1. Introduction
Fumed silica is a nanoparticle material characterized by its high specific surface area and numerous reactive hydroxyl groups on the surface. Through interactions with rubber matrices such as natural rubber (NR) and styrene-butadiene rubber (SBR), fumed silica establishes significant physical and chemical bonds that enhance the strength and elastic modulus of rubber [
1,
2,
3].
The specific surface area of fumed silica ranges from 200 to 500 m
2/g, with particle sizes typically between 10 and 40 nm. Smaller particle sizes lead to larger specific surface areas, increasing contact with rubber matrices and providing enhanced reinforcement [
4]. When dispersed in rubber, fumed silica boosts the material’s elastic modulus, improving resistance to deformation. The rigid nanoparticles dissipate stresses during deformation through inter-particle friction, reducing direct matrix damage. Furthermore, the nanoparticles endure a portion of the frictional forces during wear, improving the abrasion resistance and longevity of rubber products [
5,
6,
7].
In addition to reinforcement, the interfacial interactions between fumed silica and rubber molecules generate physical constraints. These interactions form numerous rubber–filler interfaces, restricting the mobility of rubber chains and enhancing tensile strength and wear resistance [
8]. The high modulus of fumed silica particles helps distribute and absorb external stresses, reducing localized stress concentrations and preventing premature failure of the rubber material [
9,
10].
At higher filler loadings, silica particles form a three-dimensional (3D) network through physical contacts and van der Waals forces [
11,
12,
13]. This structure increases material rigidity, modulates particle spacing under dynamic stress, and reduces internal stress concentrations, delaying fatigue failure and improving tear resistance and dimensional stability. Additionally, the internal dissipation of mechanical energy during dynamic deformation enhances damping properties and reduces rolling resistance [
14,
15].
Under specific temperature and pressure conditions, fumed silica filler may induce micro-phase separation within the rubber matrix [
16,
17]. This creates additional crosslinking points or interaction zones, strengthening the rubber matrix and increasing its adhesion and viscosity [
18,
19,
20,
21].
The use of nanosilica as a filler to enhance the properties of rubber materials has been widely studied. However, reinforcement with a single filler has limited the application of rubber composites, as it may not meet the requirements of specific environments. As a result, nanocomposite fillers have attracted attention from researchers, such as silica/carbon black, silica/clay, and silica/carbon nanotube composites. These hybrid fillers, when added to rubber, make the material suitable for more specialized applications, thereby broadening its use across various industries.
Nanocomposites refer to materials in which at least one phase has a one-dimensional size ranging from 1 to 100 nm. The unique properties of nanocomposites, such as their excellent overall performance and specific functionalities, arise from the small size effect of the dispersed phase, their large surface area, as well as interface effects and quantum effects, including macroscopic quantum tunneling.
However, simple mechanical blending alone does not significantly enhance the performance of rubber materials. As a result, the preparation of composite materials has become a focal point in rubber filler research [
22].
Kong et al. [
23] used multi-walled carbon nanotubes and fumed silica as dual-component fillers. Prior to blending, the components were pretreated to create chemical bonds between MWCNTs and SiO
2. This study was the first to modify MWCNTs with methyl diethoxy silane (MDES) and SiO
2 with vinyltriethoxysilane, followed by a platinum-catalyzed hydrosilylation reaction to create the CNTs-b-FSiO
2 binary nanofiller. The results indicated that the CNTs-b-FSiO
2 composite significantly improved the mechanical properties of sulfur rubber, such as tensile strength, tear strength, and Young’s modulus. This enhancement is primarily due to the strong chemical bonding between CNTs and FSiO
2, which leads to a strong interaction, while the TEVS molecules on FSiO
2 improve the interfacial interaction between the composite filler and the SR matrix.
Sahar Ziraki et al. [
24] reinforced high-temperature vulcanized silicone rubber with polypropylene fibers and nanosilica. The results showed that the composite effectively reduced the compression set of the silicone rubber while slightly increasing the rubber’s glass transition temperature (Tg). Cao et al. [
25] prepared a composite material by filling graphene oxide (RGO) and KH550- and Si69-modified nanosilica into natural rubber. The composite effectively enhanced the energy storage modulus and wear resistance of the natural rubber while reducing the loss factor and elongation at break. Adding 1% by weight of this composite to car tires increased the tire’s wear resistance by 44.5%, reduced rolling resistance by 5.1%, and improved wet skid resistance by 14.6%.
