Development of Gelatin-Based Renewable Packaging with Melaleuca alternifolia Essential Oil for Chicken Breast Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antimicrobial Activity
2.2.1. Halo Test
2.2.2. Minimum Inhibitory Concentration (MIC)
2.3. Antioxidant Activity
2.3.1. FRAP
2.3.2. ABTS
2.4. Oil Characterization
2.5. Film Preparation
2.6. Film Characterization
2.6.1. Water Vapor Permeability
2.6.2. Solubility
2.6.3. Mechanical Proprieties
2.7. Packaging of Chicken Breast
2.7.1. Color Parameters
2.7.2. pH
2.7.3. Texture Analysis
2.7.4. Mass Loss
2.7.5. Antimicrobial
2.8. Statistical Analysis
3. Results
3.1. Antimicrobial Activity of Melaleuca alternifolia Essential Oil
3.2. Antioxidant Activity of Melaleuca alternifolia Essential Oil
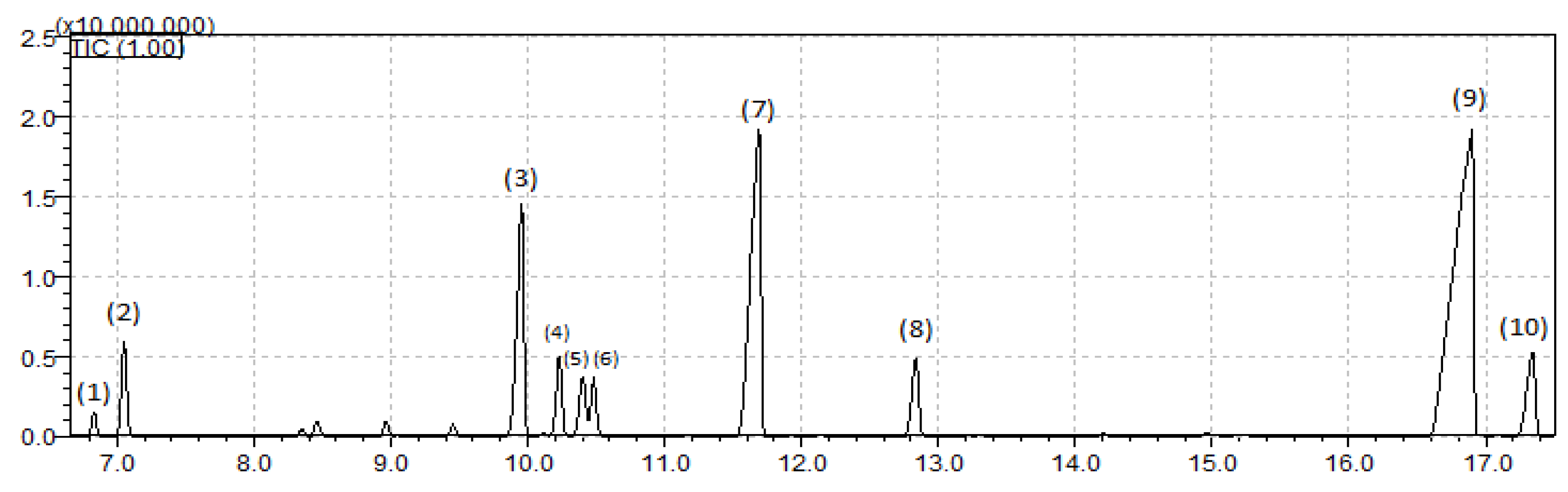
3.3. Oil Characterization
3.4. Characterization of Packaging Films
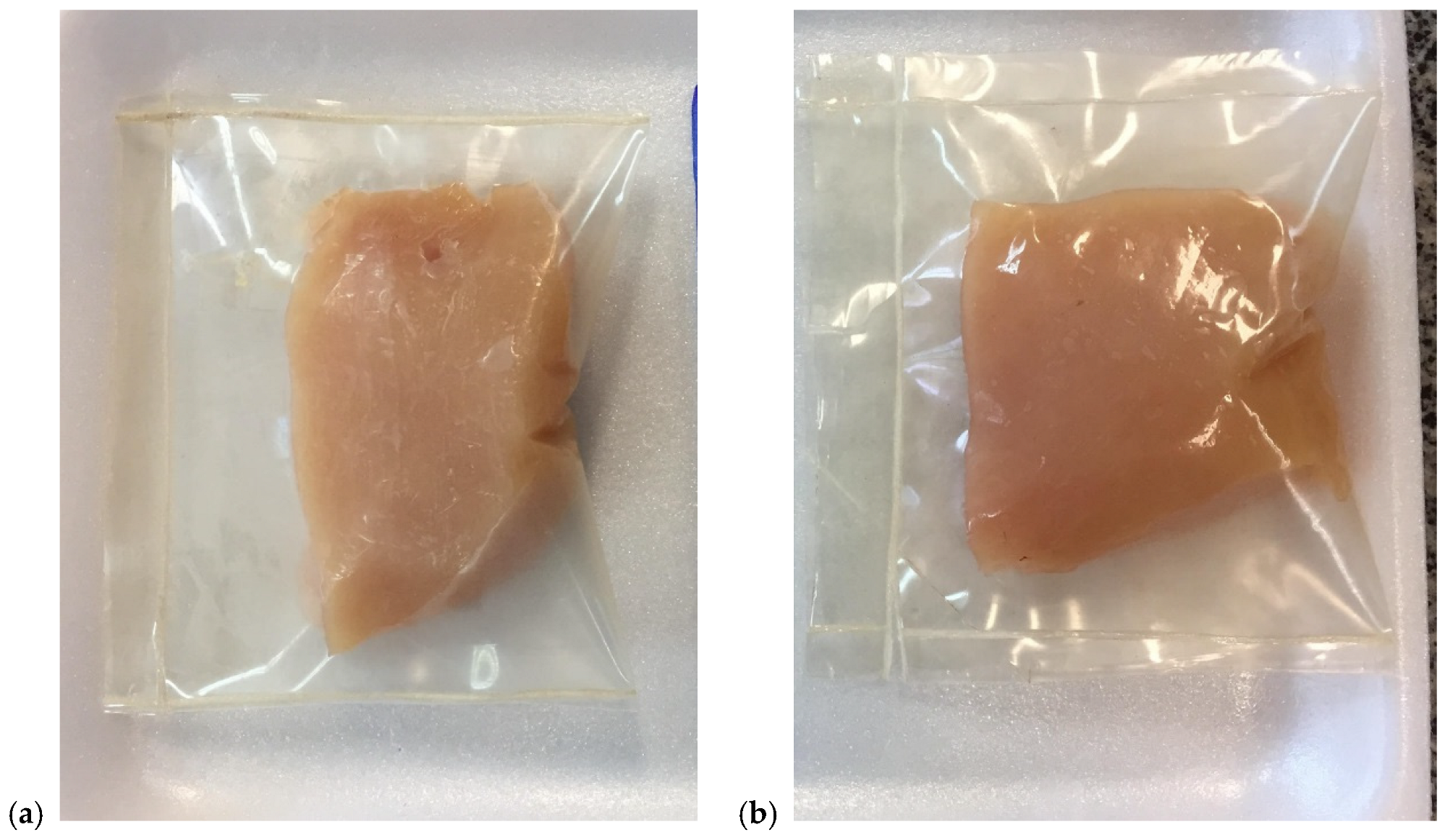
3.4.1. Visual Assessment
3.4.2. Water Vapor Permeability (WVP) and Solubility
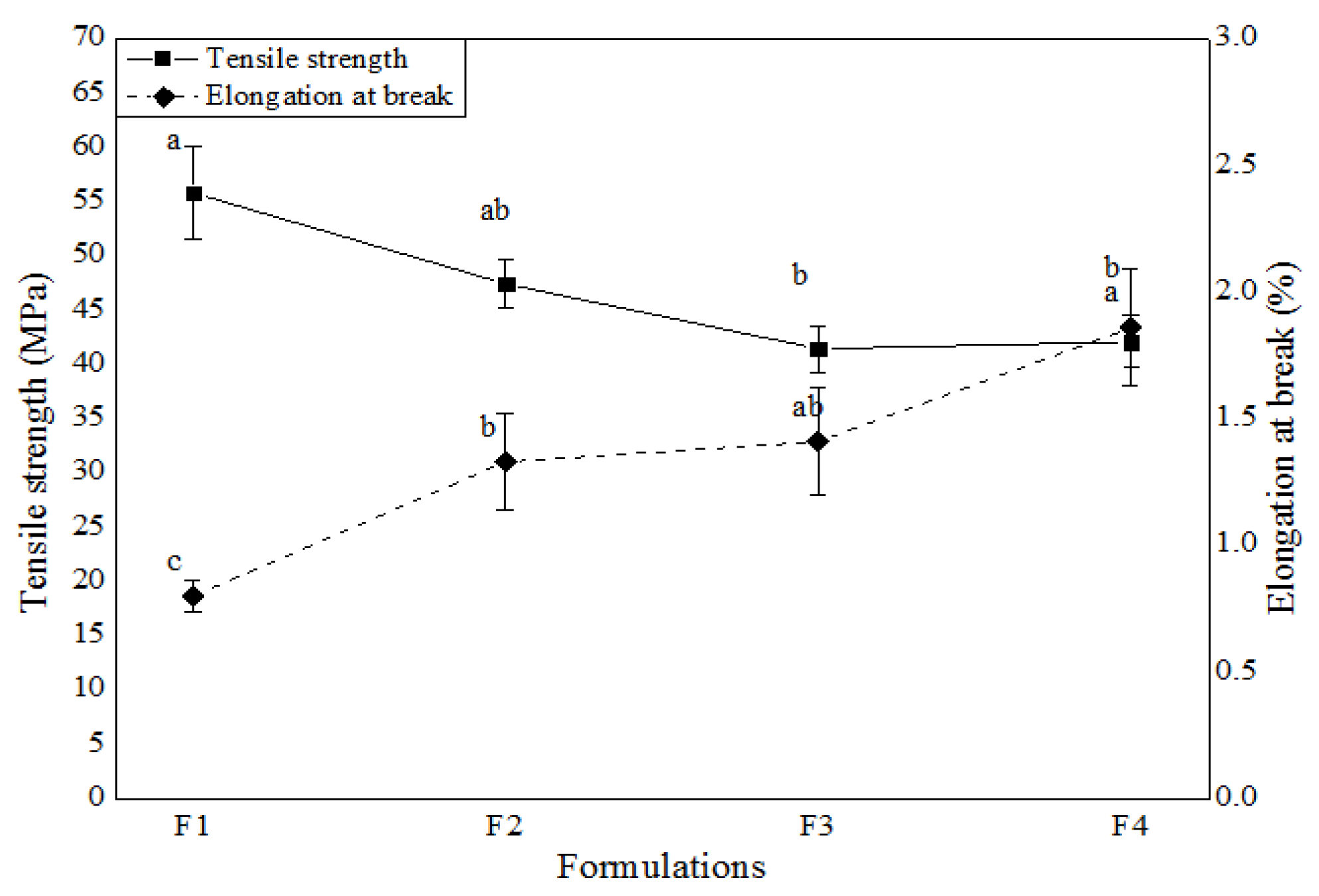
3.4.3. Mechanical Proprieties
3.4.4. Antioxidant Activity
3.5. Application of Packaging on Chicken Breast
3.5.1. Color Parameters
3.5.2. pH
3.5.3. Texture Analysis
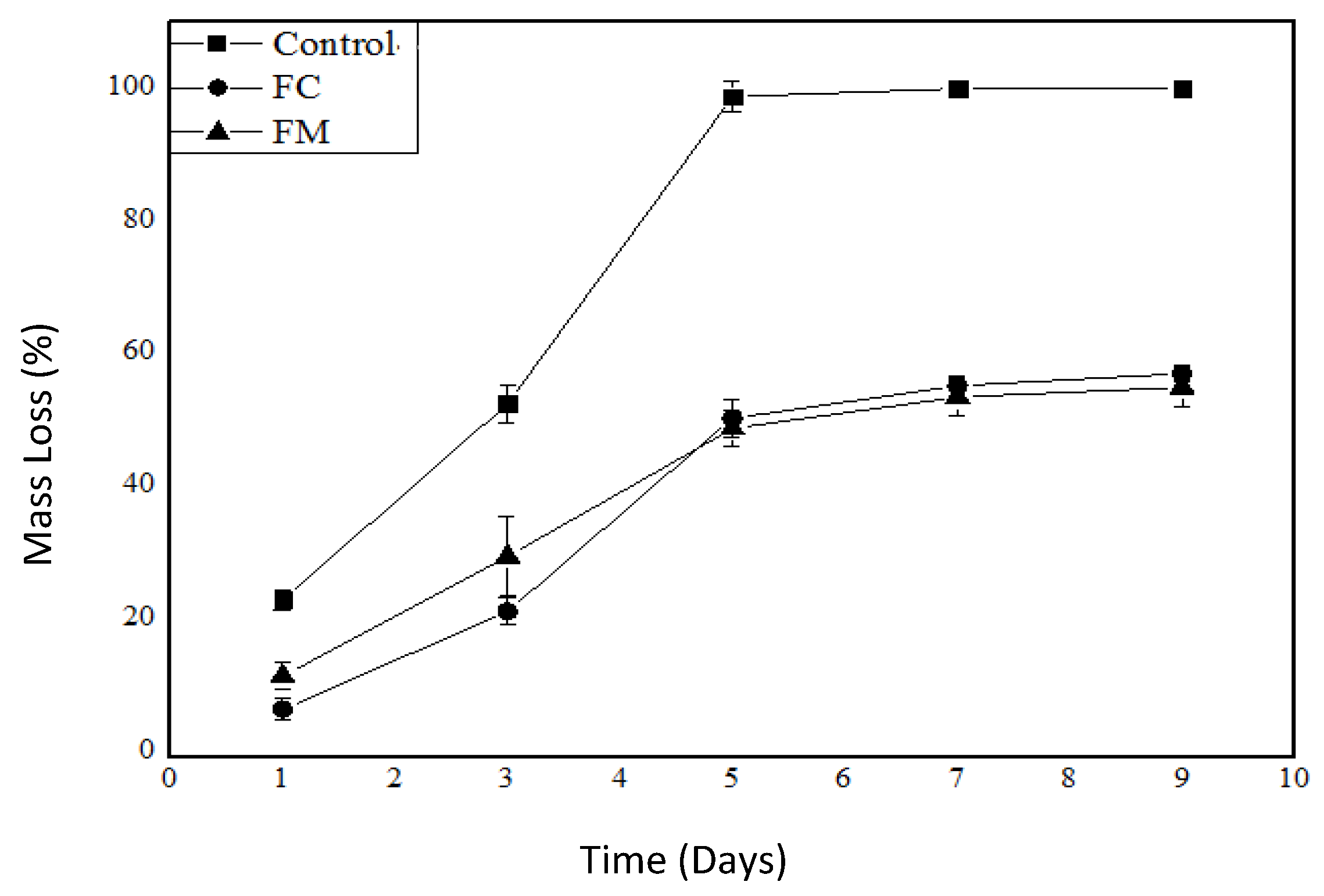
3.5.4. Mass Loss
3.5.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/Potato Starch Edible Biocomposite Films: Correlation between Morphology and Physical Properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Mousavi, Z.; McClements, D.J. Beeswax: A Review on the Recent Progress in the Development of Superhydrophobic Films/Coatings and Their Applications in Fruits Preservation. Food Chem. 2023, 424, 136404. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and Antimicrobial Cellulose Film from Ginger Nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Balachander, R. Starch Bioplastic Film as an Alternative-Packaging Material. J. Achiev. Mater. Manuf. Eng. 2016, 75, 78–84. [Google Scholar] [CrossRef]
- Chia, M.R.; Phang, S.W.; Ahmad, I. Influence of Polyaniline and Cellulose Nanocrystals on Starch Biopolymer Film for Intelligent Food Packaging. Food Biosci. 2023, 56, 103212. [Google Scholar] [CrossRef]
- Montalvo-Paquini, C.; Rangel-Marrón, M.; Palou, E.; López-Malo, A. Physical and Chemical Properties of Edible from Faba Bean Protein. Int. J. Biol. Biomed. Eng. 2014, 8, 125–131. [Google Scholar]
