Enhanced Hydrophobicity, Thermal Stability, and X-Ray Shielding Efficiency of BaSO4/P(VDF-HFP) Nanocomposites for Advanced Lead-Free Radiation Protection
Abstract
1. Introduction
2. Materials and Methods
Materials and Film Preparation
- A.
- Sample characterization
- 1.
- Surface Morphology
- 2.
- Hydrophobicity
- 3.
- Crystal Structures
- 4.
- Mechanical Property
- 5.
- Thermal Stability
- 6.
- Absorption performance
3. Results and Discussion
3.1. Surface Morphology
3.1.1. SEM Analysis
3.1.2. AFM Analysis
3.2. Hydrophobicity
3.3. Crystal Structure
3.3.1. FTIR Analysis
3.3.2. XRD Analysis
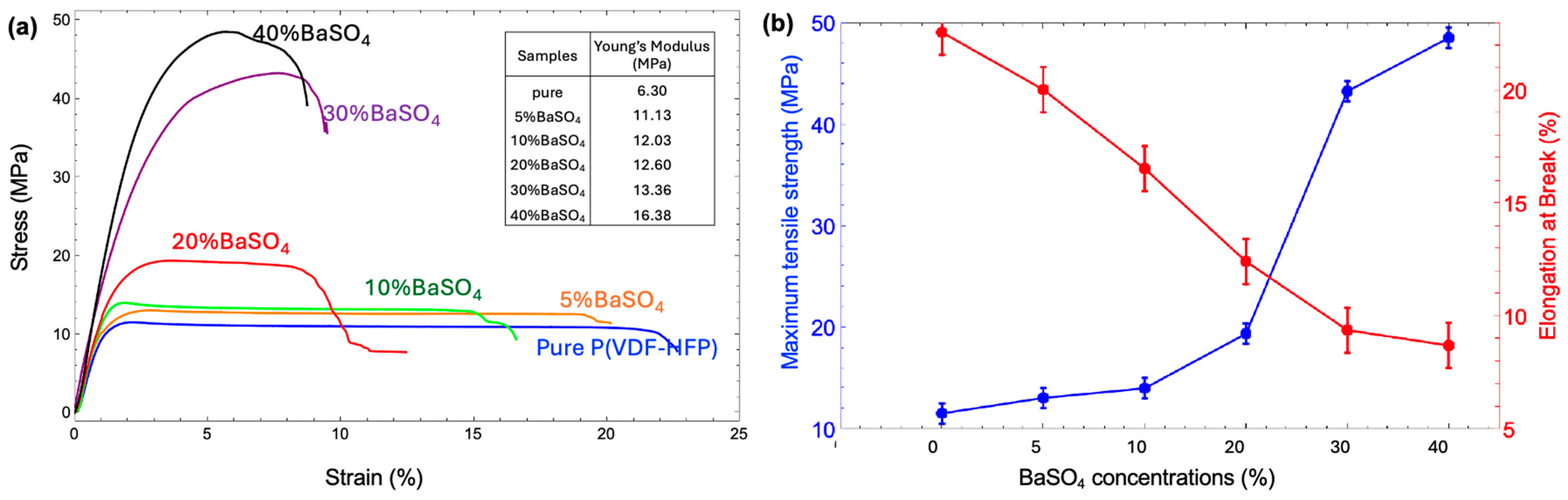
3.4. Mechanical Property
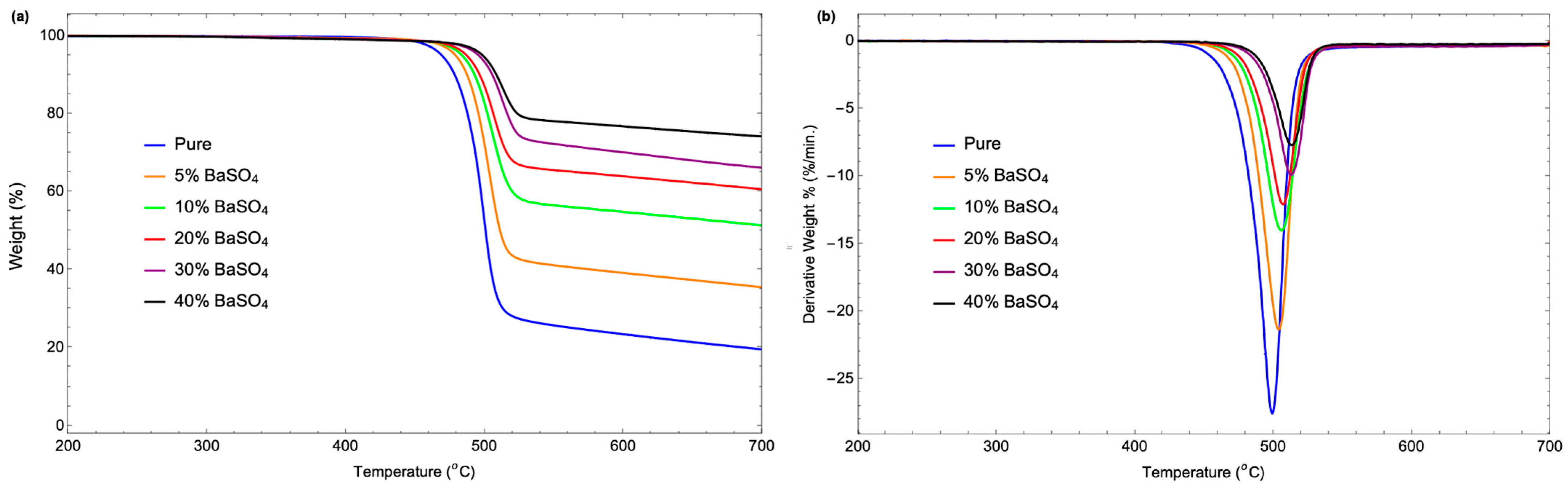
3.5. Thermal Stability
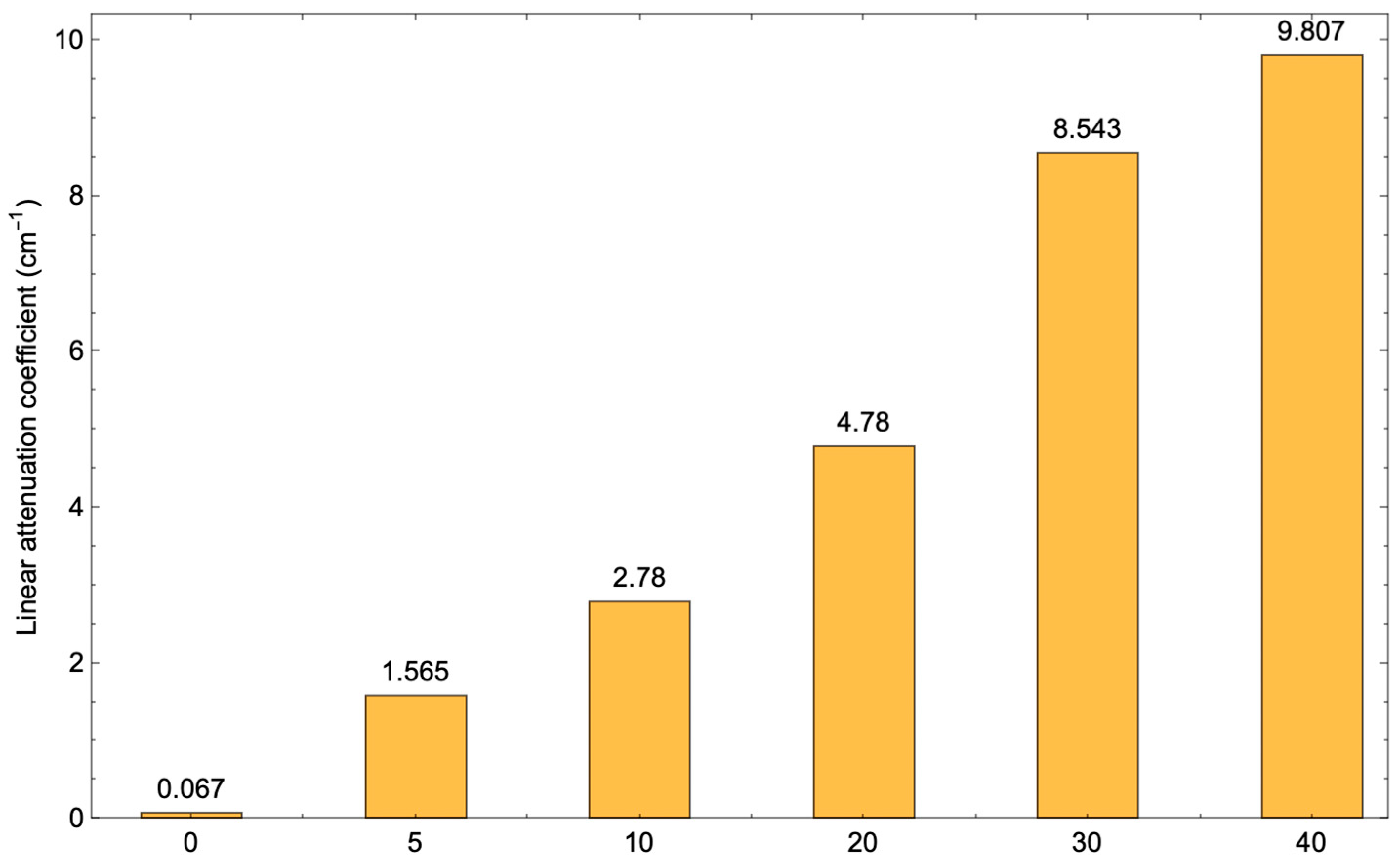
3.6. Absorption Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghamdi, A.; Alsharari, Z.; Almatari, M.; Alkhalailah, M.; Alamri, S.; Alghamdi, A.; Ghuwayr, M.; Alabthani, I. Radiation Risk Awareness Among Health Care Professionals: An Online Survey. J. Radiol. Nurs. 2020, 39, 132–138. [Google Scholar] [CrossRef]
- Miller, D.; Schauer, D. The ALARA principle in medical imaging. AAPM Newsl. 2015, 40, 38–40. [Google Scholar]
- Mortazavi, S.; Bevelacqua, J.J.; Rafiepour, P.; Sina, S.; Moradgholi, J.; Mortazavi, A.; Welsh, J.S. Lead-free, multilayered, and nanosized radiation shields in medical applications, industrial, and space research. In Advanced Radiation Shielding Materials; Elsevier: Amsterdam, The Netherlands, 2024; pp. 305–322. [Google Scholar]
- Singh, A.K.; Singh, R.K.; Sharma, B.; Tyagi, A.K. Characterization and biocompatibility studies of lead free X-ray shielding polymer composite for healthcare application. Radiat. Phys. Chem. 2017, 138, 9–15. [Google Scholar] [CrossRef]
