Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CV@AC Nanohybrid
2.3. Preparation of Films
2.4. Physicochemical Characterization of CV@AC Nanohybrid and Pure AC Powders, as Well as of All Obtained Films
2.4.1. CV Desorption Release Kinetics of CV@AC and PLA/TEC/xCV@AC Films
2.4.2. X-Ray Diffraction (XRD) Analysis of CV@AC Nanohybrid and Pure AC Powders as Well as of All Obtained Films
2.4.3. Fourier-Transform Infrared (FTIR) Analysis of CV@AC Nanohybrid and Pure AC Powders as Well as of All Obtained Films
2.4.4. Scanning Electron Microscopy Images of Films
2.4.5. Instrumentation for Mechanical and Thermomechanical Properties of Films
2.5. Characterization of Active Packaging Properties of Films
2.5.1. Instrumentation for Water/Oxygen Barrier Properties of Films
2.5.2. Antioxidant Activity of PLA/TEC/xCV@AC Films
2.5.3. Instrumentation for Antibacterial Activity of PLA/TEC/xCV@AC Films
2.6. Shelf Life of Fresh Pork Minced Meat
2.7. Statistical Analysis
3. Results
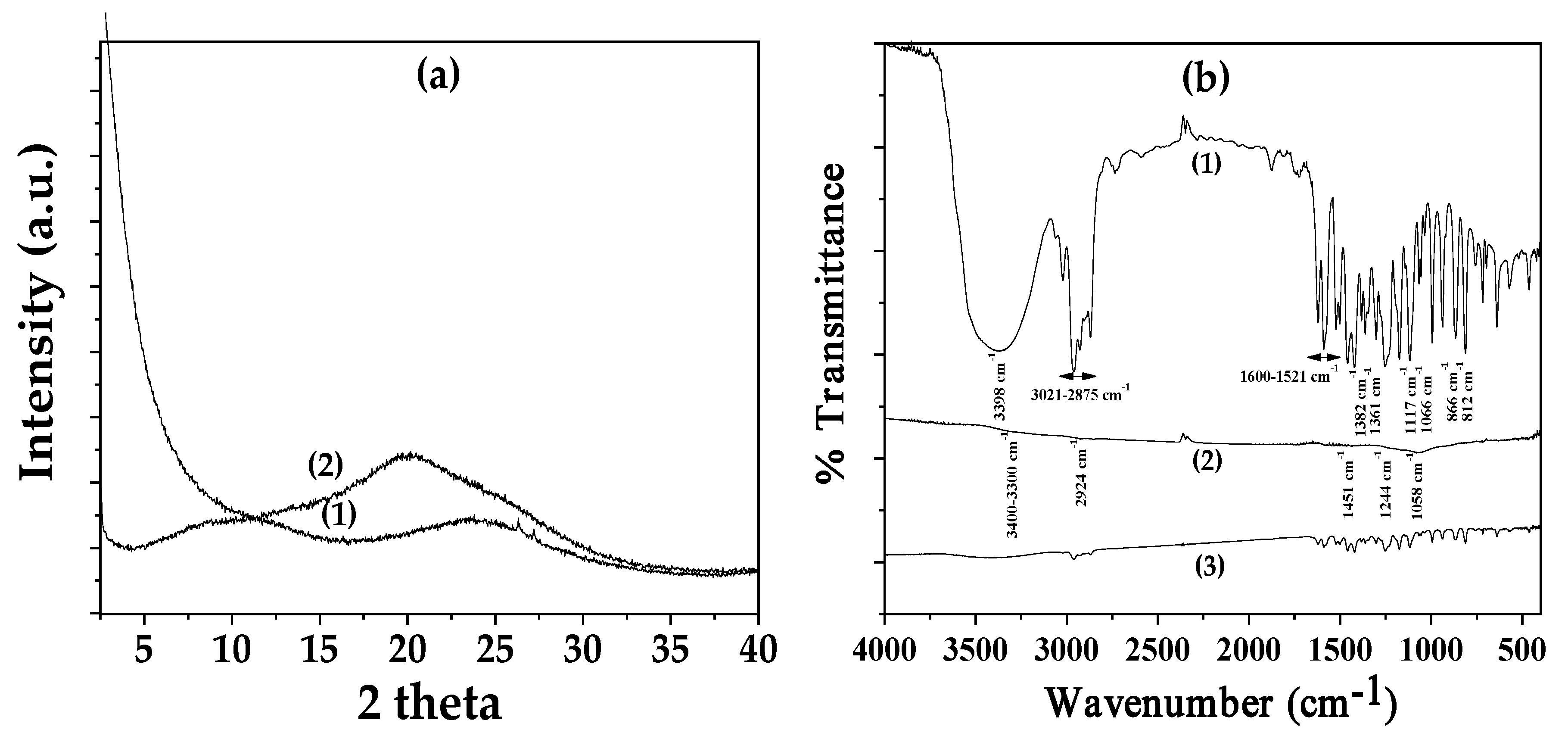
3.1. Physicochemical Characterization of CV@AC Nanohybrid
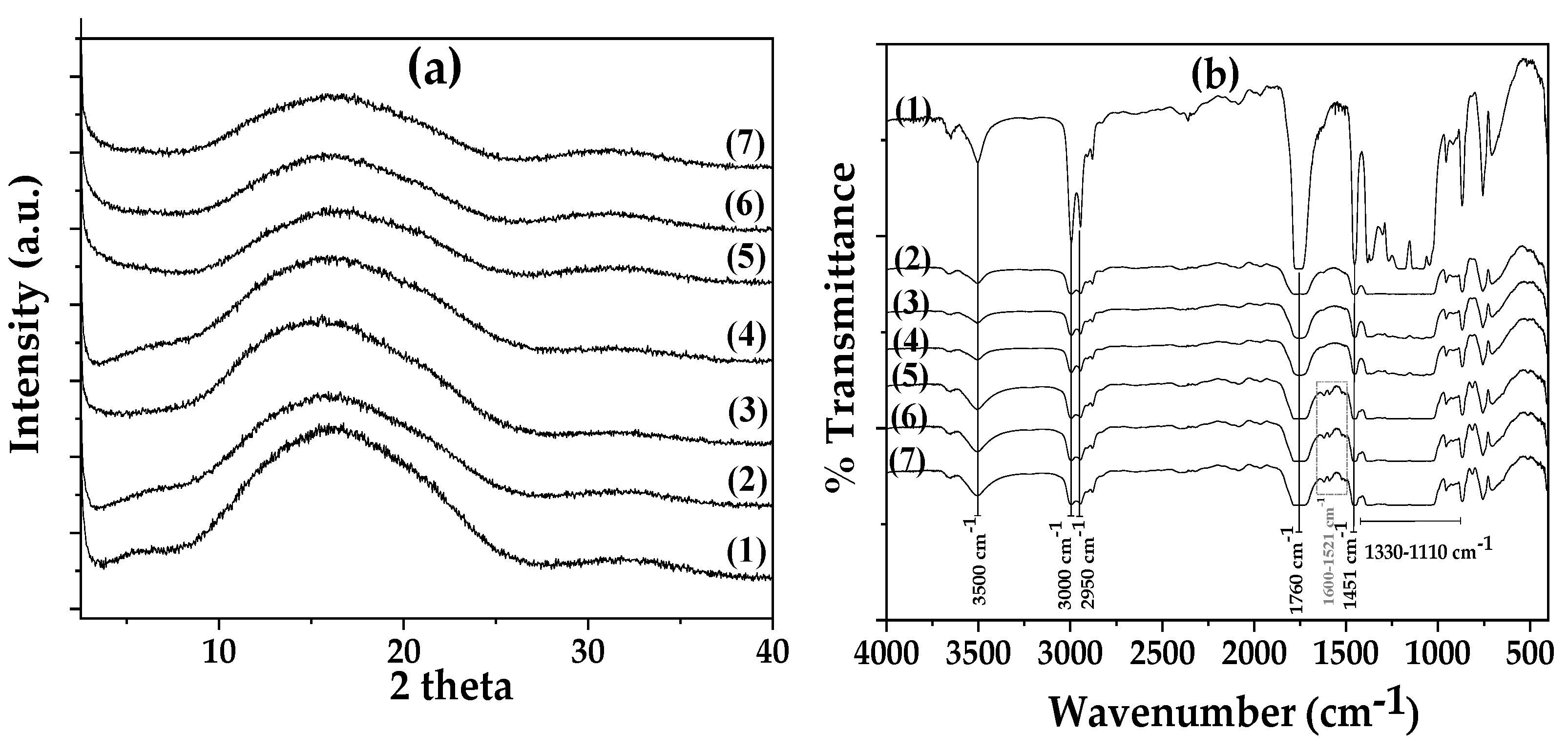
3.2. Physicochemical Characterization of Films
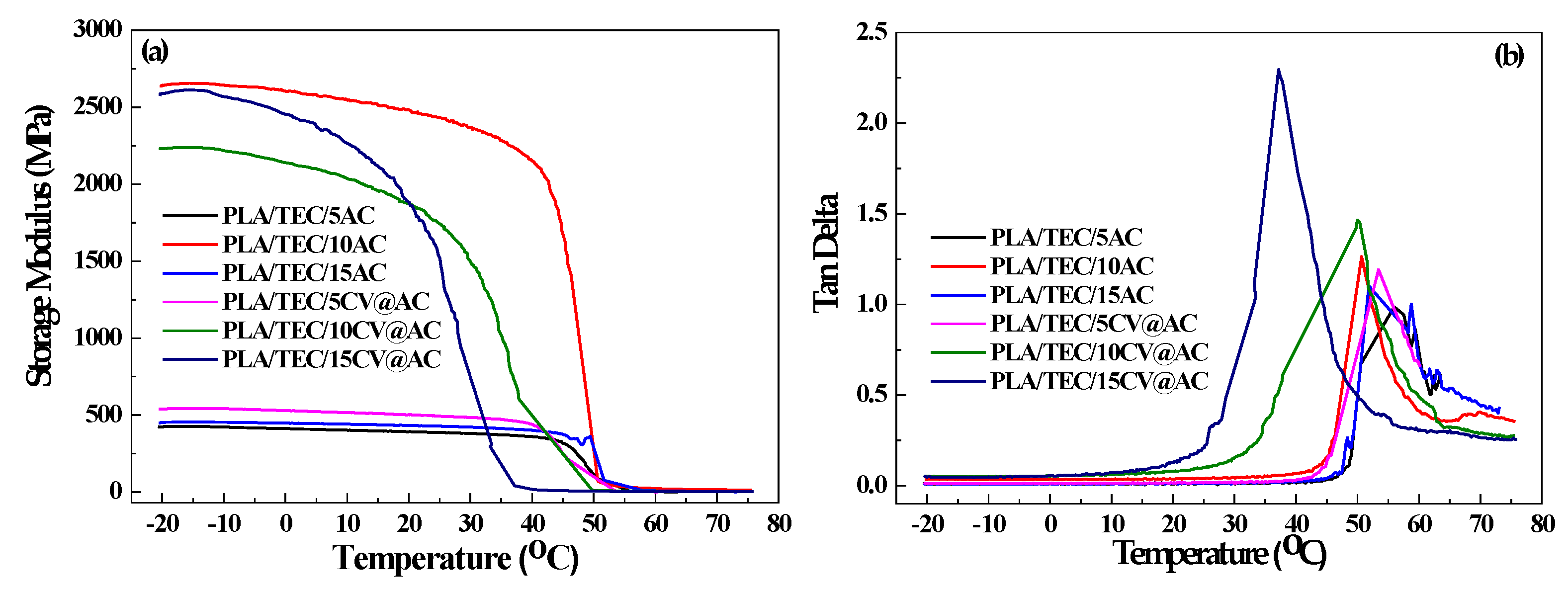
3.3. Results for Mechanical and Thermomechanical Properties of Films
3.4. Water/Oxygen Barrier Properties of Films
3.5. Antioxidant Activity of PLA/TEC/xCV@AC Films
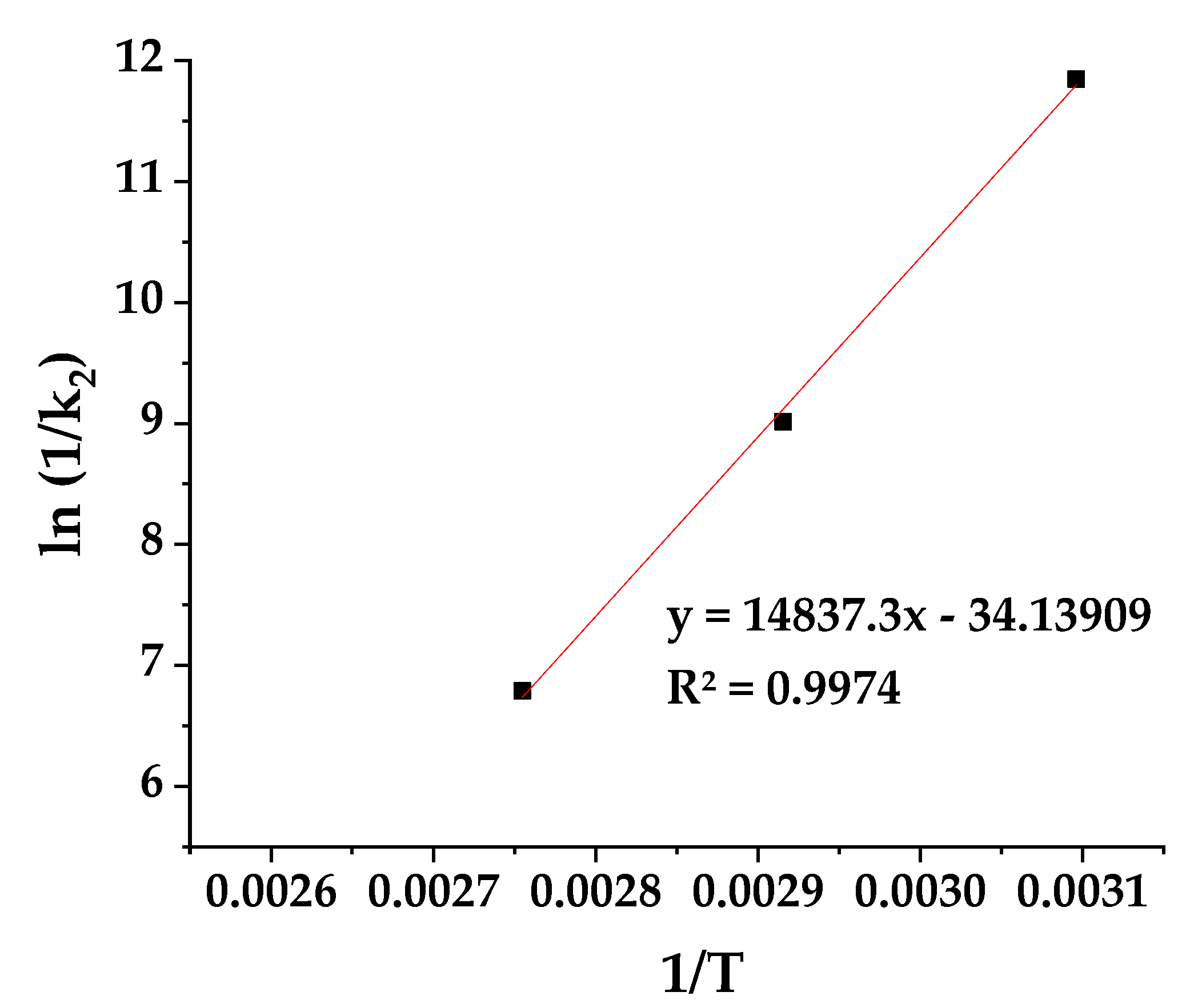
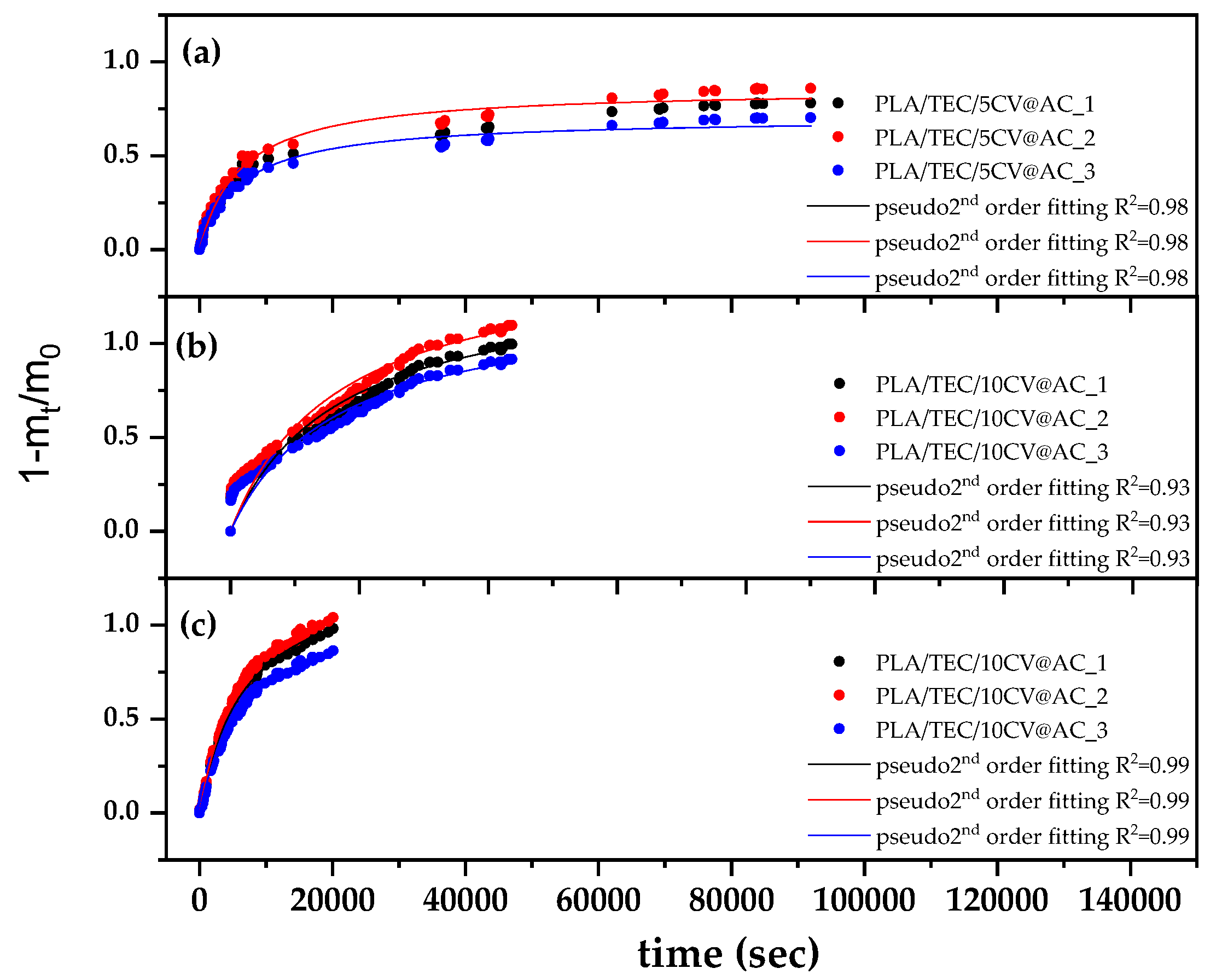
3.6. CV Release Kinetics of PLA/TEC/xCV@AC Films
3.7. Antibacterial Activity of PLA/TEC/xCV@AC Films
3.8. Preservation of Fresh Minced Pork Meat
3.8.1. Microbiological Evaluation of Minced Pork
3.8.2. Lipid Oxidation and Heme Iron Contents of Minced Pork
3.8.3. Sensory Analysis of Minced Pork