- Nogueira, G.F.; Fakhouri, F.M.; Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta arundinaceae L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Halal, E.; Mello, S.L.; Colussi, R.; Biduski, B.; Evangelho, J.A.D.; Bruni, G.P.; Zavareze, E.D.R. Morphological, Mechanical, Barrier and Properties of Films Based on Acetylated Starch and Cellulose from Barley. J. Sci. Food Agric. 2017, 97, 411–419. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative Study of Processing Methods for Starch/Gelatin Films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Extending Cheese Shelf-Life Using Eco-Friendly Sodium Alginate-Gelatin Films Reinforced with Nanoclay. Food Biosci. 2023, 56, 103304. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO Nanoparticles Stabilized with Gelatin for Potential Use in Food Packaging Applications. Sci. Rep. 2022, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Eddine, L.S.; Souhaila, M.; Hasan, G.G.; Kir, I.; Abdullah, J.A.A. Green Synthesis of SnO2 Nanoparticles from Laurus nobilis L. Extract for Enhanced Gelatin-Based Films and CEF@SnO2 for Efficient Antibacterial Activity. Food Bioprocess. Technol. 2024, 17, 1364–1382. [Google Scholar] [CrossRef]
- Brito, R.M. Elaboração e Caracterização de Filmes a Base de Amido, Gelatina, Glicerol e Óleo Essencial; Trabalho de Conclusão de Curso, Universidade Tecnológica Federal do Paraná: Curitiba, Brazil, 2013. [Google Scholar]
- Bertolo, M.R.V.; Dias, L.D.; Lima, A.R.; Aguiar, A.S.N.; Alves, F.; de Souza, M.; Napolitano, H.B.; Bagnato, V.S.; Junior, S.B. Photoantimicrobial Chitosan-Gelatin-Pomegranate Peel Extract Films for Strawberries Preservation: From Microbiological Analysis to in Vivo Safety Assessment. Int. J. Biol. Macromol. 2023, 253, 127085. [Google Scholar] [CrossRef]
- Bona, E.A.M.D.; Pinto, F.G.D.S.; Fruet, T.K.; Jorge, T.C.M.; Moura, A.C.D. Comparação de Métodos Para Avaliação Da Atividade Antimicrobiana e Determinação Da Concentração Inibitória Mínima (Cim) de Extratos Vegetais Aquosos e Etanólicos. Arq. Inst. Biol. 2014, 81, 218–225. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Szablewski, T.; Przybylska-Balcerek, A.; Szwajkowska-Michałek, L.; Krzyżaniak, M.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z. Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine. Molecules 2023, 28, 244. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for Screening and Evaluation of Antimicrobial Activity: A Review of Protocols, Advantages, and Limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; Neri-Numa, I.A.; de Araújo, F.F.; Pastore, G.M. A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef]