D’Arienzo et al. [
26] used polyhedral oligomeric silsesquioxane (POSS) to composite with modified nanosilica, creating POSS@SiO
2 nanocomposites. First, they surface-modified nanosilica with 3-(trimethoxysilyl)propyl methacrylate (TMMS) and grafted varying amounts of POSS onto the surface of nanosilica. Similarly to silane coupling agents, the organic groups of the cage-like POSS structure tightly bound the nanosilica to styrene-butadiene rubber (SBR). During vulcanization, the curing agent dicumyl peroxide (DCP) prevented the acrylate structure in POSS from being degraded, ensuring that the silica nanoparticles remained tightly linked within the POSS nanocage. This improved the interaction between the filler and the rubber matrix. The authors incorporated the POSS@SiO
2 nanocomposite into an SBR matrix, resulting in the POSS@SiO
2/SBR composite. Their research showed that the presence of POSS significantly improved the modulus of the rubber composite under different strain conditions.
1.1. Surface Modification and Interaction Mechanisms
The surface of fumed silica contains numerous silanol (Si–OH) groups, which can chemically bond with rubber molecules to form strong interfacial interactions. To enhance compatibility with hydrophobic rubber matrices, silane coupling agents such as sulfur-containing silane (TESPT) are often used. These agents possess dual functional groups: one reacts with silica’s hydroxyl groups to form covalent bonds, while the other reacts with the unsaturated double bonds in rubber during vulcanization, creating a robust chemical network [
27,
28,
29,
30,
31].
Fumed silica has high surface energy and a strong tendency to agglomerate within the rubber matrix, leading to poor dispersion [
32]. Silane coupling agents modify silica’s surface, reducing its surface energy and minimizing agglomeration. This ensures uniform filler distribution and enhances mechanical and dynamic properties such as tensile strength, tear resistance, and fatigue resistance [
33,
34].
1.2. Industrial Challenges and Solutions
Fumed silica’s extremely high specific surface area and nanometric particle size (10–40 nm) result in significant dusting during handling, negatively affecting industrial production environments [
35,
36]. Direct use as a filler requires prolonged mixing times to achieve uniform dispersion in rubber. To address this, this study introduces a pretreatment process: fumed silica is mixed with deionized water, dried, and ball-milled. This process transforms the material into a viscous gel, reduces its volume, and eliminates dust formation [
16,
37,
38].
Upon contact with water, hydroxyl groups on the silica surface interact with water molecules to form hydrated silica. This hydration enhances surface polarity, improves dispersion within rubber matrices, and reduces the shear modulus difference (ΔG′) between low- and high-strain states [
39]. Consequently, rubber maintains a stable elastic modulus over a wide range of deformations, improving its performance under dynamic loads and complex conditions [
40].
1.3. Experimental Results
This study demonstrates that the hydration–ball milling process significantly reduces production challenges without compromising the mechanical properties of the rubber compound. The vulcanizates showed slight improvements in wet skid performance due to hydration, as water molecules formed hydrogen bonds with silica’s hydroxyl groups, increasing surface polarity and hydrophilicity.
1.4. Applications and Benefits
Fumed silica modified with silane coupling agents serves as an effective reinforcing filler, improving the mechanical strength, thermal stability, and hydrophobicity of rubber composites. Its high electrical resistivity (approximately 10
12 Ω·m) makes it an ideal insulating material for rubber compounds [
41,
42]. Additionally, the inclusion of fumed silica in formulations for industrial rubber products, such as fluororubber, hydrogenated nitrile butadiene rubber (HNBR), and BR/SBR/IR blends, enhances their Payne effect, damping properties, and dynamic performance, striking a balance between rolling resistance and wet skid resistance [
43,
44,
45].