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Gabric, A.J. The Climate Change Crisis: A Review of Its Causes and Possible Responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable Materials Alternative to Petrochemical Plastics Pollution: A Review Analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Jain, P.C. Greenhouse Effect and Climate Change: Scientific Basis and Overview. Renew. Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-Based Biodegradable Packaging Materials: A Review of Their Preparation, Characterization and Diverse Applications in the Food Industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and Food Packaging: A Review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(Lactic Acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304–1343. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Lactic Acid: A Comprehensive Review of Production to Purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. An Overview of Biodegradable Poly (Lactic Acid) Production from Fermentative Lactic Acid for Biomedical and Bioplastic Applications. Biomass Conv. Bioref. 2024, 14, 3057–3076. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of Lactic Acid Based Polymers and Their Correlation with Composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7—Extrusion of Biopolymers for Food Applications. In Advances in Biopolymers for Food Science and Technology; Pal, K., Sarkar, P., Cerqueira, M.Â., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 137–169. ISBN 978-0-443-19005-6. [Google Scholar]
- O’Loughlin, J.; Doherty, D.; Herward, B.; McGleenan, C.; Mahmud, M.; Bhagabati, P.; Boland, A.N.; Freeland, B.; Rochfort, K.D.; Kelleher, S.M.; et al. The Potential of Bio-Based Polylactic Acid (PLA) as an Alternative in Reusable Food Containers: A Review. Sustainability 2023, 15, 15312. [Google Scholar] [CrossRef]