- Yu, L.; Yap, P.L.; Santos, A.; Tran, D.; Losic, D. Lightweight polyester fabric with elastomeric bismuth titanate composite for high-performing lead-free X-ray shielding. Radiat. Phys. Chem. 2022, 205, 110726. [Google Scholar] [CrossRef]
- Palanisami, S.; Jayachandran, V.; Kalpana, G.; Elango, M.; Godlaveeti, S.K.; Sangaraju, S.; Tawfeek, A.M. Lead-free X-Ray shielding aprons using Zn-doped SnO2 epoxy nanocomposite: A promising alternative to traditional heavy and lead-based materials. Opt. Mater. 2023, 145, 114496. [Google Scholar] [CrossRef]
- Jayakumar, S.; Saravanan, T.; Philip, J. A review on polymer nanocomposites as lead-free materials for diagnostic X-ray shielding: Recent advances, challenges and future perspectives. Hybrid Adv. 2023, 4, 100100. [Google Scholar] [CrossRef]
- Alsaab, A.H.; Zeghib, S. Analysis of X-ray and gamma ray shielding performance of prepared polymer micro-composites. J. Radiat. Res. Appl. Sci. 2023, 16, 100708. [Google Scholar] [CrossRef]
- Kim, S.-C. Construction of a Medical Radiation-Shielding Environment by Analyzing the Weaving Characteristics and Shielding Performance of Shielding Fibers Using X-ray-Impermeable Materials. Appl. Sci. 2021, 11, 1705. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wu, J.; Hu, J.; Mou, J.; Li, L.; Feng, Y.; Deng, Z. Preparation of eGaIn NDs/TPU Composites for X-ray Radiation Shielding Based on Electrostatic Spinning Technology. Materials 2024, 17, 272. [Google Scholar] [CrossRef]