By incorporating hydrated and surface-modified fumed silica, this study provides a practical solution to the challenges of industrial rubber production, ensuring enhanced performance and environmental safety. The pretreatment process of fumed silica is shown in
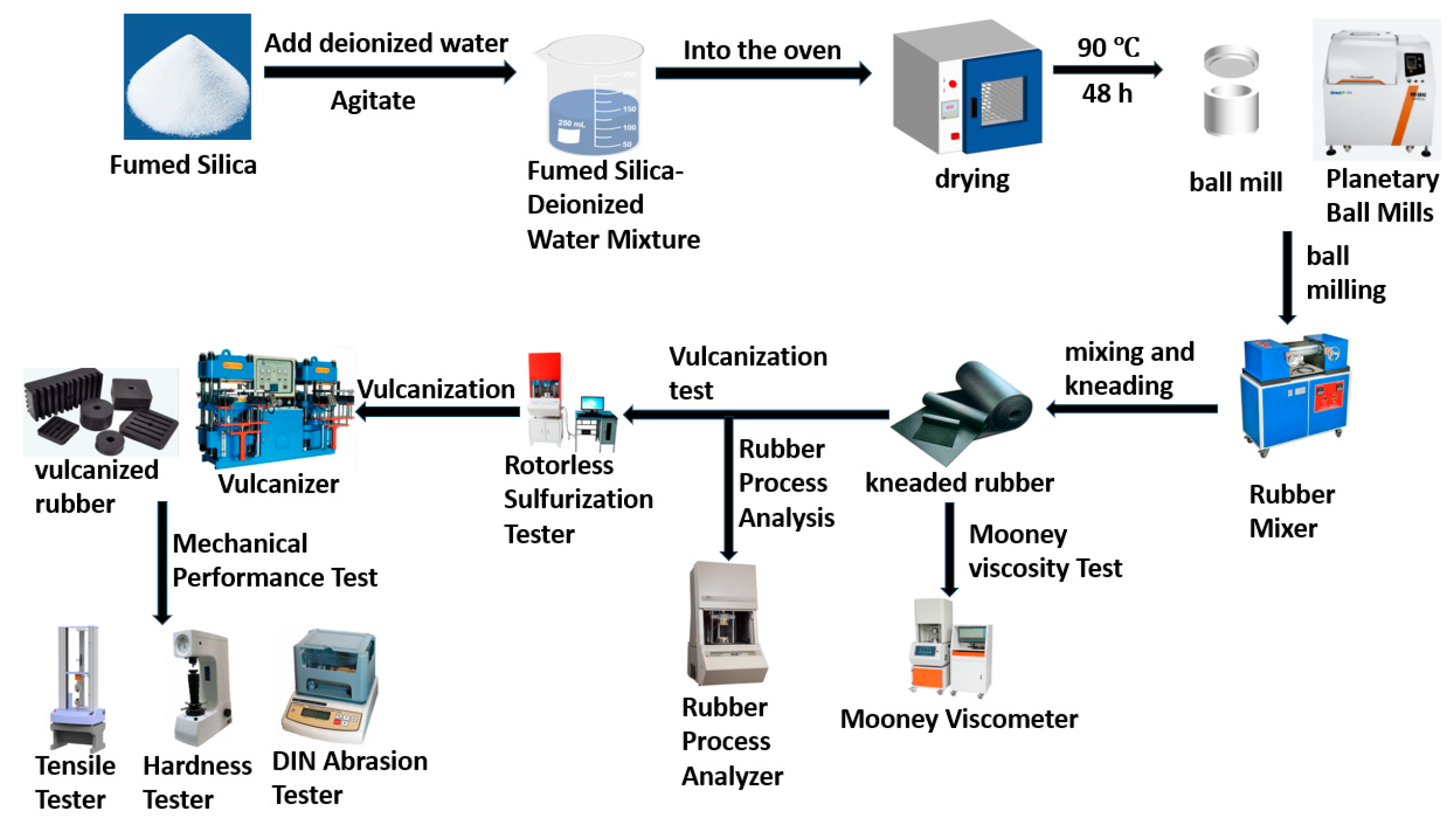
Figure 1 2. Experimental Methods
2.1. Experimental Process and Testing
The experimental workflow is illustrated in
Figure 2.
