Recycling Textiles: From Post-Consumer Polyester Garments to Materials for Injection Molding
Abstract
1. Introduction
- Material Sorting: It is essential to sort homogeneous materials (monomaterials). In fact, when PET is mixed with cotton or other synthetic polymers, polymer composites or blends are formed in reprocessing [16]. Due to the different chemical structures, the compatibility between the polymers is limited, resulting in poor mechanical properties [17]. In the past several reactive processing methodologies were developed by researchers to overcome this issue in PET blends recycling [18,19,20,21]. Nowadays, sorting techniques based on spectroscopy [22], like for instance near-infrared spectroscopy [23,24] coupled with artificial intelligence systems [25], can be applied on an industrial scale.
- Compounding: The material obtained must be compounded by extrusion with appropriate additives, depending on the final application. This step could allow the flakes to be transformed into pellets suitable for injection molding, compression molding, or additive manufacturing. However, the material thus obtained should show the desired rheological properties. Generally, PET undergoes chain scission during compounding operations, resulting in a material with very high fluidity, not suitable to be processed in manufacturing technologies where a significant melt strength is required.
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Characterization
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granskog, A.; Lee, L. The Fashion Industry Can Reduce Emissions Across the Entire Valuechain; 2020 MacKinsey& Company Report; MacKinsey& Company: New York, NY, USA, 2020; Available online: https://www.mckinsey.com/capabilities/sustainability/our-insights/sustainability-blog/the-fashion-industry-can-reduce-emissions-across-the-entire-value-chain (accessed on 22 January 2025).
- Sajn, N. Environmental Impact of the Textile and Clothing Industry; European Parliamentary Research Service: Bruxelles, Belgium, 2019; Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2019/633143/EPRS_BRI(2019)633143_EN.pdf (accessed on 22 January 2025).
- Amed, I.; D’Auria, G.; Bain, M. The State of Fashion 2025; BOF Report 2022; MacKinsey & Company: New York, NY, USA, 2022; Available online: https://www.mckinsey.com/~/media/mckinsey/industries/retail/our%20insights/state%20of%20fashion/2025/the-state-of-fashion-2025-v2.pdf?shouldIndex=false (accessed on 22 January 2025).
- Global Fiber Production Reached an All-Time High of 124 Million Tonnes in 2023, According to Textile Exchange’s Materials Market Report. 2024. Textile Enchange. Available online: https://textileexchange.org/news/textile-exchange-releases-2024-materials-market-report/ (accessed on 22 January 2025).
- Hossain, M.T.; Shahid, M.A.; Limon, M.G.M.; Hossain, I.; Mahmud, N. Techniques, applications, and challenges in textiles for a sustainable future. J. Open Innov. Technol. Mark. Complex. 2024, 10, 100230. [Google Scholar] [CrossRef]
- Frazier, R.M.; Vivas, K.A.; Azuaje, I.; Vera, R.; Pifano, A.; Forfora, N.; Jameel, H.; Ford, E.; Pawlak, J.J.; Venditti, R.; et al. Beyond cotton and polyester: An evaluation of emerging feedstocks and conversion methods for the future of fashion industry. J. Bioresour. Bioprod. 2024, 9, 130–159. [Google Scholar] [CrossRef]
- Aizenshtein, E.M. World production and consumption of polyester fibres and thread. Fibre Chem. 2006, 38, 264–271. [Google Scholar] [CrossRef]
- Majumdar, A.; Shukla, S.; Singh, A.A.; Arora, S. Circular Fashion: Properties of Fabrics Made from Mechanically Recycled Poly-Ethylene Terephthalate (PET) Bottles. Resour. Conserv. Recycl. 2020, 161, 104915. [Google Scholar] [CrossRef]
- Massoud, T.; Dsilva, J. Closing the PET Plastic Recycling Loop: A Sustainable Transformation from Plastic to Fiber. Next Sustain. 2025, 6, 100095. [Google Scholar] [CrossRef]
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 27 January 2025).