- Bawazeer, O.; Baatiyah, B.; Al Amoudi, S.; Aga, Z.; Matar, Z.; Khan, S.; Al-Qahtani, S.; Algethami, M.; Adil, S.F.; Alomari, A.; et al. Evaluation of X-ray radiation shielding performance of Bi2O3 and BaTiO3 embedded in PVP and PEG polymer nanocomposite. Radiat. Eff. Defects Solids 2024, 1–13. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Neoh, K.G.; Shi, Z.; Kang, E.T. Antibacterial activities of surface modified electrospun poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) fibrous membranes. Appl. Surf. Sci. 2009, 255, 3854–3858. [Google Scholar] [CrossRef]
- Kazemi, D.; Yaftian, M.R. PVDF-HFP-based polymer inclusion membrane functionalized with D2EHPA for the selective extraction of bismuth (III) from sulfate media. Sci. Rep. 2024, 14, 11622. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.; Tay, J.-H. Effect of additives on the fabrication of poly(vinylidene fluoride- co-hexafluropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2008, 315, 195–204. [Google Scholar] [CrossRef]
- Toh, M.J.; Oh, P.C.; Shaufi, M.I.S.M. Preparation of Highly Hydrophobic PVDF-HFP Membrane with Anti-Wettability Characteristic. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012176. [Google Scholar] [CrossRef]
- Gharissah, M.S.; Ardiansyah, A.; Pauziah, S.R.; Muhammad, N.A.; Rahmat, R.; Heryanto, H.; Tahir, D. Composites cement/BaSO4/Fe3O4/CuO for improving X-ray absorption characteristics and structural properties. Sci. Rep. 2022, 12, 19169. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Shi, J.; Shi, H.; Liu, Y. Effect of Barium Sulfate Nanoparticles on Mechanical Properties and Crystallization Behaviour of HDPE. Polym. Polym. Compos. 2010, 18, 145–152. [Google Scholar] [CrossRef]
- Maghrabi, H.A.; Vijayan, A.; Mohaddes, F.; Deb, P.; Wang, L. Evaluation of X-ray radiation shielding performance of barium sulphate-coated fabrics. Fibers Polym. 2016, 17, 2047–2054. [Google Scholar] [CrossRef]