- Battisti, M.A.; Caon, T.; Machado de Campos, A. A Short Review on the Antimicrobial Micro- and Nanoparticles Loaded with Melaleuca Alternifolia Essential Oil. J. Drug Deliv. Sci. Technol. 2021, 63, 102283. [Google Scholar] [CrossRef]
- Russell, M.; Southwell, I. Monoterpenoid Accumulation in Melaleuca Alternifolia Seedlings. Phytochemistry 2002, 59, 709–716. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Muniandy, Y.; Sapuan, S.M.; Sahari, J. TeaTree (Melaleuca Alternifolia) Fiber as Novel Reinforcement Material for Sugar Palm Biopolymer Based Composite Films. Bio Resour. 2017, 12, 3751–3765. [Google Scholar]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of Easy-Removing Antioxidant Films of Chitosan with Melaleuca Alternifolia Essential Oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Upadhyay, P. GC-MS Chemical Profile, Antioxidant Activity, and Sun Protection Factor of Essential Oil of Tea Tree (Melaleuca Alternifolia) and Rosemary (Rosmarinus officinalis L.). Orient. J. Chem. 2022, 38, 1266–1275. [Google Scholar] [CrossRef]
- Li, X.L.; Shen, Y.; Hu, F.; Zhang, X.X.; Thakur, K.; Rengasamy, K.R.R.; Khan, M.R.; Busquets, R.; Wei, Z.J. Fortification of Polysaccharide-Based Packaging Films and Coatings with Essential Oils: A Review of Their Preparation and Use in Meat Preservation. Int. J. Biol. Macromol. 2023, 242, 124767. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.; Franco, B.D.G.d.M. Doencas Microbianas de Origem Alimentar Provocadas Por Enteropatogenos. Rev. Cienc. Farm. 1996, 17, 77–113. [Google Scholar]
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- Silva, J.A. Microrganismos Patogênicos Em Carne de Frangos. Hig. Aliment. 1998, 12, 9–14. [Google Scholar]
- Silva, M.C.D. Salmonella Sp Em Ovos e Carcaças de Frangos “in Natura” 454 Comercializadas Em Maceió. Hig. Aliment. 2004, 18, 80–84. [Google Scholar]
- Weerasooriya, G.; Dulakshi, H.M.T.; de Alwis, P.S.; Bandara, S.; Premarathne, K.R.P.S.; Dissanayake, N.; Liyanagunawardena, N.; Wijemuni, M.I.; Priyantha, M.A.R. Persistence of Salmonella and Campylobacter on Whole Chicken Carcasses under the Different Chlorine Concentrations Used in the Chill Tank of Processing Plants in Sri Lanka. Pathogens 2024, 13, 664. [Google Scholar] [CrossRef]