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Dou, M.; Wang, C. Abundance and removal characteristics of microplastics at a wastewater treatment plant in Zhengzhou. Environ. Sci. Pollut. Res. 2020, 27, 36295–36305. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.-T.; Huang, Q.-S.; Ni, B.-J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Res. 2019, 163, 114881. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Bianchi, S.; Bartoli, F.; Bruni, C.; Fernandez-Avila, C.; Rodriguez-Turienzo, L.; Mellado-Carretero, J.; Spinelli, D.; Coltelli, M.-B. Opportunities and Limitations in Recycling Fossil Polymers from Textiles. Macromol 2023, 3, 120–148. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Harrats, C.; Aglietto, M.; Groeninckx, G. Influence of compatibilizer precursor structure on the phase distribution of low density poly(ethylene) in a poly(ethylene terephthalate) matrix. Polym. Eng. Sci. 2008, 48, 1424–1433. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Della Maggiore, I.; Savi, S.; Aglietto, M.; Ciardelli, F. Modified Styrene-Butadiene-Styrene Block Copolymer as Compatibiliser Precursor in Polyethylene/Poly(Ethylene Terephthalate) Blends. Polym. Degrad. Stab. 2005, 90, 211–223. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Giani, M.; Lochiatto, F.; Aglietto, M.; Savi, S.; Ciardelli, F. Postconsumer Polyethylene Terephthalate (PET)/Polyolefin Blends through Reactive Processing. J. Mater. Cycles Waste Manag. 2004, 6, 13–19. [Google Scholar] [CrossRef]
- Coba-Daza, S.; Otaegi, I.; Aramburu, N.; Guerrica-Echevarria, G.; Irusta, L.; González, A.; Neubauer, L.; Ramer, G.; Lendl, B.; Hubner, G.; et al. Unlocking Superior Properties in Polypropylene/Polyethylene Terephthalate (PP/PET) Blends Using an Ethylene-Butylene-Acrylate Terpolymer Reactive Compatibilizer. Polym. Test. 2024, 130, 108293. [Google Scholar] [CrossRef]
- Luo, L.-B.; Chen, R.; Lian, Y.-X.; Wu, W.-J.; Zhang, J.-H.; Fu, C.-X.; Sun, X.-L.; Xiao, L.-R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers 2024, 16, 1052. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of Polymer Technologies for Improving the Recycling and Upcycling Efficiency of Plastic Waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- Grigore, M.E. Methods of Recycling, Properties and Applications of Recycled Thermoplastic Polymers. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Gigante, V.; Aliotta, L.; Lazzeri, A. Recyclability Perspectives of the Most Diffused Biobased and Biodegradable Plastic Materials. Macromol 2024, 4, 401–419. [Google Scholar] [CrossRef]
- Robinson, M.; Ghosh, S.; Goswami, P.; Vallati, M. A Novel Methodology for AI-based Sorting of Post-Consumer Textile Using Spectrophotometer. CEUR Workshop Proc. 2024, 3883, 1–8. [Google Scholar]
- Mäkelä, M.; Rissanen, M.; Sixta, H. Machine vision estimates the polyester content in recyclable waste textiles. Resour. Conserv. Recycl. 2020, 161, 105007. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile recognition and sorting for recycling at an automated line using near infrared spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Du, W.; Zheng, J.; Li, W.; Liu, Z.; Wang, H.; Han, X. Efficient Recognition and Automatic Sorting Technology of Waste Textiles Based on Online Near infrared Spectroscopy and Convolutional Neural Network. Resour. Conserv. Recycl. 2022, 180, 106157. [Google Scholar] [CrossRef]
- Bartoli, F.; Bianchi, S.; Minei, P.; Pinna, M. Spin-PET srl, Method for Producing Plastic Granules from Recycling of Waste Fabrics and/or Yarns, EP24188242.2. Available online: https://www.patentguru.com/inventor/pinna-michele (accessed on 22 January 2025).
- Vadood, M.; Haji, A. A hybrid artificial intelligence model to predict the color coordinates of polyester fabric dyed with madder natural dye. Expert Syst. Appl. 2022, 193, 116514. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Bianchi, S.; Aglietto, M. Poly(ethylene terephthalate) (PET) degradation during the Zn catalysed transesterification with dibutyl maleate functionalized polyolefins. Polymer 2007, 48, 1276–1286. [Google Scholar] [CrossRef]
- Jae Young Jang, K.S.; Seo, J. Chain-Extending Modification for Value-Added Recycled PET: A Review. Polym. Rev. 2022, 62, 860–889. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Bertolini, A.; Aliotta, L.; Gigante, V.; Vannozzi, A.; Lazzeri, A. Chain Extension of Poly(Lactic Acid) (PLA)–Based Blends and Composites Containing Bran with Biobased Compounds for Controlling Their Processability and Recyclability. Polymers 2021, 13, 3050. [Google Scholar] [CrossRef]
- Frenz, V.; Scherzer, D.; Villalobos, M.; Awojulu, A.A.; Edison, M.; Van Der Meer, R. Multifunctional Polymers as Chain Extenders and Compatibilizers for Polycondensates and Biopolymers. Tech. Pap. Reg. Tech. Conf.—Soc. Plast. Eng. 2008, 3, 1678–1682. [Google Scholar]