- Agarwal, H.; Yadav, S.; Jaiswar, G. Effect of nanoclay and barium sulfate nanoparticles on the thermal and morphological properties of polyvinylidene fluoride nanocomposites. J. Therm. Anal. Calorim. 2017, 129, 1471–1479. [Google Scholar] [CrossRef]
- Silva, L.A.; Batista, A.M.S.; Serodre, T.; Neto, A.T.B.; Furtado, C.A.; Faria, L.O. Enhancement of X-ray Shielding Properties of PVDF/BaSO4 Nanocomposites Filled with Graphene Oxide. MRS Adv. 2019, 4, 169–175. [Google Scholar] [CrossRef]
- Banerjee, S. Simple derivation of Young, Wenzel and Cassie-Baxter equations and its interpretations. arXiv 2008, arXiv:0808.146. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Yuennan, J.; Tohluebaji, N.; Putson, C.; Muensit, N.; Channuie, P. Enhanced electroactive β-phase and dielectric properties in P(VDF-HFP) composite flexible films through doping with three calcium chloride salts: CaCl2, CaCl2·2H2O, and CaCl2·6H2O. Polym. Adv. Technol. 2024, 35, e6437. [Google Scholar] [CrossRef]
- Selvakumar, K.; Manimuthu, R. Investigation on meta-polybenzimidazole blend with sulfonated PVdF-HFP proton conducting polymer electrolytes for HT-PEM fuel cell application. J. Mater. Sci. Mater. Electron. 2018, 29, 15163–15173. [Google Scholar] [CrossRef]
- ISO 37-2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Li, H.; Lim, S. Boosting Performance of Self-Polarized Fully Printed Piezoelectric Nanogenerators via Modulated Strength of Hydrogen Bonding Interactions. Nanomaterials 2021, 11, 1908. [Google Scholar] [CrossRef]