- Almeida, I.C.; Gonçalves, P.M.R.; Franco, R.M.; Carvalho, J.C.A.d.P. Isolation and Presence of Salmonella in Fresh Chicken Samples, by a Rapid Method. Hig. Aliment. 2000, 14, 59–62. [Google Scholar]
- Baú, A.C.; Carvalhal, J.B.; Aleixo, J.A.G. Prevalence of Salmonella in Chicken Products and Hen’s Eggs from Pelotas, Rs, Brazil. Ciência Rural 2001, 31, 303–307. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Moreno, L.Z.; Castellanos, L.R.; Chattaway, M.A.; McLauchlin, J.; Lodge, M.; OGrady, J.; Zamudio, R.; Doughty, E.; Petrovska, L.; et al. Dynamics of Salmonella Enterica and Antimicrobial Resistance in the Brazilian Poultry Industry and Global Impacts on Public Health. PLoS Genet. 2022, 18, e1010174. [Google Scholar] [CrossRef] [PubMed]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Santos Filho, D.; Sarti, S.J.; Bastos, J.K.; Leitäo Filho H de, F.; Machado, J.O.; Araújo ML, C.; Lopes, W.D.; Abreu, J.E. Atividade antibacteriana de extratos vegetais. Rev. Ciênc. Farm 1990, 12, 47–69. [Google Scholar]
- Suffredini, I.B.; Sader, H.S.; Gonçalves, A.G.; Reis, A.O.; Gales, A.C.; Varella, A.D.; Younes, R.N. Screening of Antibacterial Extracts from Plants Native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res. 2004, 37, 379–384. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.d.G.; Perez-Jimenez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Total Em Frutas Método de Redução Do (FRAP); Technical Report 125; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2006. [Google Scholar]
- Dong, Y.; Rao, Z.; Liu, Y.; Zheng, X.; Tang, K.; Liu, J. Soluble Soybean Polysaccharide/Gelatin Active Edible Films Incorporated with Curcumin for Oil Packaging. Food Packag. Shelf Life 2023, 35, 101039. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; Sousa De Brito, E.; Maia De Morais, S.; De Goes Sampaio, C.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Determinação Da Atividade Antioxidante Total Em Frutas Pela Captura Do Radical Livre ABTS +; Technical Report 128; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2007. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Business Media: Carol Stream, IL, USA, 2017; ISBN 9781932633214. [Google Scholar]
- Fakhouri, F.M. Bioplásticos Flexíveis e Biodegradáveis à Base de Amido e Gelatina. Ph.D. Thesis, Universidade Estadual de Campinas (UNICAMP), Campinas, Brazil, 2009. [Google Scholar]