- Bamps, B.; Buntinx, M.; Peeters, R. Seal Materials in Flexible Plastic Food Packaging: A Review. Packag. Technol. Sci. 2023, 36, 507–532. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Liu, Q.; Dong, H.; Duan, Y.; Zhang, J. Plasticization of Poly (Lactic) Acid Film as a Potential Coating Material. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022062. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(Lactic Acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [PubMed]
- Mele, G.; Bloise, E.; Cosentino, F.; Lomonaco, D.; Avelino, F.; Marcianò, T.; Massaro, C.; Mazzetto, S.E.; Tammaro, L.; Scalone, A.G.; et al. Influence of Cardanol Oil on the Properties of Poly(Lactic Acid) Films Produced by Melt Extrusion. ACS Omega 2019, 4, 718–726. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An Environmentally Sustainable Plasticizer Toughened Polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef] [PubMed]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing Effects of Citrate Esters on Properties of Poly(Lactic Acid). J. Polym. Eng. 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Moschovas, D.; Karydis-Messinis, A.; Leontiou, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers 2024, 16, 1130. [Google Scholar] [CrossRef]
- Giannakas, A.E. Plant Extracts-Based Food Packaging Films. In Natural Materials for Food Packaging Application; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 23–49. ISBN 978-3-527-83730-4. [Google Scholar]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of Essential Oils as Preservatives in Food Systems: Challenges and Future Prospectives—A Review. Phytochem. Rev. 2022, 21, 1209–1246. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial activity of essential plant oils and their major components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Chacha, J.S.; Ofoedu, C.E.; Xiao, K. Essential Oil-Based Active Polymer-Based Packaging System: A Review of Its Effect on the Antimicrobial, Antioxidant, and Sensory Properties of Beef and Chicken Meat. J. Food Process. Preserv. 2022, 46, e16933. [Google Scholar] [CrossRef]
