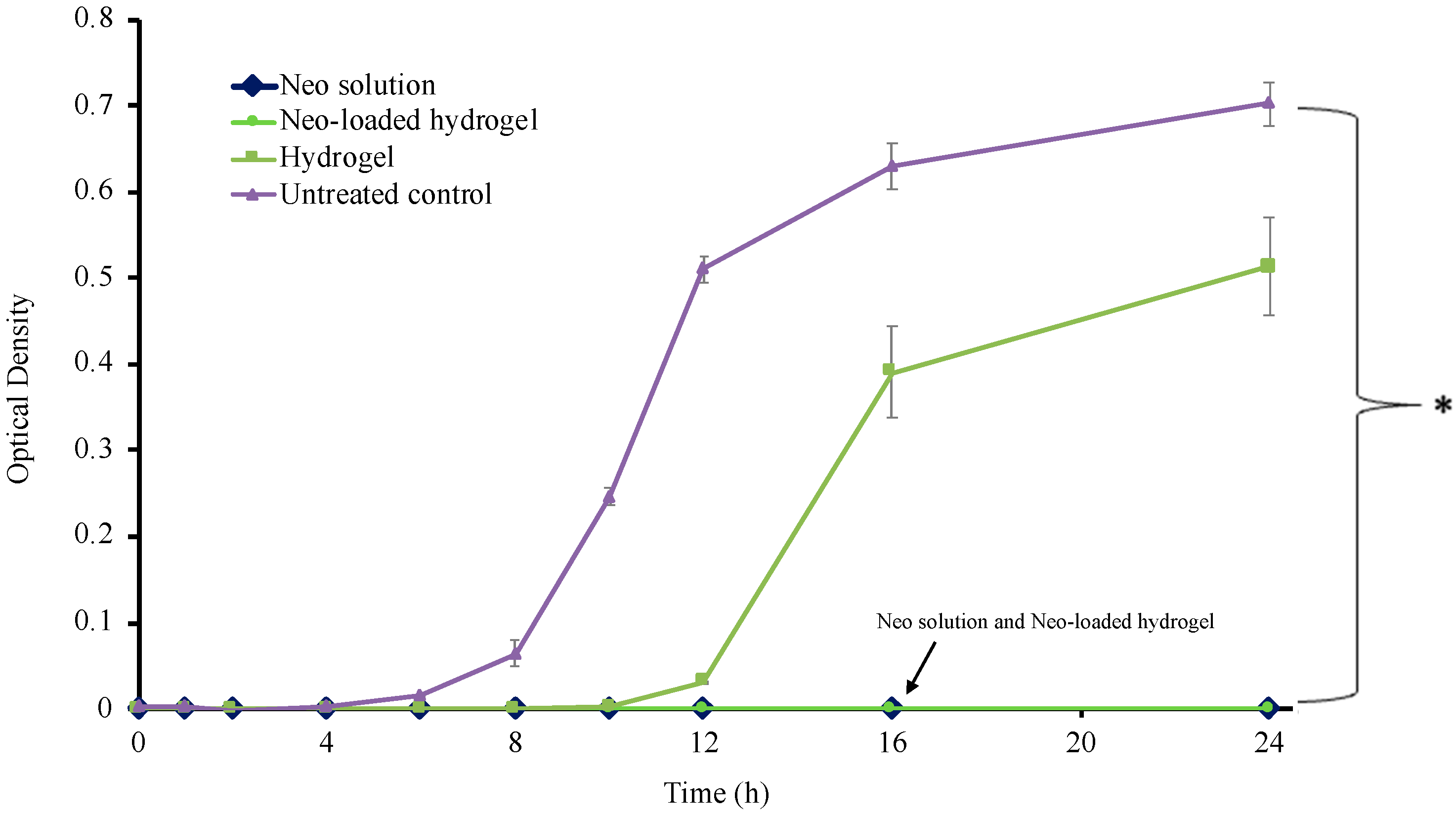
3.10. Time Kill Assay
4. Conclusions
5. Limitations and Future Directions
- 1.
- Though biocompatible and biodegradable polymers and crosslinker was used in this study, an examination performed after they have been combined to form the hydrogel would be able to ascertain the formulation’s safety profile.
- 2.