- American Society for Testing and Materials (ASTM). Method E-96: Standardtest Methods for Water Vapor Transmission of Materials; Annual Book of ASTM Standards: Conshohocken, PA, USA, 1980. [Google Scholar]
- Gennadios, A.; McHugh, T.H.; Weller, C.L.; Krochta, J.M. Edible coatings and films based on proteins. In Edible Coatings and Films to Improve Food Quality; Krochta, J.M., Baldwin, E.A., Nisperos-Carriedo, M., Eds.; Technomic Publishing Company: Lancaster, PA, USA, 1994; pp. 201–277. [Google Scholar]
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Edible Composite Films of Wheat Gluten and Lipids: Water Vapour Permeability and Other Physical Properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Method for Tensile Properties of Thin Plastic Sheeting; Annual Book of ASTM Standards: Conshohocken, PA, USA, 1980. [Google Scholar]
- Simões, R.P.; Groppo, F.C.; Sartorato, A.; Fiol, F.D.S.D.; Mattos Filho, T.D.; Ramacciato, J.C.; Rodrigues, M.V.N. Effect of Melaleuca Alternifolia over Staphylococcal Infection. Lecta 2002, 20, 143–152. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal Activity of the Components of Melaleuca Alternifolia (Tea Tree) Oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Packer, J.F.; Da Luz, M.M.S. Evaluation and Research Method for Natural Products Inhibitory Activity. Rev. Bras. Farmacogn. 2007, 17, 102–107. [Google Scholar] [CrossRef]
- Alzoreky, N.S.; Nakahara, K. Antibacterial Activity of Extracts from Some Edible Plants Commonly Consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Nakai, S.A.; Siebert, K.J. Organic Acid Inhibition Models for Listeria Innocua, Listeria Ivanovii, Pseudomonas Aeruginosa and Oenococcus Oeni. Food Microbiol. 2004, 21, 67–72. [Google Scholar] [CrossRef]
- Bloomfield, S.J.; Palau, R.; Holden, E.R.; Webber, M.A.; Mather, A.E. Genomic Characterization of Pseudomonas Spp. on Food: Implications for Spoilage, Antimicrobial Resistance and Human Infection. BMC Microbiol. 2024, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S. Desenvolvimento de Uma Formulação Farmacêutica Utilizando Óleo 443 Essencial de Melaleuca Alternifolia. In Proceedings of the Anais do XIII 444 INIC, IX EPG E III INIC JR, UNIVAP, Vale do Paraiba, Brasil, 2009. [Google Scholar]