- Wu, W.J.; Sun, X.L.; Chen, Q.; Qian, Q. Recycled Poly(Ethylene Terephthalate) from Waste Textiles with Improved Thermal and Rheological Properties by Chain Extension. Polymers 2022, 14, 510. [Google Scholar] [CrossRef]
- Vozniak, A.; Hosseinnezhad, R.; Vozniak, I.; Galeski, A. PET Mechanical Recycling. A New Principle for Chain Extender Introduction. Sustain. Mater. Technol. 2024, 40, e00886. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Chain extension of polyesters PET and PBT With Two New Diimidodiepoxides. II. J. Polym. Sci. 1996, 34, 1337–1342. [Google Scholar] [CrossRef]
- Raffa, P.; Coltelli, M.B.; Castelvetro, V. Expanding the Application Field of Post-Consumer Poly(Ethylene Terephthalate) through Structural Modification by Reactive Blending. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Heuzey, M.C.; Ghanbari, A.; Carreau, P.J. Polyethylene Terephthalate/Organoclay Nanocomposites: Improvement of Morphology and Viscoelastic Properties by Using a Chain-Extender. Appl. Clay Sci. 2022, 225, 106551. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, S.; Cui, X.; Sun, S.; Zhang, H. Application of Macromolecular Chain Extender and Contribution to the Toughening of Poly(Ethylene Terephthalate). J. Thermoplast. Compos. Mater. 2016, 29, 833–849. [Google Scholar] [CrossRef]
- Veselova, E.V.; Andreeva, T.I.; Strelkova, M.V. Reaction Modification of Recycled Polyethylene Terephthalate with 1,3-Phenylene-Bis-Oxazoline. Int. Polym. Sci. Technol. 2014, 41, 37–40. [Google Scholar] [CrossRef]
- Odet, F.; Ylla, N.; Delage, K.; Cassagnau, P. Influence of Chain Extenders on Recycled Standard and Opaque PET Rheology and Melt-Spun Filament Properties. ACS Appl. Polym. Mater. 2022, 4, 8290–8302. [Google Scholar] [CrossRef]
- Karl, C.W.; Arstad, B.; Shamsuyeva, M.; Lecinski, J.; Olafsen, K.; Larsen, Å.G.; Kubowicz, S.; Comerford, J.; Endres, H.J. Upgrading and Enhancement of Recycled Polyethylene Terephthalate with Chain Extenders: In-Depth Material Characterization. Ind. Eng. Chem. Res. 2024, 63, 12277–12287. [Google Scholar] [CrossRef]
- Härth, M.; Dörnhöfer, A.; Kaschta, J.; Münstedt, H.; Schubert, D.W. Molecular Structure and Rheological Properties of a Poly(Ethylene Terephthalate) Modified by Two Different Chain Extenders. J. Appl. Polym. Sci. 2021, 138, 50110. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Y.; Wang, K.; Xia, Y.; Gao, H.; Luo, K.; Cao, Z.; Qi, J. Effect of the Trifunctional Chain Extender on Intrinsic Viscosity, Crystallization Behavior, and Mechanical Properties of Poly(Ethylene Terephthalate). ACS Omega 2020, 5, 19247–19254. [Google Scholar] [CrossRef]
- Raffa, P.; Coltelli, M.-B.; Savi, S.; Bianchi, S.; Castelvetro, V. Chain Extension and Branching of Poly(Ethylene Terephthalate) (PET) with Di- and Multifunctional Epoxy or Isocyanate Additives: An Experimental and Modelling Study. React. Funct. Polym. 2012, 72, 50–60. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Q. Effects of Bifunctional Chain Extender on the Crystallinity and Thermal Stability of PET. J. Mater. Sci. Chem. Eng. 2013, 1, 9–15. [Google Scholar] [CrossRef]
- Van der Maas, K.; Weinland, D.H.; van Putten, R.J.; Wang, B.; Gruter, G.J.M. Catalyst Free PET and PEF Polyesters Using a New Traceless Oxalate Chain Extender. Green Chem. 2024, 26, 11182–11195. [Google Scholar] [CrossRef] [PubMed]
- El-shazly, R.I.; Kamal, R.S.; Farag, R.K. Structural and viscosity studies of dendritic hyper branched polymer as viscosity index improvers. BMC Chem. 2024, 18, 107. [Google Scholar] [CrossRef]
- Guclu, M.; Alkan Göksu, Y.; Özdemir, B.; Ghanbari, A.; Nofar, M. Thermal Stabilization of Recycled PET Through Chain Extension and Blending with PBT. J. Polym. Environ. 2022, 30, 719–727. [Google Scholar] [CrossRef]
- Arayesh, H.; Golshan Ebrahimi, N.; Khaledi, B.; Khabazian Esfahani, M. Introducing four different branch structures in PET by reactive processing—A rheological investigation. J. Appl. Polym. Sci. 2020, 137, 49243. [Google Scholar] [CrossRef]
- Qu, M.; Lu, D.; Deng, H.; Wu, Q.; Han, L.; Xie, Z.; Qin, Y.; Schubert, D.W. A comprehensive study on recycled and virgin PET melt-spun fibers modified by PMDA chain extender. Mater. Today Commun. 2021, 29, 103013. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(lactic acid) (PLA)/Poly(butylene succinate-co-adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Bonacchi, D.; Coltelli, M.-B.; Lazzeri, A. Analysis, Development, and Scaling-Up of Poly(lactic acid) (PLA) Biocomposites with Hazelnuts Shell Powder (HSP). Polymers 2021, 13, 4080. [Google Scholar] [CrossRef]
- ASTM D1238-10; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM: West Conshohocken, PA, USA, 2013.
- ISO 527-2:2012; Plastics—Determination of Tensile Properties Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2012.
- ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- Negoro, T.; Thodsaratpreeyakul, W.; Takada, Y.; Thumsorn, S.; Inoya, H.; Hamada, H. Role of Crystallinity on Moisture Absorption and Mechanical Performance of Recycled PET Compounds. Energy Procedia 2016, 89, 323–327. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Harzallah, O.; Lallam, A. Progress in Polyethylene Terephthalate Recycling; Polyethylene Terephthalate: Uses, Properties and Degradation; Nova Science Publishers: Hauppauge, NY, USA, 2017; Available online: https://hal.science/hal-02953197v1 (accessed on 9 February 2025).
- Kwon, K.; Isayev, A.I.; Kim, K.H. Theoretical and experimental studies of anisotropic shrinkage in injection moldings of various polyesters. J. Appl. Polym. Sci. 2006, 102, 3526–3544. [Google Scholar] [CrossRef]
- Rizal, N.R.S.; Rosman, M.A.; Jumahat, A.; Yusoff, N. Investigation of the injection moulding plastic flows behaviour of PETCylindrical containers with multiple-cavity mould. Pertanika J. Sci. Technol. 2017, 25, 239–250. [Google Scholar]
- Torres, N.; Robin, J.J.; Boutevin, B. Study of thermal and mechanical properties of virgin and recycled poly(ethylene terephthalate) before and after injection molding. Eur. Polym. J. 2000, 36, 2075–2080. [Google Scholar] [CrossRef]
- Annicchiarico, D.; Alcock, J.R. Review of Factors that Affect Shrinkage of Molded Part in Injection Molding. Mater. Manuf. Process. 2014, 29, 662–682. [Google Scholar] [CrossRef]
- Dobrovszky, K.; Ronkay, F. Effects of Phase Inversion on Molding Shrinkage, Mechanical, and Burning Properties of Injection-molded PET/HDPE and PS/HDPE Polymer Blends. Polym.-Plast. Technol. Eng. 2017, 56, 1147–1157. [Google Scholar] [CrossRef]
- Mahdavipour, Z.; Karimi, M. A robust relation between mechanical modulus and thermal shrinkage force of PET filaments based on entropy stress. Polymer 2024, 296, 126781. [Google Scholar] [CrossRef]
- Mittermeier, C.; Lion, A. Challenges in the experimental investigation of the caloric and thermomechanical behaviour of semi-crystalline polymers: A study on the example of polyethylene terephthalate (PET). Polym. Test. 2020, 81, 106252. [Google Scholar] [CrossRef]