- Mälzer, T.; Mathies, L.; Band, T.; Gorgas, R.; Leipner, H.S. Influence of Different Solvents and High-Electric-Field Cycling on Morphology and Ferroelectric Behavior of Poly(Vinylidene Fluoride-Hexafluoropropylene) Films. Materials 2021, 14, 3884. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, H. Femtosecond laser processed superhydrophobic surface. J. Manuf. Process. 2024, 109, 250–287. [Google Scholar] [CrossRef]
- Souza, P.S.; Santos, A.J.; Cotrim, M.A.P.; Abrão, A.M.; Câmara, M.A. Analysis of the surface energy interactions in the tribological behavior of ALCrN and TIAlN coatings. Tribol. Int. 2020, 146, 106206. [Google Scholar] [CrossRef]
- Bala, H.; Fu, W.; Guo, Y.; Zhao, J.; Jiang, Y.; Ding, X.; Yu, K.; Li, M.; Wang, Z. In situ preparation and surface modification of barium sulfate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 71–76. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Shi, L.; Li, J.; Guo, Z. Advances in the theory of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 20112–20127. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Vibrational spectrum of PVDF and its interpretation. Polym. Test. 2004, 23, 791–796. [Google Scholar] [CrossRef]
- Ramesh, D. One-step fabrication of biomimetic PVDF-BaTiO3 nanofibrous composite using DoE. Mater. Res. Express 2018, 5, 085308. [Google Scholar] [CrossRef]
- Siva, D.; Shakthi, T.; Hemalatha, J. Synthesis and ferroelectric investigations of poly(vinylidene fluoride-co-hexafluoropropylene)-Mg(NO3)2films. J. Appl. Polym. Sci. 2016, 133, 44008. [Google Scholar] [CrossRef]
- Sifontes, Á.B.; Cañizales, E.; Toro-Mendoza, J.; Ávila, E.; Hernández, P.; Delgado, B.A.; Gutiérrez, G.B.; Díaz, Y.; Cruz-Barrios, E. Obtaining Highly Crystalline Barium Sulphate Nanoparticles via Chemical Precipitation and Quenching in Absence of Polymer Stabilizers. J. Nanomater. 2015, 2015, 510376. [Google Scholar] [CrossRef]