- Oliva, B.; Piccirilli, E.; Ceddia, T.; Pontieri, E.; Aureli, P.; Ferrini, A.M. Antimycotic Activity of Melaleuca Alternifolia Essential Oil and Its Major Components. Lett. Appl. Microbiol. 2003, 37, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Mondello, F.; Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In Vitro and in Vivo Activity of Tea Tree Oil against Azole-Susceptible and -Resistant Human Pathogenic Yeasts. J. Antimicrob. Chemother. 2003, 51, 1223–1229. [Google Scholar] [CrossRef]
- Kokina, M.; Salevic, A.; Kaluševic, A.; Levic, S.; Pantic, M.; Pljevljakušic, D.; Šavikin, K.; Shamtsyan, M.; Nikšic, M.; Nedovic, V. Characterization, Antioxidant and Antibacterial Activity of Essential Oils and Their Encapsulation into Biodegradable Material Followed by Freeze Drying. Food Technol. Biotechnol. 2019, 57, 282. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential Oils as Antioxidants: Their Evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-Carotene Bleaching Methods. Monatsh Chem. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Gondim, R.F.A.; Da Silva, J.D.; De Sá Silva, C. Applicationof Tea Tree Essential (Melaleuca Alternifolia) and Copaiba (Copaifera Officinalis) Butter Oil to Control Staphylococcusaureusin Cooked Chicken Meat. Contrib. Cienc. Soc. 2023, 16, 2531–2550. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical Properties, Antimicrobial Activity and Oil Release of Fish Gelatin Films Incorporated with Cinnamon Essential Oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Kilinc, D.; Ocak, B.; Özdestan-Ocak, Ö. Preparation, Characterization and Antioxidant Properties of Gelatin Films Incorporated with Origanum onites L. Essential Oil. J. Food Meas. Charact. 2021, 15, 795–806. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Physical and Functional Characterization of Active Fish Gelatin Films Incorporated with Lignin. Food Hydrocoll. 2013, 30, 163–172. [Google Scholar] [CrossRef]
- Filgueiras, C.T.; Fakhouri, F.M.; Garcia, V.A.d.S.; Velasco, J.I.; Nogueira, G.F.; Ramos da Silva, L.; Oliveira, R.A. Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis. Polymers 2024, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Roça, R.O. Desenvolvimento de método para avaliação da absorção de água em carcaças e cortes de frangos; Apostila; Universidade Estadual Paulista: Botucatu, Brazil, 2006; 10p. [Google Scholar]
| Bacteria | Inhibition Zone Diameter (mm) |
|---|---|
| Escherichia coli | Ø |
| Staphylococcus aureus | Ø |
| Pseudomonas aeruginosa | 17 |
| Salmonella sp. | 9 |
| Bacteria | Minimum Inhibitory Concentration (%) |
|---|---|
| Pseudomonas aeruginosa | 10 |
| Salmonella sp. | 15 |