- EAFUS. A Food Additive Database; Centre for Food Safety, and Applied Nutrition, US Food, and Drug Administration: Washington, DC, USA, 2006. [Google Scholar]
- Council, E.P. Regulation (EC) No 2232/96 the European Parliament, and of the Council on 28 October 1996, Commission Decision of 23 February 1999 Adopting a Register of Flavouring Substances Used in or on Foodstuffs. Off. J. Eur. Commun. 1996, 39, 1–37. [Google Scholar]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef]
- Alkan Tas, B.; Sehit, E.; Erdinc Tas, C.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Carvacrol Loaded Halloysite Coatings for Antimicrobial Food Packaging Applications. Food Packag. Shelf Life 2019, 20, 100300. [Google Scholar] [CrossRef]
- Wrona, M.; Manso, S.; Silva, F.; Cardoso, L.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. New Active Packaging Based on Encapsulated Carvacrol, with Emphasis on Its Odour Masking Strategies. Food Packag. Shelf Life 2023, 40, 101177. [Google Scholar] [CrossRef]
- López-Gómez, A.; Navarro-Martínez, A.; Garre, A.; Iguaz, A.; Maldonado-Guzmán, P.; Martínez-Hernández, G.B. Kinetics of Carvacrol Release from Active Paper Packaging for Fresh Fruits and Vegetables under Conditions of Open and Closed Package. Food Packag. Shelf Life 2023, 37, 101081. [Google Scholar] [CrossRef]
- Krümmel, A.; Pagno, C.H.; Malheiros, P. da S. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods 2024, 13, 1141. [Google Scholar] [CrossRef]
- Laroque, D.A.; de Aragão, G.M.F.; de Araújo, P.H.H.; Carciofi, B.A.M. Active Cellulose Acetate-Carvacrol Films: Antibacterial, Physical and Thermal Properties. Packag. Technol. Sci. 2021, 34, 463–474. [Google Scholar] [CrossRef]