- Staicu, L.; Bajda, T.; Drewniak, L.; Charlet, L. Power Generation: Feedstock for High-Value Sulfate Minerals. Minerals 2020, 10, 188. [Google Scholar] [CrossRef]
- Li, D.; Liao, M. Study on the dehydrofluorination of vinylidene fluoride (VDF) and hexafluoropropylene (HFP) copolymer. Polym. Degrad. Stab. 2018, 152, 116–125. [Google Scholar] [CrossRef]
- Mohammed, A.; Salman, S.; Noori, F.M. Preparation and Characterizations of Poly (vinylidene fluoride)(PVDF)/Ba0.6Sr0.4TiO3(BST) Nanocomposites. Int. J. Appl. Eng. Res. 2018, 13, 5008–5013. [Google Scholar]
- Chang, Q.; Guo, S.; Zhang, X. Radiation shielding polymer composites: Ray-interaction mechanism, structural design, manufacture and biomedical applications. Mater. Des. 2023, 233, 112253. [Google Scholar] [CrossRef]
- Baeyens, A.; Abrantes, A.M.; Ahire, V.; Ainsbury, E.A.; Baatout, S.; Baselet, B.; Botelho, M.F.; Boterberg, T.; Chevalier, F.; Da Pieve, F.; et al. Basic Concepts of Radiation Biology. In Radiobiology Textbook; Baatout, S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 25–81. [Google Scholar]
- Jaiyen, S.; Phunpueok, A.; Thongpool, V. Determination of radiation attenuation coefficients of BaSO4/PVC and BaSO4/PS for X-ray shielding. J. Phys. Conf. Ser. 2019, 1380, 012133. [Google Scholar] [CrossRef]

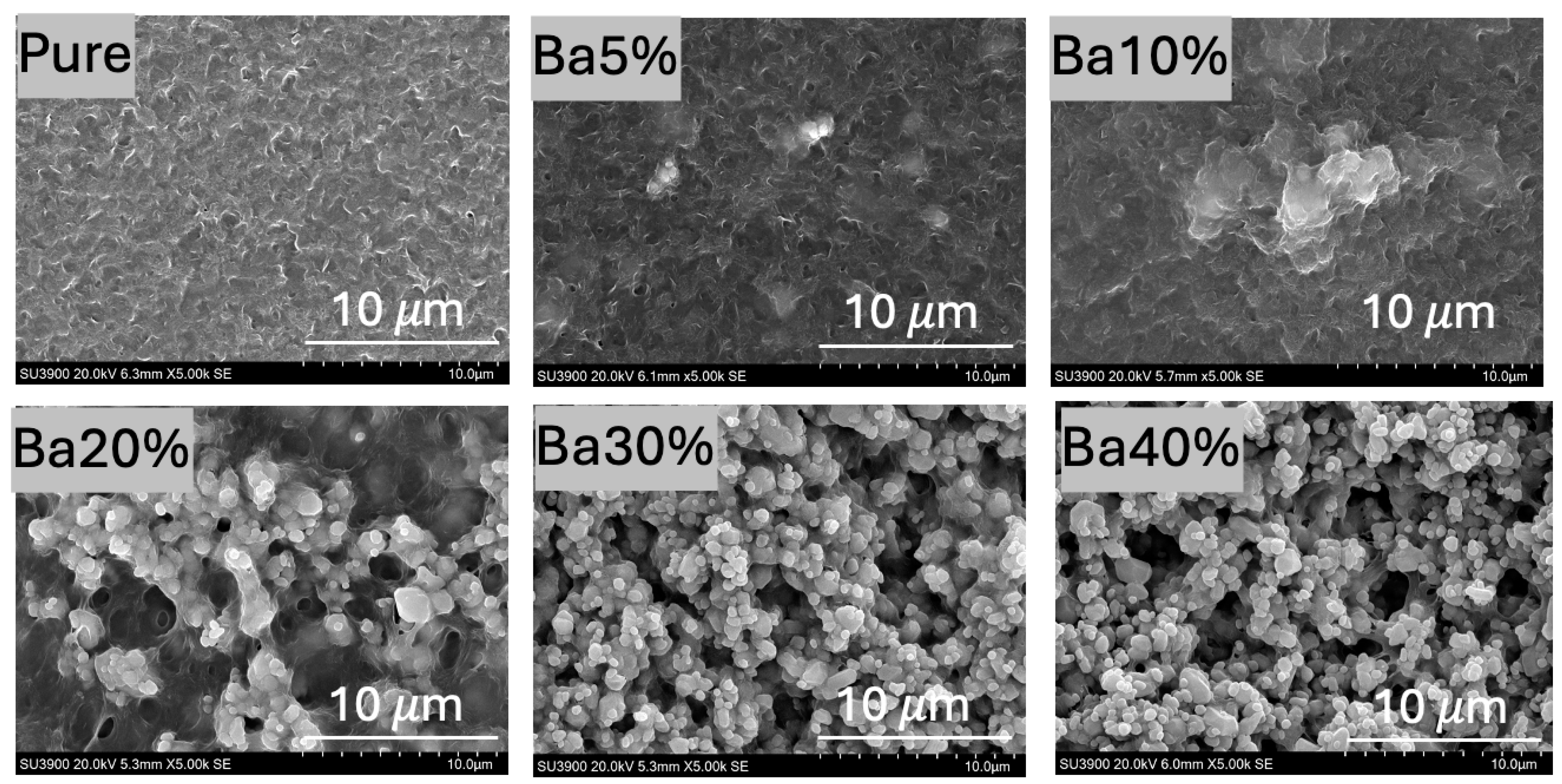

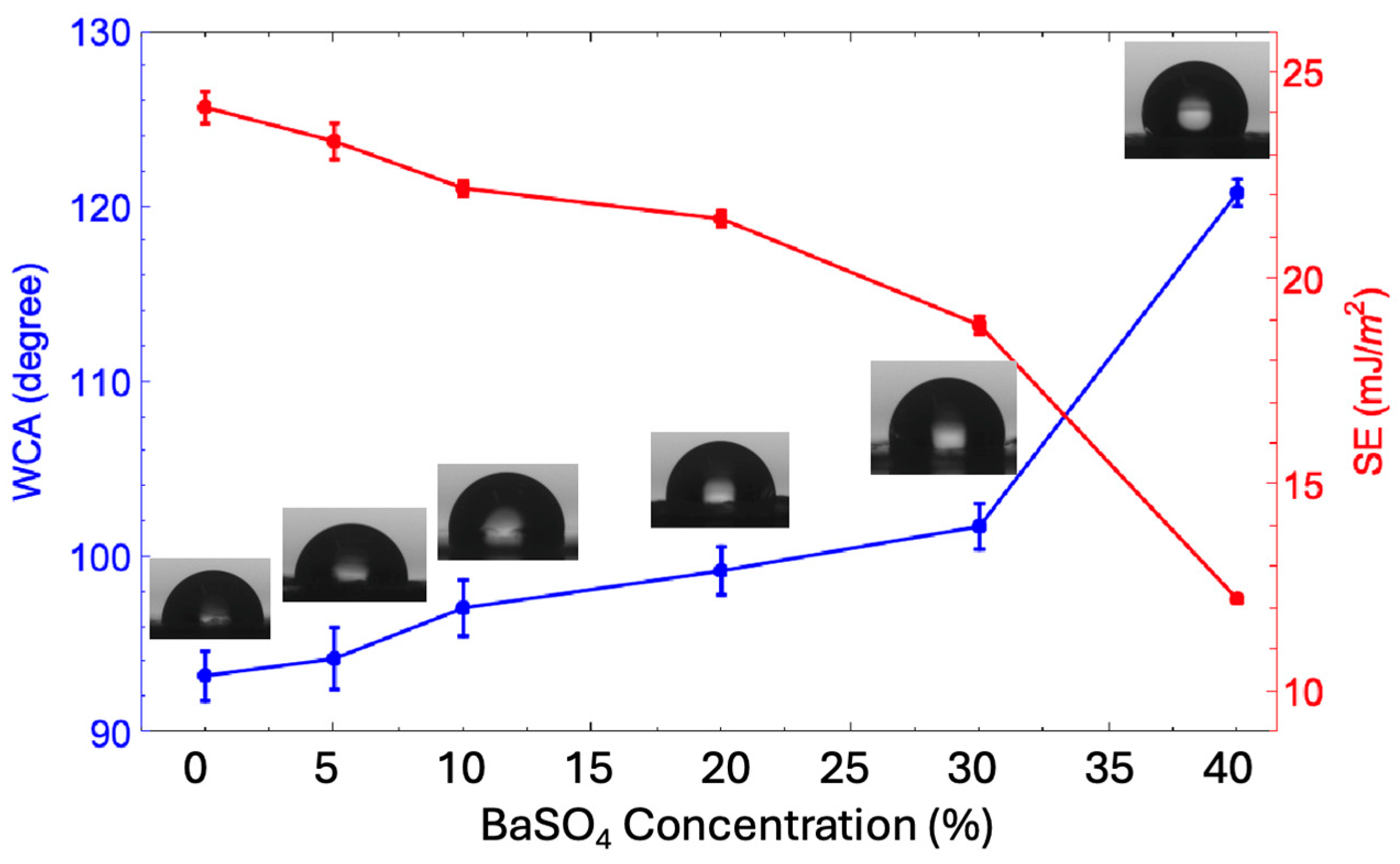
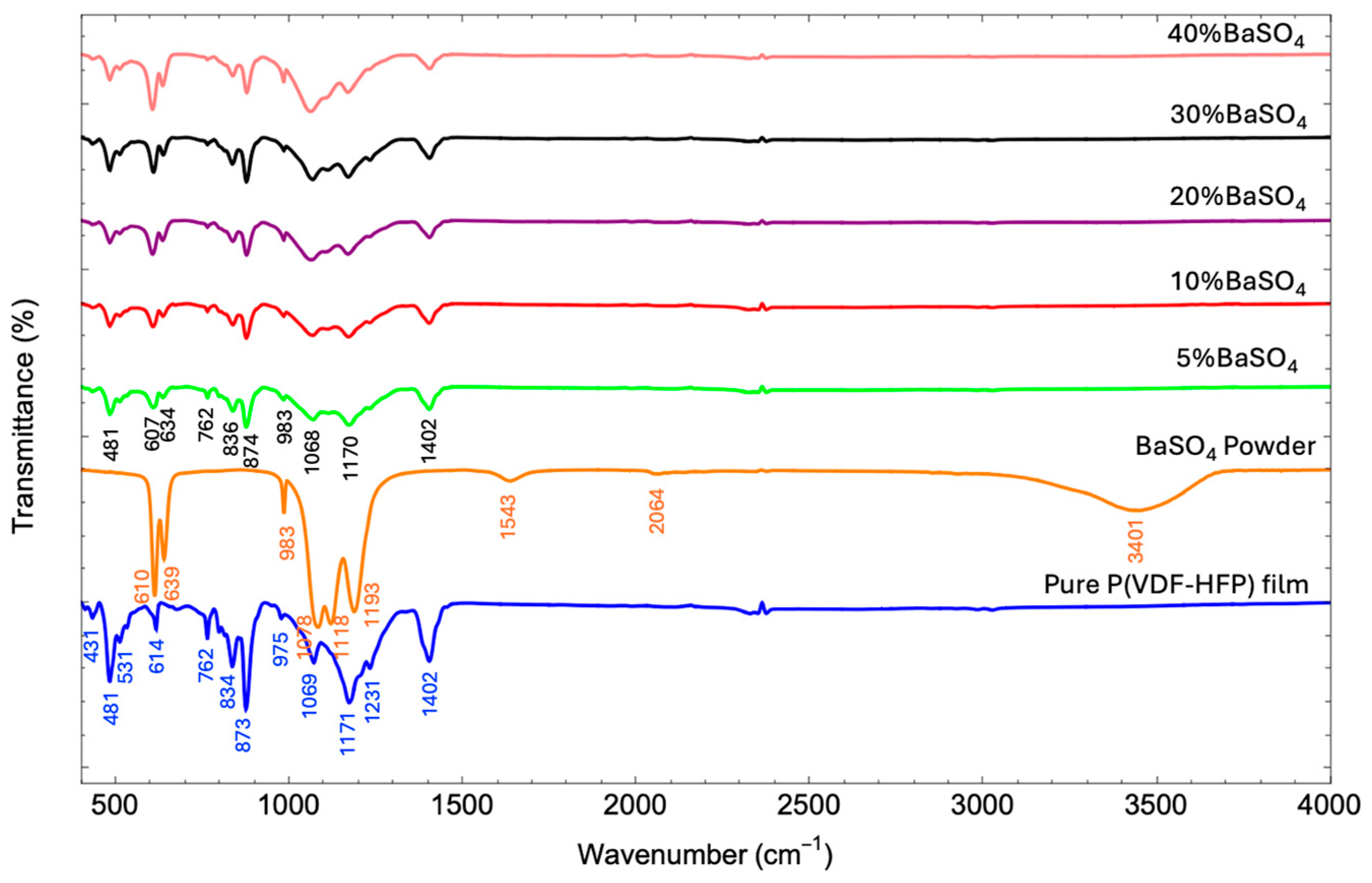
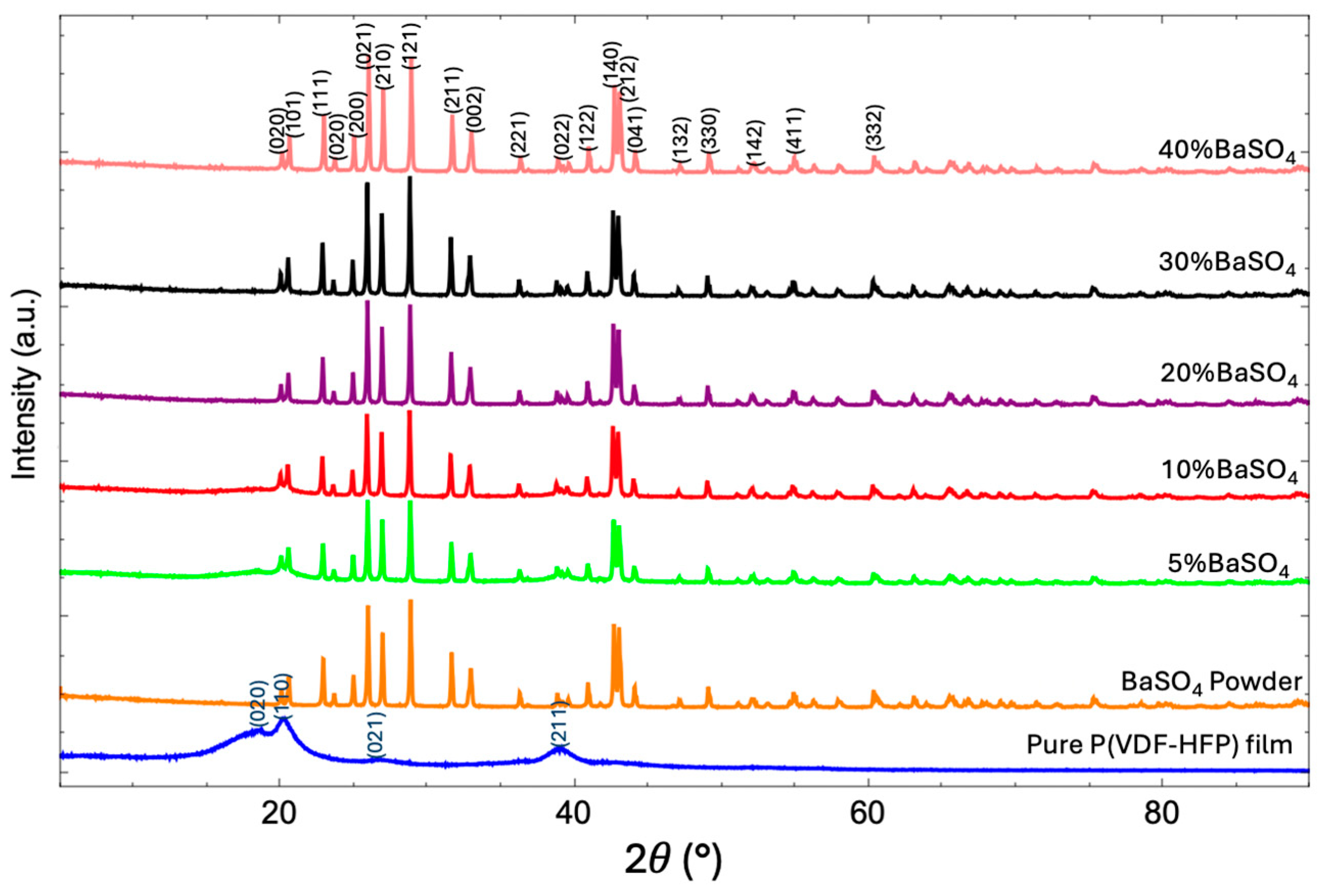
| Sample | Porosity (%) | Root Means Square Roughness (Rq) (nm) | Crystallinity (Xc) (%) |
|---|---|---|---|
| Pure | 0.30 | 66.57 | 60.10 |
| 5% BaSO4 | 1.60 | 138.93 | 71.24 |