| FRAP (μM FeSO4/g) | ABTS (μM Trolox/g) | |
|---|---|---|
| Melaleuca EO | 446 ± 5.78 | 1309 ± 18.0 |
| Compounds (mg/g of GF) | Concentration | |||
|---|---|---|---|---|
| 5% | 10% | 15% | 20% | |
| α-Thujene | --- | --- | 1.00 | 1.50 |
| α-Pineno | 1.50 | 3.10 | 4.70 | 6.60 |
| Canrphene | --- | --- | --- | --- |
| Sabinene | --- | --- | --- | 1.00 |
| β-Pinene | --- | --- | --- | 1.00 |
| Myrcene | --- | --- | --- | 1.00 |
| 2-Carene | 6.40 | 11.50 | 18.20 | 23.40 |
| α-Terpinene | 1.50 | 3.10 | 4.50 | 6.40 |
| Limoneno | 1.40 | 2.70 | 4.10 | 5.60 |
| 1,8-Cineole | 1.20 | 2.40 | 3.60 | 4.90 |
| β-Ocimene | --- | --- | --- | --- |
| γ-Terpinene | 11.00 | 21.70 | 33.10 | 51.20 |
| Mentha-3,8-diene | 1.60 | 3.20 | 4.90 | 6.80 |
| α-Terpinene | --- | 1.10 | 1.50 | 2.10 |
| Torreyol1 | --- | 1.20 | 1.50 | 2.30 |
| Terpine-4-ol | 20.10 | 39.50 | 59.70 | 78.90 |
| α-terpineol | 2.30 | 4.70 | 6.10 | 9.20 |
| Myrrenol | --- | --- | --- | --- |
| Copene | --- | --- | --- | --- |
| Cyperene | --- | --- | --- | --- |
| Caryophyllene | --- | 1.50 | 2.30 | 3.10 |
| Aromadandrene | --- | --- | --- | 1.00 |
| Cunracrene | 1.10 | 1.40 | 2.50 | |
| α-Selinene | --- | --- | 1.00 | 1.40 |
| Cubebol | --- | --- | --- | --- |
| Spathulenol | --- | --- | --- | 1.00 |
| Globulol | --- | --- | --- | --- |
| Guaiol | --- | --- | --- | --- |
| Cedren9-one | --- | --- | --- | --- |
| Sample | Thickness (mm) | WVP (g.mm/h.m2.kPa) | Solubility (%) |
|---|---|---|---|
| Control | 0.059 ± 0.003 b | 1.72 ± 0.10 b | 30.98 ± 2.98 b |
| 5% | 0.107 ± 0.016 a | 2.90 ± 0.52 a | 44.15 ± 3.41 a |
| 10% | 0.114 ± 0.022 a | 2.96 ± 0.32 a | 44.46 ± 2.67 a |
| 15% | 0.127 ± 0.033 a | 3.33 ± 0.77 a | 44.61 ± 0.51 a |
| 20% | 0.126 ± 0.004 a | 3.32 ± 0.27 a | 43.34 ± 5.57 a |
| Sample | FRAP (μM FeSO4/g) | ABTS (%) |
|---|---|---|
| Control | 6.23 ± 4.28 a | - |
| 5% | 23.95 ± 0.75 b | 2.42 ± 0.79 a |
| 10% | 50.17 ± 7.10 c | 3.92 ± 0.60 a |
| 15% | 78.43 ± 4.91 d | 26.39 ± 1.64 b |
| 20% | 136.26 ± 1.38 e | 54.39 ± 2.97 c |
| Time (Days) | Time (Days) | L* | a* | b* | ΔE* | ΔE** |
|---|---|---|---|---|---|---|
| 0 | Control FC FM | 59.79 ± 2.44 55.41 ± 1.05 58.01 ± 1.74 | 4.45 ± 1.43 4.21 ± 1.26 2.97 ± 0.74 | 6.70 ± 1.71 9.27 ± 1.24 9.69 ± 2.15 | 12.83 4.22 6.37 | - 5.08 4.00 |
| 9 | Control FC FM | 49.68 ± 1.97 55.25 ± 2.95 53.05 ± 1.96 | 9.87 ± 2.63 6.65 ± 1.34 6.97 ± 1.09 | 12.45 ± 2.27 12.71 ± 1.43 12.94 ± 1.79 | - - - | 6.44 4.47 |