- Ozogul, F.; Çetinkaya, A.; EL Abed, N.; Kuley, E.; Durmus, M.; Ozogul, İ.; Ozogul, Y. The Effect of Carvacrol, Thymol, Eugenol and α-Terpineol in Combination with Vacuum Packaging on Quality Indicators of Anchovy Fillets. Food Biosci. 2024, 59, 104008. [Google Scholar] [CrossRef]
- Karam, L.; Roustom, R.; Abiad, M.G.; El-Obeid, T.; Savvaidis, I.N. Combined Effects of Thymol, Carvacrol and Packaging on the Shelf-Life of Marinated Chicken. Int. J. Food Microbiol. 2019, 291, 42–47. [Google Scholar] [CrossRef]
- Gokoglu, N. Novel Natural Food Preservatives and Applications in Seafood Preservation: A Review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Sasidharan, A. Applying Innovative Technological Interventions in the Preservation and Packaging of Fresh Seafood Products to Minimize Spoilage—A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e29066. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- Sundar, S.K.; Parikh, J.K. Advances and Trends in Encapsulation of Essential Oils. Int. J. Pharm. 2023, 635, 122668. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf Life Extension of Lamb Meat Using Thyme or Oregano Essential Oils and Modified Atmosphere Packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial Effect of Thymol and Carvacrol Added to a Vinegar-Based Marinade for Controlling Spoilage of Marinated Beef (Shawarma) Stored in Air or Vacuum Packaging. Int. J. Food Microbiol. 2020, 332, 108769. [Google Scholar] [CrossRef]
- Giannakas, A.E. 7—Bionanocomposites with Hybrid Nanomaterials for Food Packaging Applications. In Advances in Biocomposites and Their Applications; Karak, N., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2024; pp. 201–225. ISBN 978-0-443-19074-2. [Google Scholar]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Baikousi, M.; Karabagias, V.K.; Karageorgou, I.; Iordanidis, G.; Emmanouil-Konstantinos, C.; Leontiou, A.; Karydis-Messinis, A.; Zafeiropoulos, N.E.; Kehayias, G.; et al. Low-Density Polyethylene-Based Novel Active Packaging Film for Food Shelf-Life Extension via Thyme-Oil Control Release from SBA-15 Nanocarrier. Nanomaterials 2024, 14, 423. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Activated Carbon for Food Packaging Application: Review. Walailak J. Sci. Technol. (WJST) 2018, 15, 255–271. [Google Scholar] [CrossRef]
- Saad, H.; Ayed, A.; Srasra, M.; Attia, S.; Srasra, E.; Bouhtoury, F.C.-E.; Tabbene, O.; Saad, H.; Ayed, A.; Srasra, M.; et al. New Trends in Clay-Based Nanohybrid Applications: Essential Oil Encapsulation Strategies to Improve Their Biological Activity. In Nanoclay—Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2022; ISBN 978-1-80356-558-3. [Google Scholar]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of Clove Essential Oil Immobilized in Mesoporous Silica Nanoparticles on the Functional Properties of Poly(Lactic Acid) Biocomposite Food Packaging Film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous Activated Carbon Derived via Pyrolysis Process of Spent Coffee: Structural Characterization. Investigation of Its Use for Hexavalent Chromium Removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Mitura, K.; Kornacka, J.; Kopczyńska, E.; Kalisz, J.; Czerwińska, E.; Affeltowicz, M.; Kaczorowski, W.; Kolesińska, B.; Frączyk, J.; Bakalova, T.; et al. Active Carbon-Based Nanomaterials in Food Packaging. Coatings 2021, 11, 161. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Van Den Top, T.; Zhou, R. Development of an Activated Carbon-Based Nanocomposite Film with Antibacterial Property for Smart Food Packaging. Mater. Today Commun. 2020, 23, 101124. [Google Scholar] [CrossRef]
- Salmas, C.E.; Leontiou, A.; Kollia, E.; Zaharioudakis, K.; Kopsacheili, A.; Avdylaj, L.; Georgopoulos, S.; Karabagias, V.K.; Karydis-Messinis, A.; Kehayias, G.; et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings 2023, 13, 1503. [Google Scholar] [CrossRef]