- Viana, J.C.; Alves, N.M.; Mano, J.F. Morphology and Mechanical Properties of Injection Molded Poly(Ethylene Terephthalate). Polym. Eng. Sci. 2004, 44, 12. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Katalagarianakis, A.; Huysman, S.; Toncheva, A.; Raquez, J.-M.; Duretek, I.; Holzer, C.; Cardon, L.; Bernaerts, K.V.; Van Hemelrijck, D.; et al. Effect of extrusion and fused filament fabrication processing parameters of recycled poly(ethylene terephthalate) on the crystallinity and mechanical properties. Addit. Manuf. 2022, 50, 102518. [Google Scholar] [CrossRef]
- Fang, F.; Wang, H.; Wang, H.; Huang, W.M.; Chen, Y.; Cai, N.; Chen, X.; Chen, X. Stimulus-Responsive Shrinkage in Electrospun Membranes: Fundamentals and Control. Micromachines 2021, 12, 920. [Google Scholar] [CrossRef]
- Viora, L.; Combeau, M.; Pucci, M.F.; Perrin, D.; Liotier, P.-J.; Bouvard, J.-L.; Combeaud, C. A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers 2023, 15, 4613. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and Mechanical Properties of the Recycled and Virgin PET—Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, S.M.; Dehghani, A.; Al-Maadeed, M.A.; Wahit, M.U.; Hassan, A. Mechanical and thermal properties of recycled poly(ethylene terephthalate) reinforced newspaper fiber composites. Fibers Polym. 2014, 15, 1531–1538. [Google Scholar] [CrossRef]
- Aging, T.; Panowicz, R.; Konarzewski, M.; Durejko, T.; Szala, M.; Łazi, M. Properties of Polyethylene Terephthalate (PET) after Thermo-Oxidative Aging. Materials 2021, 14, 3833. [Google Scholar] [CrossRef]
- Snell, H.; Nassour, A.; Nelles, M. Qualitative comparison of polyethylene terephthalate flakes from various collection systems in Germany. Waste Manag. Res. 2017, 35, 163–171. [Google Scholar] [CrossRef]
- Elamri, A.; Lallam, A.; Harzallah, O.; Bencheikh, L. Mechanical characterization of melt spun fibers from recycled and virgin PET blends. J. Mater. Sci. 2007, 42, 8271–8278. [Google Scholar] [CrossRef]