| Time (Days) | Control 1 | FC 2 | FM 3 |
|---|---|---|---|
| 0 | 5.81 ± 0.06 Ca | 5.81 ± 0.06 Ca | 5.81 ± 0.06 Ca |
| 1 | 6.02 ± 0.01 Aa | 5.86 ± 0.02 BCb | 5.82 ± 0.03 BCc |
| 3 | 6.01 ± 0.09 Aba | 5.96 ± 0.13 ABa | 5.88 ± 0.08 ABa |
| 5 | 5.94 ± 0.05 Bab | 5.97 ± 0.04 Aa | 5.88 ± 0.06 ABb |
| 7 | 5.97 ± 0.06 Aba | 5.98 ± 0.06 Aa | 5.90 ± 0.02 Ab |
| 9 | 5.84 ± 0.06 Ca | 5.70 ± 0.11 Db | 5.59 ± 0.01 Dc |
| Time (Days) | Sample | Control | 20% |
|---|---|---|---|
| 0 | 0.402 ± 0.02 Ca | 0.402 ± 0.02 Ca | 0.402 ± 0.01 Ca |
| 1 | 0.414 ± 0.02 Ca | 0.412 ± 0.01 Ca | 0.413 ± 0.01 Ba |
| 3 | 0.410 ± 0.01 Cb | 0.412 ± 0.01 Ca | 0.412 ± 0.01 Ba |
| 5 | 0.472 ± 0.02 ABa | 0.417 ± 0.03 ABb | 0.435 ± 0.01 Ab |
| 7 | 0.482 ± 0.01 Aa | 0.418 ± 0.01 Ab | 0.428 ± 0.01 Ab |
| 9 | 0.462 ± 0.07 Ba | 0.423 ± 0.01 Bb | 0.424 ± 0.01 ABb |
| Time (Days) | ||||
|---|---|---|---|---|
| 0 | 9 | |||
| Coliformes, 45°C (UFC/g) | Salmonella sp. | Coliformes, 45 °C (UFC/g) | Salmonella sp. | |
| Control 1 | <1.0 × 102 | Ø | <1.0 × 102 | Ø |
| FC 2 | <1.0 × 102 | Ø | <1.0 × 102 | Ø |
| FM 3 | <1.0 × 102 | Ø | <1.0 × 102 | Ø |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.P.d.; Carrea, D.d.A.; Garcia, V.A.d.S.; Tostes Filgueiras, C.; Matta Fakhouri, F.; Velasco, J.I. Development of Gelatin-Based Renewable Packaging with Melaleuca alternifolia Essential Oil for Chicken Breast Preservation. Polymers 2025, 17, 646. https://doi.org/10.3390/polym17050646
Lima RPd, Carrea DdA, Garcia VAdS, Tostes Filgueiras C, Matta Fakhouri F, Velasco JI. Development of Gelatin-Based Renewable Packaging with Melaleuca alternifolia Essential Oil for Chicken Breast Preservation. Polymers. 2025; 17(5):646. https://doi.org/10.3390/polym17050646
Chicago/Turabian StyleLima, Rene Pereira de, Daniela de Almeida Carrea, Vitor Augusto dos Santos Garcia, Cristina Tostes Filgueiras, Farayde Matta Fakhouri, and José Ignacio Velasco. 2025. "Development of Gelatin-Based Renewable Packaging with Melaleuca alternifolia Essential Oil for Chicken Breast Preservation" Polymers 17, no. 5: 646. https://doi.org/10.3390/polym17050646
APA StyleLima, R. P. d., Carrea, D. d. A., Garcia, V. A. d. S., Tostes Filgueiras, C., Matta Fakhouri, F., & Velasco, J. I. (2025). Development of Gelatin-Based Renewable Packaging with Melaleuca alternifolia Essential Oil for Chicken Breast Preservation. Polymers, 17(5), 646. https://doi.org/10.3390/polym17050646