- Raul, P.K.; Thakuria, A.; Das, B.; Devi, R.R.; Tiwari, G.; Yellappa, C.; Kamboj, D.V. Carbon Nanostructures As Antibacterials and Active Food-Packaging Materials: A Review. ACS Omega 2022, 7, 11555–11559. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K.W.; Belcher, J.N. 6—Advances in the Packaging of Fresh and Processed Meat Products. In Advances in Meat, Poultry and Seafood Packaging; Woodhead Publishing Series in Food Science, Technology and Nutrition; Kerry, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 173–204. ISBN 978-1-84569-751-8. [Google Scholar]
- Saleh, T.A. Chapter 3—Kinetic Models and Thermodynamics of Adsorption Processes: Classification. In Interface Science and Technology; Saleh, T.A., Ed.; Surface Science of Adsorbents and Nanoadsorbents; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 65–97. [Google Scholar]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) Sorption Properties of Activated Carbon Produced via Pyrolysis of the “Posidonia oceanica” Seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef]
- Frenkel, J. Theorie der Adsorption und verwandter Erscheinungen. Z. Phys. 1924, 26, 117–138. [Google Scholar] [CrossRef]
- Knopf, D.A.; Ammann, M. Technical Note: Adsorption and Desorption Equilibria from Statistical Thermodynamics and Rates from Transition State Theory. Atmos. Chem. Phys. 2021, 21, 15725–15753. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef]
- Kechagias, A.; Salmas, C.E.; Chalmpes, N.; Leontiou, A.A.; Karakassides, M.A.; Giannelis, E.P.; Giannakas, A.E. Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials 2024, 14, 1938. [Google Scholar] [CrossRef]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM E96/E 96M-05; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D 3985; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2010.