- An examination of wound contraction rates, epithelialization, and granulation tissue formation would allow us to determine the hydrogel’s efficacy in promoting tissue repair relative to conventional wound care treatments.
- 3.
- Evaluations of the hydrogel’s antimicrobial properties against multiple bacterial species could determine its effectiveness across diverse wound microbiomes. Moreover, an assessment of bacterial clearance in infected wound models could illustrate the antibacterial properties under physiological conditions.
- 4.
- Hydrogel’s degradation, mechanical integrity, and drug stability under storage condition should be analyzed.
- 5.
- Integration of in vivo findings will provide a comprehensive understanding of hydrogel performance, supporting further optimization and potential clinical translation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Heal, C.F.; Banks, J.L.; Lepper, P.D.; Kontopantelis, E.; van Driel, M.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2016, 11, CD011426. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tonnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta. Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Topical Antibacterials in Dermatology. Indian J. Dermatol. 2021, 66, 117–125. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; An, J.; Xin, H.; Xiao, Y.; Jia, Z.; Wu, Y.; Sheng, L.; Wen, M. Cu2O Nanocubes Embedded in Polycaprolactone Nanofibers for Photo–chemotherapeutic Wound Disinfection and Regeneration. ACS Appl. Nano Mater. 2024, 7, 17707–17718. [Google Scholar] [CrossRef]
- Xin, H.; Liu, Y.; Xiao, Y.; Wen, M.; Sheng, L.; Jia, Z. Design and Nanoengineering of Photoactive Antimicrobials for Bioapplications: From Fundamentals to Advanced Strategies. Adv. Funct. Mater. 2024, 34, 2402607. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef]
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending poly(3-hydroxybutyrate) with tannic acid: Influence of a polyphenolic natural additive on the rheological and thermal behavior. Eur. Polym. J. 2015, 63, 123–131. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef] [PubMed]
- Shutava, T.; Prouty, M.; Kommireddy, D.; Lvov, Y. pH Responsive Decomposable Layer-by-Layer Nanofilms and Capsules on the Basis of Tannic Acid. Macromolecules 2005, 38, 2850–2858. [Google Scholar] [CrossRef]
- Erel-Unal, I.; Sukhishvili, S.A. Hydrogen-Bonded Multilayers of a Neutral Polymer and a Polyphenol. Macromolecules 2008, 41, 3962–3970. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Jankovic, A.; Chaudhary, G.; Goia, F. Designing the design of experiments (DOE)—An investigation on the influence of different factorial designs on the characterization of complex systems. Energy Build. 2021, 250, 111298. [Google Scholar] [CrossRef]
- Kadlimatti, H.; Mohan, B.R.; Saidutta, M. Bio-oil from microwave assisted pyrolysis of food waste-optimization using response surface methodology. Biomass Bioenergy 2019, 123, 25–33. [Google Scholar] [CrossRef]
- Ghelich, R.; Jahannama, M.R.; Abdizadeh, H.; Torknik, F.S.; Vaezi, M.R. Central composite design (CCD)-Response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Compos. Part B Eng. 2019, 166, 527–541. [Google Scholar] [CrossRef]
- Eakwaropas, P.; Myat, Y.Y.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Akkaramongkolporn, P.; Opanasopit, P. Optimization of Boesenbergia rotunda Extract-Loaded Polyvinyl Alcohol Hydrogel Wound Dressing by Box-Behnken Design. Key Eng. Mater. 2019, 819, 38–44. [Google Scholar] [CrossRef]
- Chidchai, P.; Singpanna, K.; Opanasopit, P.; Patrojanasophon, P.; Pornpitchanarong, C. Development of photo-crosslinked chitosan-methacrylate hydrogel incorporated with ciprofloxacin as dressing for infected wounds. Carbohydr. Polym. Technol. Appl. 2024, 7, 100478. [Google Scholar] [CrossRef]
- Frost, J. Regression Analysis: An Intuitive Guide for Using and Interpreting Linear Models, 1st ed.; Statistics by Jim Publishing: State College, PA, USA, 2019; p. 16. [Google Scholar]
- Kraber, S. Understanding Lack of Fit: When to Worry. Available online: https://www.statease.com/blog/understanding-lack-of-fit-when-to-worry/# (accessed on 27 December 2024).