- Lindström, F. Chemical and Physical Changes in PET Fibres Due to Exhaust Dyeing—Issues in Thermo-Mechanical Recycling of Dyed PET Textiles. Master’s Thesis, The Swedish School of Textiles, University of Borås, Borås, Sweden, 2018. [Google Scholar]
- Dawson, J.F. The Structure and Properties of Disperse Dyes in Polyester Coloration. JSDC 1983, 99, 183. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Thermal degradation of azobenzene dyes. Results Chem. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Chung, K. Mutagenicity and carcinogenicity of aromatic amines metabolically produced from Azo Dyes. J. Environ. Sci. Health Part C 2000, 18, 51–74. [Google Scholar] [CrossRef]
- Fadhel, A.M.; Hamdani, A.A.S. Al Historical Background, Literature Review on the Synthesis and Applicability of Azo-Dye Compounds: An Extensive Review. Adv. J. Chem. Sect. A 2024, 7, 687–724. [Google Scholar]
- Pinheiro, H.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Mu, B.; Yu, X.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Complete recycling of polymers and dyes from polyester/cotton blended textiles via cost-effective and destruction-minimized dissolution, swelling, precipitation, and separation. Resour. Conserv. Recycl. 2023, 199, 107275. [Google Scholar] [CrossRef]



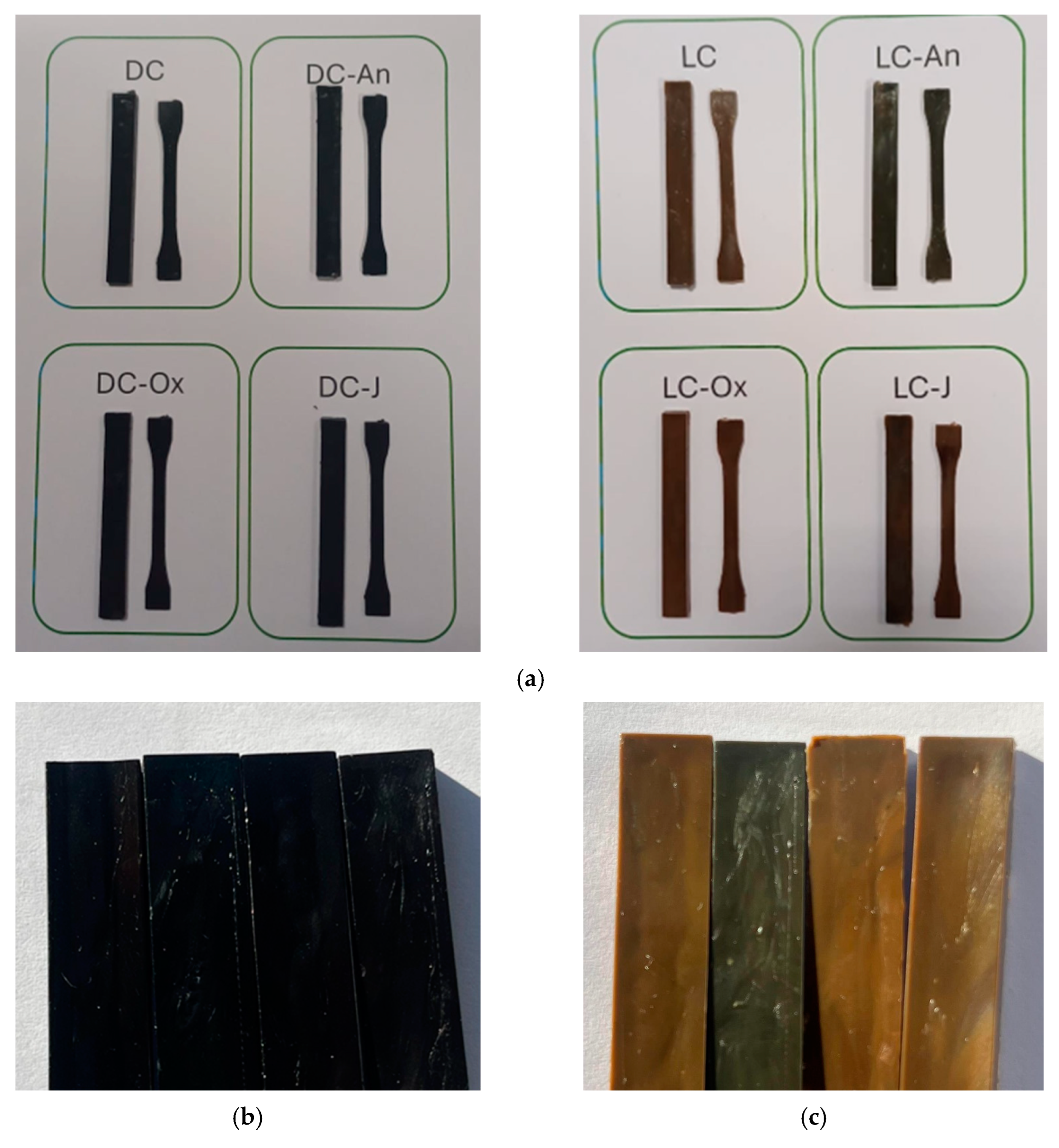

| Compound | PET Flakes (g) | Chain Extender (g) |
|---|---|---|
| DC | Dark Colored (200) | // |
| DC-An | Dark Colored (200) | Nexamite® M021200 (6) |
| DC-Ox | Dark Colored (200) | Nexamite® M992000 (6) |
| DC-J | Dark Colored (200) | Joncryl 4468 (1) |
| LC | Light Colored (200) | // |
| LC-An | Light Colored (200) | Nexamite® M021200 (6) |
| LC-Ox | Light Colored (200) | Nexamite® M992000 (6) |
| LC-J | Light Colored (200) | Joncryl 4468 (1) |
| Processing Steps for Polyester Garments | Procedures and Parameters | Final Items |
|---|---|---|
| Compaction | Heating at 150–310 °C and grinding [29] | Flakes |
| Extrusion | Drying in a ventilated oven at 150 °C for 3 h; single-screw extrusion with rotor speed set to 50 rpm, and temperature profile: 250, 260, 260, and 250 °C | Granules (pellets) |