- (1)
Preparation of Fumed Silica Samples
A beaker containing fumed silica was slowly filled with deionized water at three times the weight of the silica while continuously stirring with a glass rod. The mixture was then placed in an oven set to 90 °C for 48 h. Afterward, the material was removed, and its moisture content was tested. Once the moisture content was below 0.5%, the material was subjected to planetary ball milling for 3 h. The hydrated fumed silica obtained from this process was labeled Sample A and used as the primary filler in the third experimental group of rubber compounds.
- (2)
Preparation of Rubber Compounds
The specified quantities of smoked sheet rubber, fillers (e.g., pyrolytic carbon black or ball-milled Sample A), zinc oxide, and other rubber additives were weighed according to the formulation.
A Banbury mixer was preheated to an initial temperature of 100 °C and set to a rotational speed of 90 rpm.
Natural rubber was added and masticated for 60 s before introducing the filler (pyrolytic carbon black or Sample A), zinc oxide, and other additives.
The mixing continued until the material temperature reached 145 °C, where it was maintained for 90 s before discharging the compound.
The compound was then processed on an open mill, during which the vulcanization system (including HDOT20, accelerator DZ, and CTP) was added. The rubber sheet was folded and rolled three times to achieve uniform dispersion, forming the rubber compound.
- (3)
Preparation of Vulcanized Rubber
The rubber compounds were left to rest for over 8 h before testing. Samples weighing at least 6 g were taken for curing characteristic testing using an MDR-C rotorless rheometer. The vulcanization temperature was set to 150 °C, and key parameters such as optimum cure time were recorded
For vulcanization:
The vulcanization press was set to 150 °C with a pressure of 10 MPa.
Thin products were cured for 1.3 times the T90, while thick products were cured for 2 times the T90.
The rubber compounds were placed in the appropriate molds for vulcanization, and the rolling direction of the compounds was marked.
- (4)
Performance Characterization and Testing
Hardness: Tested following GB/T 531.1-2008 [
46] (ISO 48-5:2018) [
47].
Tensile and Tear Properties: Measured in accordance with GB/T 528-2009 [
48] and GB/T 529-2008 [
49] (ISO 37:2024 [
50] and ISO 34-1:2022 [
51]).
Dynamic Mechanical Properties: Evaluated under the following conditions:
Dynamic stress: 60 N, dynamic strain: 0.25%.
Static stress: 70 N, static strain: 5%.
Temperature range: −65 °C to 65 °C, heating rate: 2 °C/min.
Frequency: 10 Hz.
2.2. Experimental Formulations and Design
2.2.1. Control Group Formulation
The control Group formulation is shown in
Table 1.
2.2.2. Experimental Group 1
In this group, 1165MP silica was used to replace N326 carbon black on an equivalent weight basis. To ensure compatibility between SiO₂ and rubber, an appropriate amount of silane coupling agent Si69 was added. The formulation for Experimental Group 1 is shown in
Table 2.
2.2.3. Experimental Group 2
In this group, fumed silica was used to replace N326 carbon black on an equivalent weight basis. Silane coupling agent Si69 was also added to improve compatibility between SiO₂ and rubber. The formulation for Experimental Group 2 is shown in
Table 3.
2.2.4. Experimental Group 3
In this group, hydrated fumed silica (Sample A) was prepared by mixing fumed silica with deionized water in a 1:3 mass ratio. The mixture was oven-dried at 90 °C for 48 h, and the dried sample was ball-milled for 3 h using a planetary ball mill. The resulting hydrated and ball-milled fumed silica (Sample A) was used to replace N326 carbon black on an equivalent weight basis. Silane coupling agent Si69 was added to ensure compatibility between SiO₂ and rubber. The formulation for Experimental Group 3 is shown in
Table 4.
2.3. Instruments and Equipment
The different pieces of equipment used in this study are listed in
Table 5.
4. Conclusions
This study examined the effects of fumed silica and its hydration treatment on tread rubber performance, focusing on vulcanization characteristics, mechanical properties, the Payne effect, and dynamic mechanical behavior. The results were compared with conventional N326 carbon black and precipitated silica to assess their relative performance.
- 1.
Vulcanization Characteristics
Fumed silica shortens vulcanization times significantly, reducing scorch time (t10) and optimum cure time (t90) by 53% and 56%, respectively, compared to N326 carbon black and precipitated silica. While this enables more efficient production, it also presents processing challenges, such as scorch control. Additionally, fumed silica exhibits the highest crosslink density (MH-ML), leading to increased strength and hardness, which enhances the durability of tread compounds.