- Sarabia, L.A.; Ortiz, M.C. 1.12—Response Surface Methodology. In Comprehensive Chemometrics; Brown, S.D., Tauler, R., Walczak, B., Eds.; Elsevier: Oxford, UK, 2009; pp. 345–390. [Google Scholar] [CrossRef]
- Reena; Kumar, A.; Mahto, V.; Choubey, A.K. Synthesis and characterization of cross-linked hydrogels using polyvinyl alcohol and polyvinyl pyrrolidone and their blend for water shut-off treatments. J. Mol. Liq. 2020, 301, 112472. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly (vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Kopač, T.; Ručigaj, A.; Krajnc, M. The mutual effect of the crosslinker and biopolymer concentration on the desired hydrogel properties. Int. J. Biol. Macromol. 2020, 159, 557–569. [Google Scholar] [CrossRef]
- Eakwaropas, P.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Nuntharatanapong, N. Formulation and Optimal Design of Dioscorea bulbifera and Honey-Loaded Gantrez((R))/Xyloglucan Hydrogel as Wound Healing Patches. Pharmaceutics 2022, 14, 1302. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Chavarría, R.G.; Pérez-Pacheco, A.; Terán, E.; Quispe-Siccha, R.M. Study of Polyvinyl Alcohol Hydrogels Applying Physical-Mechanical Methods and Dynamic Models of Photoacoustic Signals. Gels 2023, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.V.; Relleve, L.S.; Aranilla, C.T.; Dela Rosa, A.M. Properties of radiation synthesized PVP-kappa carrageenan hydrogel blends. Radiat. Phys. Chem. 2003, 68, 901–908. [Google Scholar] [CrossRef]
- Joshi, A.; Fussell, G.; Thomas, J.; Hsuan, A.; Lowman, A.; Karduna, A.; Vresilovic, E.; Marcolongo, M. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials 2006, 27, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D. In vitro assessment of water vapour transmission of synthetic wound dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef]
- Demeter, M.; Scărișoreanu, A.; Călina, I. State of the Art of Hydrogel Wound Dressings Developed by Ionizing Radiation. Gels 2023, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Briggs, F.; Browne, D.; Asuri, P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci. 2022, 23, 4118. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Veirup, N.; Kyriakopoulos, C. Neomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sanchez, S.; Demain, A.L. 1.12—Secondary Metabolites. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 155–167. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials-A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]