| 10% BaSO4 | 4.67 | 139.96 | 73.81 |
| 20% BaSO4 | 15.05 | 180.66 | 79.00 |
| 30% BaSO4 | 20.40 | 237.45 | 80.44 |
| 40% BaSO4 | 25.21 | 590.32 | 83.53 |
| Sample | TGA Analysis Results | DTG Analysis Results | ||||
|---|---|---|---|---|---|---|
| Decomposition Temperature (°C) | Weight Loss Rate (%/°C) | Residual Mass (%) | Peak Decomposition Temperature (°C) | Maximum Weight Loss Rate (%/min) | Residual Mass (%) | |
| pure | 468.87 | 0.55 | 19.58 | 499.09 | 27.55 | −0.06 |
| 5% BaSO4 | 478.78 | 0.46 | 35.50 | 503.72 | 21.36 | −0.07 |
| 10% BaSO4 | 483.38 | 0.34 | 51.34 | 505.7 | 14.03 | −0.28 |
| 20% BaSO4 | 486.69 | 0.27 | 60.60 | 506.86 | 12.10 | −0.16 |
| 30% BaSO4 | 495.29 | 0.24 | 66.15 | 512.65 | 9.93 | −0.19 |
| 40% BaSO4 | 498.26 | 0.18 | 74.11 | 513.15 | 7.73 | −0.23 |
| BaSO4 (%) | Thickness (cm) | |||||
|---|---|---|---|---|---|---|
| 0.02 | 0.04 | 0.06 | 0.08 | 0.10 | 0.12 | |
| 0 | 211,070 | 210,862 | 209,526 | 209,749 | 210,277 | 209,406 |
| 5 | 207,830 | 200,578 | 194,822 | 188,871 | 181,691 | 178,249 |
| 10 | 200,517 | 192,060 | 180,053 | 170,326 | 160,278 | 153,130 |
| 20 | 187,772 | 170,768 | 155,874 | 144,114 | 128,009 | 116,125 |
| 30 | 169,853 | 133,643 | 111,235 | 95,902 | 81,458 | 71,207 |
| 40 | 163,051 | 126,983 | 105,969 | 87,571 | 72,480 | 60,077 |
| Thickness (cm) | Attenuation (%) | ||||
|---|---|---|---|---|---|
| 5%BaSO4 | 10%BaSO4 | 20%BaSO4 | 30%BaSO4 | 40%BaSO4 | |
| 0.02 | 1.48 | 4.95 | 10.99 | 19.48 | 22.71 |
| 0.04 | 4.92 | 8.96 | 19.05 | 36.65 | 39.81 |
| 0.06 | 7.65 | 14.65 | 26.11 | 47.27 | 49.77 |
| 0.08 | 10.47 | 19.26 | 31.68 | 54.54 | 58.49 |
| 0.10 | 13.87 | 24.02 | 39.32 | 61.39 | 65.64 |
| 0.12 | 15.50 | 27.41 | 44.95 | 66.25 | 71.52 |