| Injection molding | Drying in a ventilated oven at 150 °C for 3 h; setting 260 °C and piston speed 80 mm/s; mold at room temperature; specimens quenched by immersion in water at 0 °C. | Specimens |
| Flakes Color | Picture | MVR (cm3/10 min) | MFR (g/10 min) |
|---|---|---|---|
| WHITE |  | 16.9 ± 2.0 | 14.6 ± 2.3 |
| YELLOW |  | 10.3 ± 1.8 | 8.8 ± 2.1 |
| RED |  | 22.4 ± 3.9 | 18.9 ± 4.6 |
| BLUE |  | 51.2 ± 6.0 | 41.7 ± 7.4 |
| BLACK-1 |  | 65.5 ± 4.7 | 55.1 ± 5.6 |
| BLACK-2 |  | 74.5 ± 5.8 | 70.5 ± 6.1 |
| Compound | MVR (cm3/10 min) | MFR (g/10 min) |
|---|---|---|
| DC | 44.5 ± 2.5 | 47.5 ± 2.7 |
| DC-An | 38.2 ± 6.1 | 44.7 ± 7.1 |
| DC-Ox | 41.9 ± 3.7 | 46.5 ± 4.1 |
| DC-J | 26.1 ± 2.6 | 29.4 ± 2.9 |
| LC | 24.25 ± 0.7 | 27.7 ± 0.8 |
| LC-An | 9.9 ± 1.7 | 11.6 ± 1.9 |
| LC-Ox | 18.3 ± 2.7 | 21.0 ± 3.1 |
| LC-J | 4.8 ± 0.9 | 5.5 ± 1.0 |
| Compound | Young’s Modulus (MPa) | Stress at Yield (MPa) | Elongation at Yield (%) | Elongation at Break (%) |
|---|---|---|---|---|
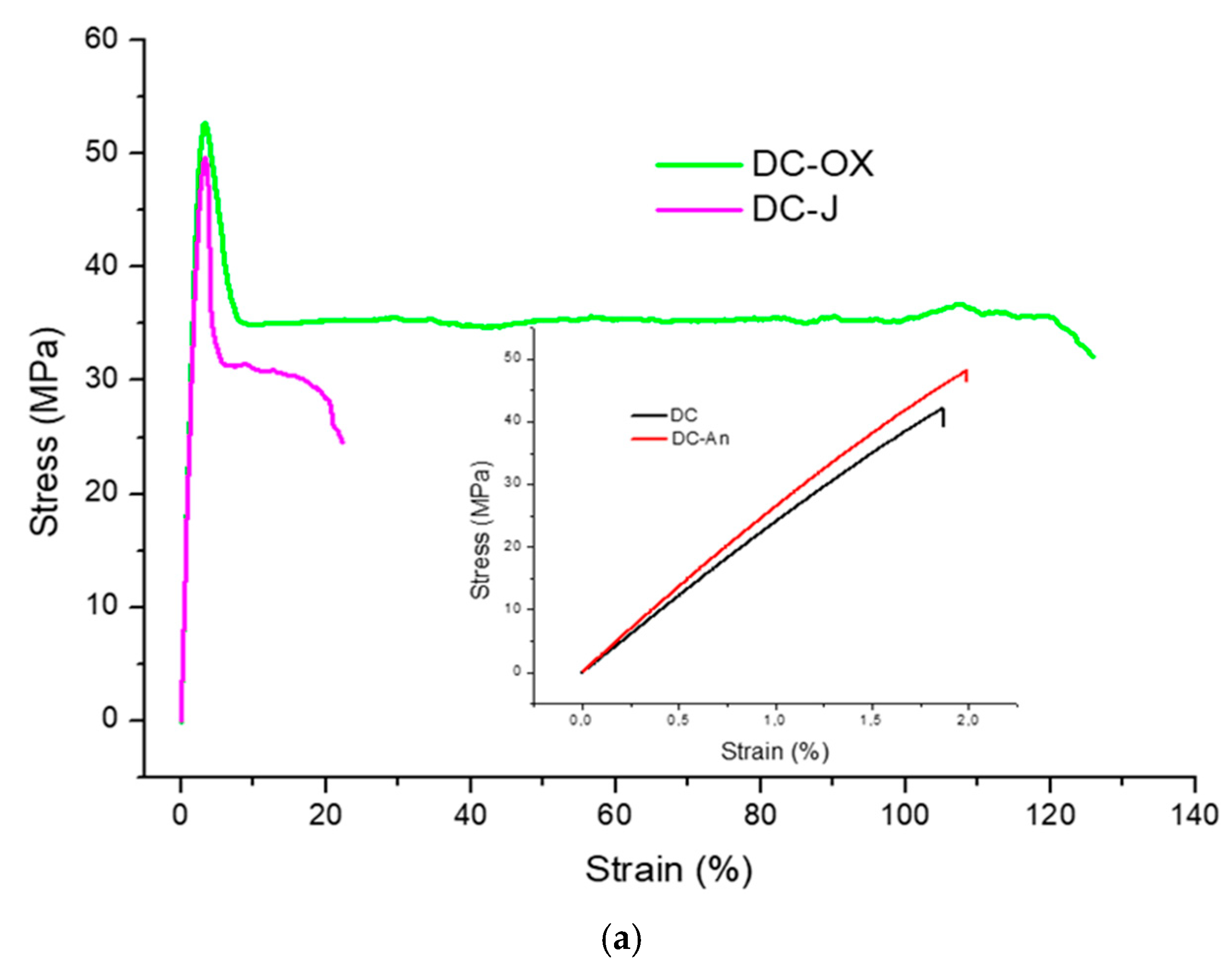
| DC | 2461 ± 191 | // | // | 1.8 ± 0.5 |
| DC-An | 2730 ± 55 | // | // | 2.0 ± 0.3 |
| DC-Ox | 2363 ± 70 | 51.5 ± 1.8 | 3.2 ± 0.1 | 152 ± 95 |
| DC-J | 2489 ± 186 | 59.4 ± 9.1 | 3.6 ± 0.2 | 35.0 ± 12 |
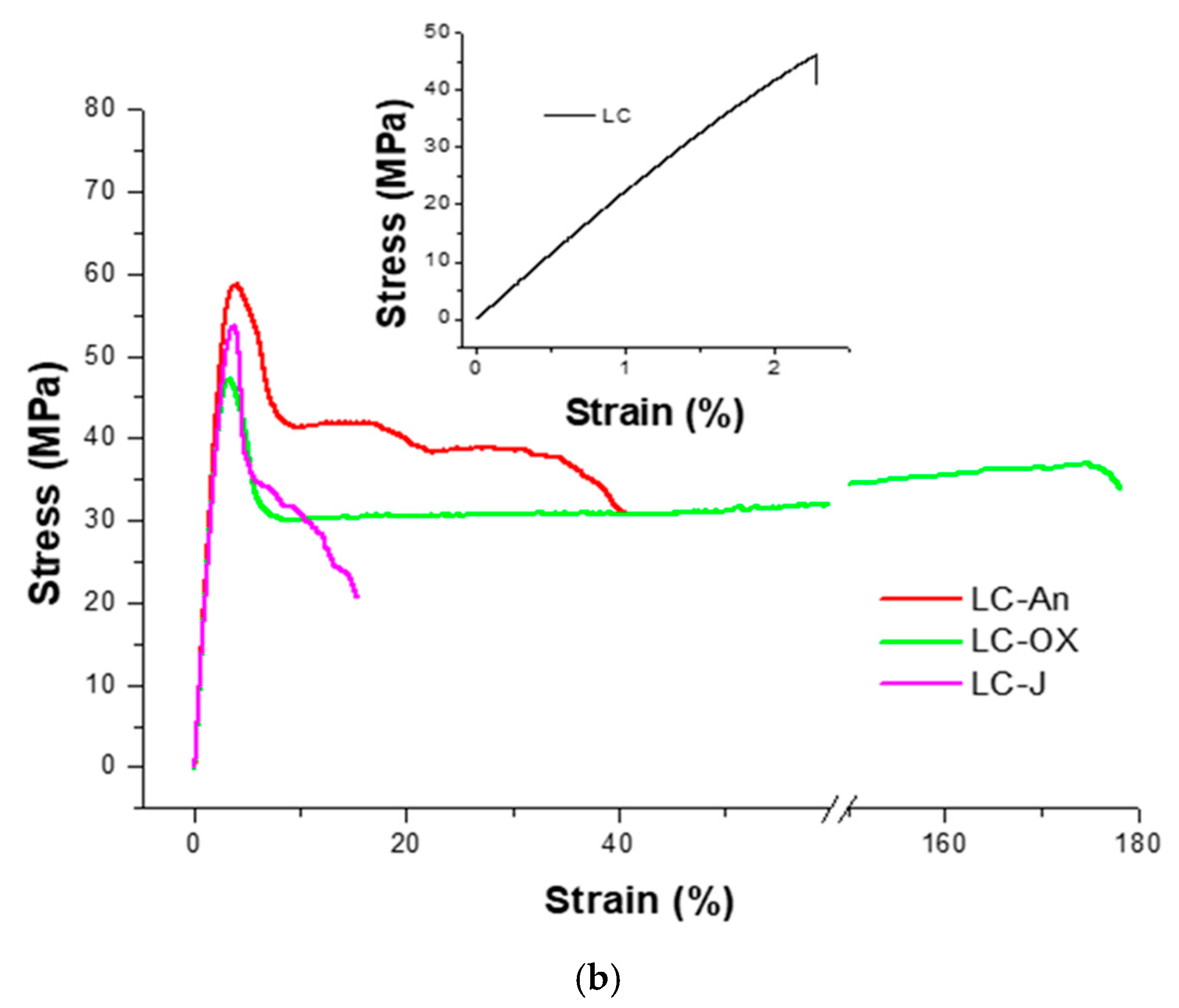
| LC | 2545 ± 231 | // | // | 2.4 ± 0.4 |
| LC-An | 2281 ± 23.0 | 57.1 ± 2.3 | 3.8 ± 0.1 | 43 ± 21 |
| LC-Ox | 2406 ± 219 | 52.5 ± 8.7 | 3.2 ± 0.3 | 171 ± 12 |
| LC-J | 2280 ± 79 | 55.7 ± 2.6 | 3.7 ± 0.1 | 11 ± 6 |
| Compound | Flexural Modulus (MPa) | Stress at Yield (MPa) | Stress at Break (%) | Deformation at Break (%) |
|---|---|---|---|---|
| DC | 1951 ± 135 | // | 54 ± 17 | 3.4 ± 1.5 |
| DC-An | 1779 ± 42 | // | 47 ± 6 | 2.9 ± 0.4 |
| DC-Ox | 1886 ± 239 | 66 ± 10 | 59 ± 11 | 6.3 ± 2.5 |
| DC-J | 2230 ± 181 | // | 54 ± 9 | 4.6 ± 1.7 |
| LC | 2289 ± 106 | // | 46 ± 5 | 2.1 ± 0.2 |
| LC-An | 2280 ± 102 | // | 49 ± 8 | 2.3 ± 0.6 |
| LC-Ox | 2355 ± 411 | 80 ± 7 | 60 ± 23 | 5.9 ± 1.3 |
| LC-J | 2323 ± 164 | // | 59 ± 8 | 2.8 ± 0.6 |
| Compound | Flow Direction (%) | Transverse Direction (%) |
|---|---|---|
| DC | 0.97 | 2.72 |