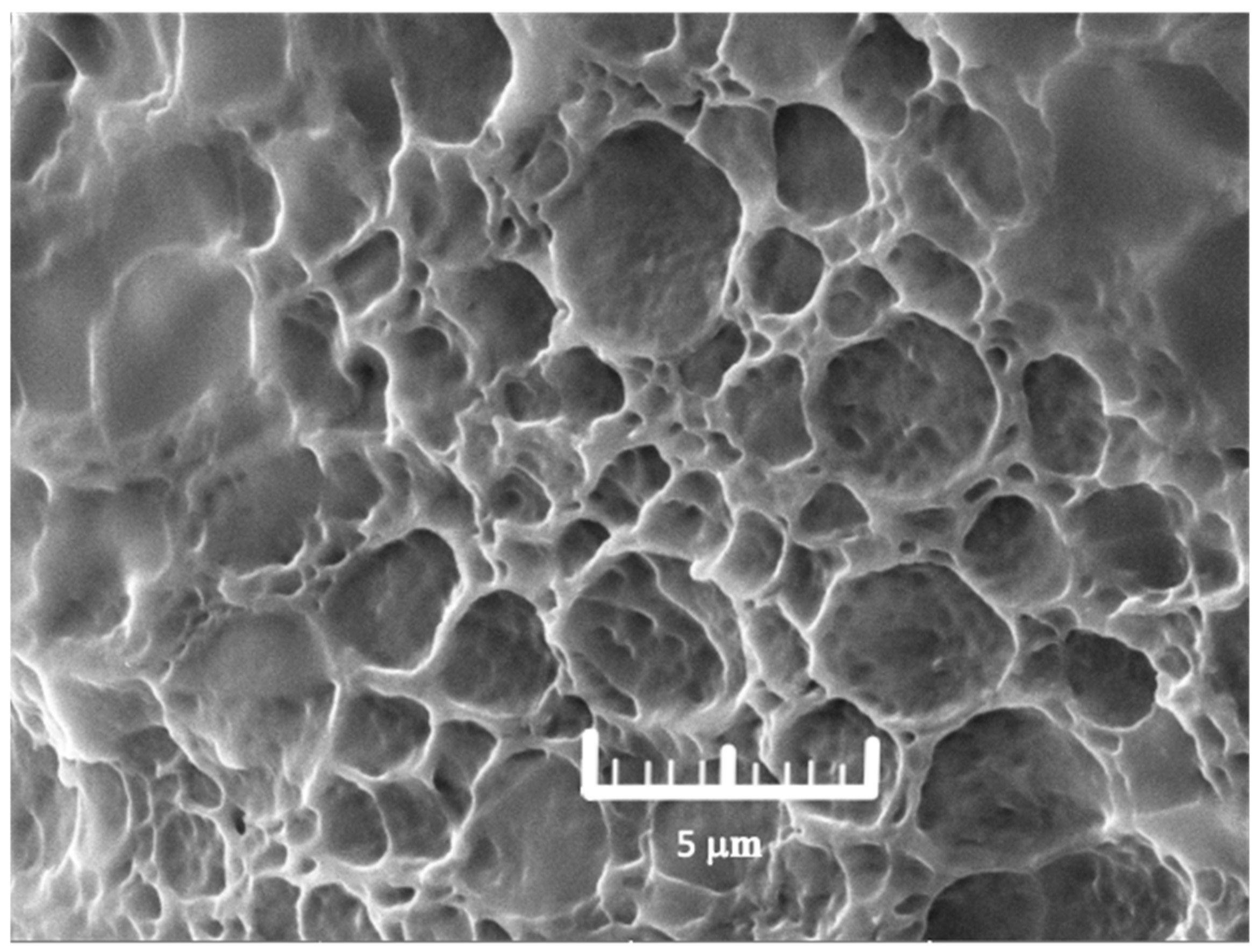
| Run | Concentration (% w/w) | Young’s Modulus (Pa, Y1) | Tensile Strength (N, Y2) | Water Content (%, Y3) | Water Absorption (%, Y4) | Erosion (%, Y5) | WVTR (g/m2/24 h, Y6) | |
|---|---|---|---|---|---|---|---|---|
| PVA | PVP | |||||||
| 1 | 15.00 | 12.50 | 149.45 | 5.60 | 72.16 | 69.44 | 42.56 | 5888 |
| 2 | 10.00 | 12.50 | 40.23 | 0.43 | 74.02 | 82.68 | 62.53 | 6210 |
| 3 | 10.00 | 20.00 | 70.30 | 1.46 | 66.89 | 77.07 | 59.23 | 5299 |
| 4 | 15.00 | 12.50 | 97.59 | 2.77 | 71.84 | 86.66 | 41.76 | 5878 |
| 5 | 15.00 | 5.00 | 149.32 | 2.50 | 80.15 | 58.00 | 30.26 | 6685 |
| 6 | 10.00 | 5.00 | 80.62 | 1.81 | 81.05 | 95.24 | 48.05 | 7370 |
| 7 | 20.00 | 5.00 | 444.23 | 7.20 | 74.45 | 127.92 | 19.12 | 5855 |
| 8 | 20.00 | 12.50 | 672.80 | 9.44 | 65.50 | 86.04 | 25.95 | 5383 |
| 9 | 15.00 | 20.00 | 417.1 | 6.78 | 63.19 | 140.41 | 45.62 | 5348 |
| 10 | 15.00 | 12.50 | 151.09 | 3.61 | 73.02 | 90.89 | 48.45 | 6004 |
| 11 | 20.00 | 20.00 | 679.90 | 8.29 | 55.96 | 158.32 | 33.11 | 4616 |
| Coefficient | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 |
|---|---|---|---|---|---|---|
| b0 | 268.42 | 4.54 | 72.24 | 97.52 | 41.51 | 5866.91 |
| b1 | 267.63 | 3.54 | −4.34 | 19.55 | −15.27 | −504.17 |
| b2 | 82.19 | 0.8367 | −8.27 | 12.44 | 6.76 | −774.50 |
| b12 | 0.00 | 0.00 | −1.08 | 0.00 | 0.00 | 208.00 |
| b11 | 0.00 | 0.00 | −2.32 | 0.00 | 0.00 | 0.00 |
| b22 | 0.00 | 0.00 | −0.4082 | 0.00 | 0.00 | 0.00 |
| p-value | 0.0014 | 0.0005 | <0.0001 | 0.0032 | <0.0001 | <0.0001 |
| R2 | 0.8057 | 0.8503 | 0.9981 | 0.8451 | 0.9267 | 0.9708 |
| Lack of fit | 0.0482 | 0.6608 | 0.8403 | 0.3678 | 0.4915 | 0.1470 |
| Variables | Criteria | Solutions | Desirability |
|---|---|---|---|
| PVA (% w/w) | In range | 20.00 | 0.879 |
| PVP (% w/w) | In range | 19.89 | |
| Young’s modulus (Pa) | None | 617.078 | |
| Tensile strength (N) | Maximize | 4.54 | |
| Water content (%) | In range | 72.24 | |
| Water absorption (%) | Maximize | 129.33 | |
| Erosion (%) | Minimize | 32.90 | |
| WVTR (g/m2/24 h) | In range | 5867 |