Fumed silica and hydrated fumed silica improve hardness, tensile strength, and dynamic performance by accelerating vulcanization and increasing crosslink density. While these benefits enhance rubber durability, they also require precise process control to manage viscosity and prevent scorch. These findings underscore the potential of fumed silica-based compounds for high-performance tire applications that demand both strength and manufacturing efficiency.
- 2.
Mechanical Properties
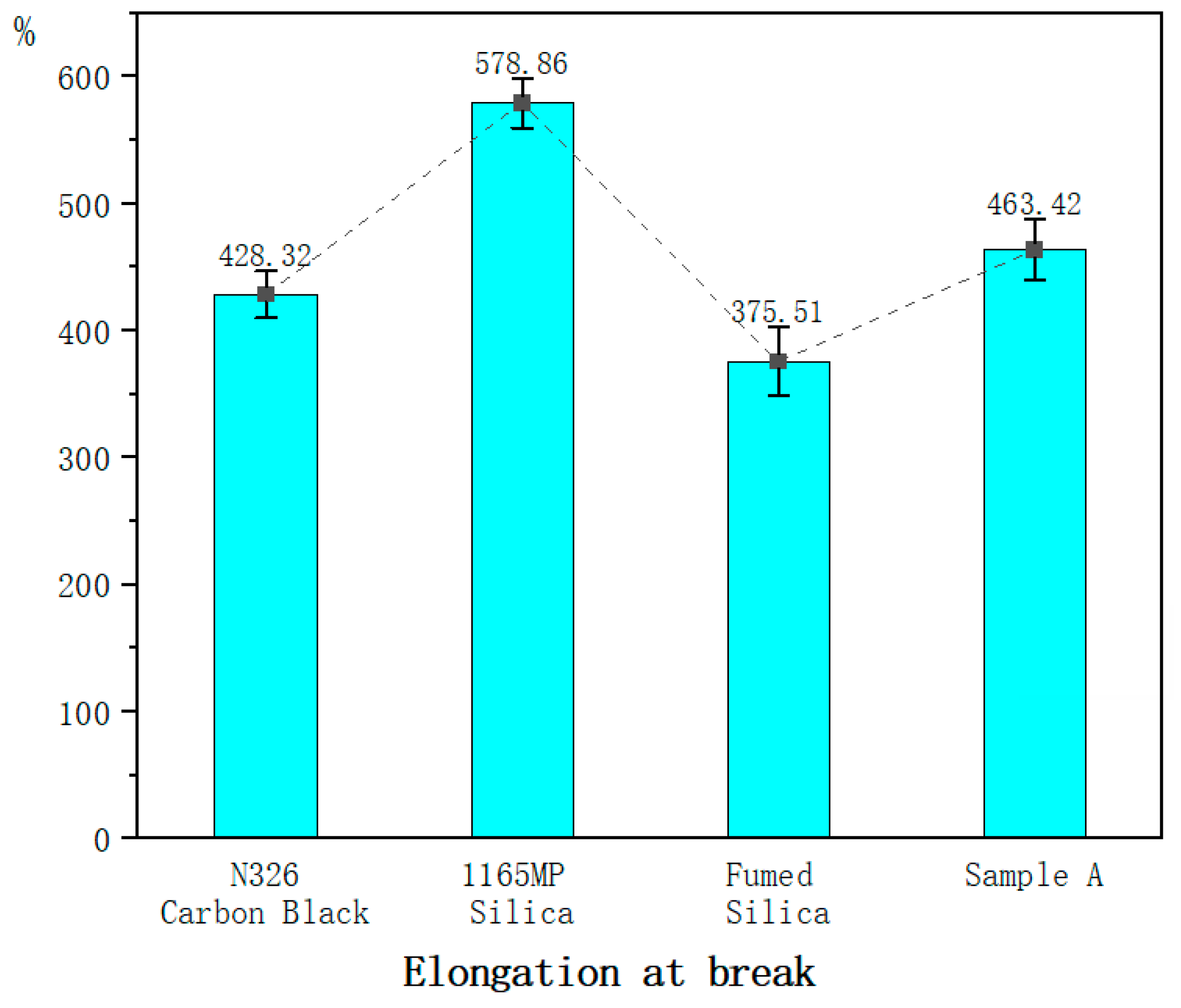
Fumed silica significantly enhances reinforcement, increasing 100% and 300% tensile stresses by 27% and 14%, respectively, while improving hardness by 20%.