| DC-An | 0.98 | 2.73 |
| DC-Ox | 0.96 | 2.81 |
| DC-J | 0.85 | 2.73 |
| LC | 0.91 | 2.60 |
| LC-An | 0.91 | 2.84 |
| LC-Ox | 0.92 | 2.36 |
| LC-J | 0.79 | 2.28 |
| Compound | Tm (°C) | Tc (°C) | ΔHm (J/g) | Crystallinity (X) (%) |
|---|---|---|---|---|
| DC | 252 | 201 | 49.8 | 35.6 |
| DC-An | 253 | 202 | 49.0 | 35.0 |
| DC-Ox | 250 | 200 | 54.2 | 39.0 |
| DC-J | 251 | 201 | 50.1 | 35.7 |
| LC | 256 | 206 | 45.1 | 32.2 |
| LC-An | 253 | 204 | 49.8 | 35.6 |
| LC-Ox | 254 | 204 | 46.7 | 33.4 |
| LC-J | 255 | 205 | 48.7 | 34.8 |
| Compound | Maximum Number of Linkages per Molecule | ΔMFR (g/10 min) | Ductility Improvement % (Tensile) |
|---|---|---|---|
| DC-An | 4 | 6.3 | 11 |
| DC-Ox | 2 | 2.6 | 8344 |
| DC-J | 23 | 18.4 | 1844 |
| LC-An | 4 | 14.35 | 1692 |
| LC-Ox | 2 | 5.95 | 7025 |
| LC-J | 23 | 19.45 | 358 |
| Chain Extender | Macromolecular Design | Melt Fluidity (g/10 min) | Ductility (Elong. %) |
|---|---|---|---|
| Ox | Linear | High (>20) | High (>150) |
| An | Slightly branched | Medium (>10) | Medium |
| J | Branched | Low (>5) | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, S.; Pinna, M.; Bartoli, F.; Minei, P.; Filidei, D.; Coltelli, M.-B. Recycling Textiles: From Post-Consumer Polyester Garments to Materials for Injection Molding. Polymers 2025, 17, 748. https://doi.org/10.3390/polym17060748
Bianchi S, Pinna M, Bartoli F, Minei P, Filidei D, Coltelli M-B. Recycling Textiles: From Post-Consumer Polyester Garments to Materials for Injection Molding. Polymers. 2025; 17(6):748. https://doi.org/10.3390/polym17060748
Chicago/Turabian StyleBianchi, Sabrina, Michele Pinna, Flavia Bartoli, Pierpaolo Minei, Daniele Filidei, and Maria-Beatrice Coltelli. 2025. "Recycling Textiles: From Post-Consumer Polyester Garments to Materials for Injection Molding" Polymers 17, no. 6: 748. https://doi.org/10.3390/polym17060748
APA StyleBianchi, S., Pinna, M., Bartoli, F., Minei, P., Filidei, D., & Coltelli, M.-B. (2025). Recycling Textiles: From Post-Consumer Polyester Garments to Materials for Injection Molding. Polymers, 17(6), 748. https://doi.org/10.3390/polym17060748