| Responses | Young’s Modulus (Pa) | Tensile Strength (N) | Water Content (%) | Water Absorption (%) | Erosion (%) | WVTR (g/m2/24 h) |
|---|---|---|---|---|---|---|
| Predicted | 617.08 ± 119.08 | 4.54 ± 1.38 | 72.24 ± 0.52 | 129.33 ± 24.74 | 32.90 ± 4.25 | 5867± 527 |
| Actual | 474.81 ± 15.24 | 5.82 ± 0.72 | 71.86 ± 2.33 | 124.96 ± 3.42 | 33.08 ± 0.88 | 5567 ± 351 |
| p-value | 0.1724 | 0.2275 | 0.7964 | 0.7895 | 0.9462 | 0.4623 |
| Models | Parameters | Neo Solution | Neo-Loaded Hydrogel |
|---|---|---|---|
| Zero order | R2 | 0.0235 | 0.6029 |
| MSC | −1.5604 | 0.3024 | |
| k | 17.618 | 13.441 | |
| First order | R2 | 0.9942 | 0.9434 |
| MSC | 3.596 | 2.2511 | |
| k | 3.325 | 0.374 | |
| Higuchi | R2 | 0.0922 | 0.9688 |
| MSC | −1.4609 | 2.8467 | |
| k | 47.84 | 33.57 | |
| Korsmeyer–Peppas | R2 | 0.9643 | 0.984 |
| MSC | 1.5245 | 3.2643 | |
| k | 83.17 | 38.14 | |
| n | 0.115 | 0.416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chidchai, P.; Singpanna, K.; Pengnam, S.; Charoenying, T.; Pamornpathomkul, B.; Patrojanasophon, P.; Chaksmithanont, P.; Pornpitchanarong, C. Experimental Optimization of Tannic Acid-Crosslinked Hydrogels for Neomycin Delivery in Infected Wounds. Polymers 2025, 17, 770. https://doi.org/10.3390/polym17060770
Chidchai P, Singpanna K, Pengnam S, Charoenying T, Pamornpathomkul B, Patrojanasophon P, Chaksmithanont P, Pornpitchanarong C. Experimental Optimization of Tannic Acid-Crosslinked Hydrogels for Neomycin Delivery in Infected Wounds. Polymers. 2025; 17(6):770. https://doi.org/10.3390/polym17060770
Chicago/Turabian StyleChidchai, Peerapat, Kanokwan Singpanna, Supusson Pengnam, Thapakorn Charoenying, Boonnada Pamornpathomkul, Prasopchai Patrojanasophon, Prin Chaksmithanont, and Chaiyakarn Pornpitchanarong. 2025. "Experimental Optimization of Tannic Acid-Crosslinked Hydrogels for Neomycin Delivery in Infected Wounds" Polymers 17, no. 6: 770. https://doi.org/10.3390/polym17060770
APA StyleChidchai, P., Singpanna, K., Pengnam, S., Charoenying, T., Pamornpathomkul, B., Patrojanasophon, P., Chaksmithanont, P., & Pornpitchanarong, C. (2025). Experimental Optimization of Tannic Acid-Crosslinked Hydrogels for Neomycin Delivery in Infected Wounds. Polymers, 17(6), 770. https://doi.org/10.3390/polym17060770