Although fumed silica exhibited slightly lower tensile strength than N326 carbon black, hydration treatment enhanced it by 16%, making it comparable to or superior to conventional fillers.
Fumed silica reduced elongation at break by 14%, but the hydration treatment counteracted this effect, increasing elongation by 19% and improving ductility.
Fumed silica and its hydrated form improve hardness and modulus by reinforcing filler–rubber interactions and restricting molecular mobility. Precipitated silica provides moderate tensile strength improvements, while fumed silica’s rigid network slightly reduces tensile strength and elongation at break. Hydration treatment enhances dispersion, reducing stiffness and improving ductility. Although silica-based compounds show higher resilience but lower abrasion resistance than carbon black, the hydration treatment improves wear resistance by optimizing filler dispersion and interfacial bonding, making it a viable approach for enhancing tread rubber performance.
- 3.
Payne Effect
The analysis of the Payne effect highlights the unique characteristics of the fumed silica filler network. Fumed silica exhibited a significantly higher initial storage modulus (G₀′) than N326 carbon black and precipitated silica, indicating a denser and stiffer filler network. However, it also showed a larger storage modulus difference (ΔG′), suggesting that its network is more prone to breakdown and dissipates more energy. The hydration treatment effectively reduced ΔG′, enhancing filler dispersion and improving dynamic performance.
The elevated ΔG′ values of fumed silica indicate poor dispersion and higher energy dissipation, which can negatively affect rolling resistance and fuel efficiency in tires. This behavior is primarily due to strong hydrogen bonding and filler agglomeration. The hydration treatment in Sample A improved silica dispersion, reducing ΔG′ and stabilizing the filler network. This resulted in lower energy loss, enhanced dynamic properties, and better industrial applicability. These findings suggest that optimizing silica dispersion through hydration can improve tread rubber performance by balancing reinforcement with lower rolling resistance and greater durability.
- 4.
Dynamic Mechanical Properties
Fumed silica improved wet skid resistance, indicated by an increased tanδ at 0 °C, and reduced rolling resistance, reflected by a lower tanδ at 60 °C. The hydration treatment further optimized these properties, making it well suited for high-performance applications.
High-performance tires require an optimal balance between damping and energy dissipation. Fumed silica enhances dynamic properties such as wet skid resistance and rolling resistance while also increasing hardness and tensile modulus. However, these enhancements affect energy dissipation and damping, which are crucial for tire performance under dynamic loading.
Incorporating fumed silica in tread rubber improves both damping and energy dissipation. Its high surface area and reinforcement ability strengthen the filler network, enhancing resistance to deformation and stabilizing shear modulus across different strain levels. This ensures consistent dynamic performance under varying loads, improving grip and ride comfort. Additionally, improved dispersion reduces rolling resistance, leading to better fuel efficiency, an essential factor in high-performance tires.
The hydration and ball-milling process developed in this study enhanced the dispersion of fumed silica in the rubber matrix, ensuring more uniform energy dissipation across the tire surface. This uniformity minimizes localized wear, maintains performance consistency over time, and optimizes the tire’s dynamic response for improved handling and comfort. The combined effects of reinforcement and controlled energy dissipation significantly enhance the performance of high-performance tires, where both handling precision and durability are critical.
Overall Findings
Fumed silica is a high-performance reinforcing filler that significantly enhances the hardness, tensile modulus, and dynamic performance of tread compounds while improving wet skid resistance and reducing rolling resistance. However, its shorter vulcanization times and higher dynamic losses may require more precise processing control. The hydration treatment of fumed silica effectively improves its dispersion and dynamic performance, making it more suitable for applications demanding a balance of mechanical and dynamic properties. These results highlight the potential of hydrated fumed silica for use in high-performance tires and other advanced rubber products.