| X-Ray Energy (keV) | Attenuation (%) of the 0.2 mm Thickness | ||||
|---|---|---|---|---|---|
| 5%BaSO4 | 10%BaSO4 | 20%BaSO4 | 30%BaSO4 | 40%BaSO4 | |
| 60 | 1.48 | 4.95 | 10.99 | 19.48 | 22.71 |
| 80 | 2.28 | 2.12 | 7.33 | 10.96 | 13.95 |
| 100 | 0.04 | 1.20 | 3.74 | 6.29 | 7.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaew-on, C.; Yuennan, J.; Tohluebaji, N.; Channuie, P.; Ruangdit, S.; Samran, R.; Tochomphoo, T.; Siri, R. Enhanced Hydrophobicity, Thermal Stability, and X-Ray Shielding Efficiency of BaSO4/P(VDF-HFP) Nanocomposites for Advanced Lead-Free Radiation Protection. Polymers 2025, 17, 723. https://doi.org/10.3390/polym17060723
Kaew-on C, Yuennan J, Tohluebaji N, Channuie P, Ruangdit S, Samran R, Tochomphoo T, Siri R. Enhanced Hydrophobicity, Thermal Stability, and X-Ray Shielding Efficiency of BaSO4/P(VDF-HFP) Nanocomposites for Advanced Lead-Free Radiation Protection. Polymers. 2025; 17(6):723. https://doi.org/10.3390/polym17060723
Chicago/Turabian StyleKaew-on, Chaiporn, Jureeporn Yuennan, Nikruesong Tohluebaji, Phongpichit Channuie, Soraya Ruangdit, Ritiron Samran, Thanaphorn Tochomphoo, and Ratchanewan Siri. 2025. "Enhanced Hydrophobicity, Thermal Stability, and X-Ray Shielding Efficiency of BaSO4/P(VDF-HFP) Nanocomposites for Advanced Lead-Free Radiation Protection" Polymers 17, no. 6: 723. https://doi.org/10.3390/polym17060723
APA StyleKaew-on, C., Yuennan, J., Tohluebaji, N., Channuie, P., Ruangdit, S., Samran, R., Tochomphoo, T., & Siri, R. (2025). Enhanced Hydrophobicity, Thermal Stability, and X-Ray Shielding Efficiency of BaSO4/P(VDF-HFP) Nanocomposites for Advanced Lead-Free Radiation Protection. Polymers, 17(6), 723. https://doi.org/10.3390/polym17060723