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-Lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; De, G.C.R. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Elahi, M.G.; Hekmati, M.; Esmaeili, D.; Ziarati, P.; Yousefi, M. Evaluation and Efficacy Modified Carvacrol and Anti-Cancer Peptide Against Cell Line Gastric AGS. Int. J. Pept. Res. Ther. 2022, 28, 125. [Google Scholar] [CrossRef]
- Dittmann, D.; Saal, L.; Zietzschmann, F.; Mai, M.; Altmann, K.; Al-Sabbagh, D.; Schumann, P.; Ruhl, A.S.; Jekel, M.; Braun, U. Characterization of Activated Carbons for Water Treatment Using TGA-FTIR for Analysis of Oxygen-Containing Functional Groups. Appl. Water Sci. 2022, 12, 203. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, İ. Surface Properties of Activated Carbon Prepared from Wastes. Surf. Interface Anal. 2008, 40, 612–615. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Zych, A.; Athanassiou, A. Effect of Green Plasticizer on the Performance of Microcrystalline Cellulose/Polylactic Acid Biocomposites. ACS Appl. Polym. Mater. 2021, 3, 3071–3081. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, A.; Świetlicki, M.; Prószyński, A.; Gładyszewski, G. Physical Properties of Starch/Powdered Activated Carbon Composite Films. Polymers 2021, 13, 4406. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, F.; Zhai, M.; Mitomo, H.; Yoshii, F. Study on CM-Chitosan/Activated Carbon Hybrid Gel Films Formed with EB Irradiation. Radiat. Phys. Chem. 2008, 77, 622–629. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Cen, Z.; Wei, L. Characterization of Nanocellulose and Activated Carbon Nanocomposite Films’ Biosensing Properties for Smart Packaging. Carbohydr. Polym. 2019, 225, 115189. [Google Scholar] [CrossRef]
- dos Santos, J.W.S.; Garcia, V.A.d.S.; Venturini, A.C.; Carvalho, R.A.d.; da Silva, C.F.; Yoshida, C.M.P. Sustainable Coating Paperboard Packaging Material Based on Chitosan, Palmitic Acid, and Activated Carbon: Water Vapor and Fat Barrier Performance. Foods 2022, 11, 4037. [Google Scholar] [CrossRef]
- Khodaei, N.; Houde, M.; Bayen, S.; Karboune, S. Exploring the Synergistic Effects of Essential Oil and Plant Extract Combinations to Extend the Shelf Life and the Sensory Acceptance of Meat Products: Multi-Antioxidant Systems. J. Food Sci. Technol. 2023, 60, 679–691. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Befa Kinki, A.; Atlaw, T.; Haile, T.; Meiso, B.; Belay, D.; Hagos, L.; Hailemichael, F.; Abid, J.; Elawady, A.; Firdous, N. Preservation of Minced Raw Meat Using Rosemary (Rosmarinus officinalis) and Basil (Ocimum basilicum) Essential Oils. Cogent Food Agric. 2024, 10, 2306016. [Google Scholar] [CrossRef]
- Wang, L.; Heising, J.; Fogliano, V.; Dekker, M. Fat Content and Storage Conditions Are Key Factors on the Partitioning and Activity of Carvacrol in Antimicrobial Packaging. Food Packag. Shelf Life 2020, 24, 100500. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential Oils and Its Antibacterial, Antifungal and Anti-Oxidant Activity Applications: A Review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]




| Sample Name | PLA (g) | TEC (mL-% v/w) | AC (g-% w/w) | CV@AC (g-% w/w) | Twin Extruder Operating Conditions | ||
|---|---|---|---|---|---|---|---|
| T (°C) | Speed (rpm) | Time (min) | |||||
| PLA/TEC | 4 | 0.6–15 | - | - | 180 | 120 | 5 |
| PLA/TEC/5AC | 4 | 0.6–15 | 0.2–5 | - | 180 | 120 | 5 |
| PLA/TEC/10AC | 4 | 0.6–15 | 0.4–10 | - | 180 | 120 | 5 |
| PLA/TEC/15AC | 4 | 0.6–15 | 0.6–15 | - | 180 | 120 | 5 |
| PLA/TEC/5CV@AC | 4 | 0.6–15 | - | 0.2–5 | 180 | 120 | 5 |
| PLA/TEC/10CV@AC | 4 | 0.6–15 | - | 0.4–10 | 180 | 120 | 5 |
| PLA/TEC/15CV@AC | 4 | 0.6–15 | - | 0.6–15 | 180 | 120 | 5 |
| CV@AC | 50 °C/323 K | 70 °C/343 K | 90 °C/363 K | 110 °C/383 K |
|---|---|---|---|---|
| k2 (s−1) | 7.17 × 10−6 ± 1.97 × 10−6 | 1.22 × 10−4 ± 3.53 × 10−5 | 1.12 × 10−3 ± 9.67 × 10−4 | 1.73 × 10−3 ± 1.2 × 10−4 |
| qe | 0.919 ± 0.006 | 0.943 ± 0.010 | 0.960 ± 0.006 | 0.989 ± 0.006 |
| Sample Name | E (Mpa) | σuts (MPa) | %ε |
|---|---|---|---|
| PLA/TEC | 564.1 ± 93.7 a | 8.0 ± 1.9 a | 436.3 ± 285.6 a |
| PLA/TEC/5 AC | 3391.9 ± 228.7 b | 59.7 ± 7.4 b | 3.1 ± 0.5 b |
| PLA/TEC/10 AC | 2498.7 ± 538.1 c | 42.6 ± 4.9 c | 2.6 ± 0.6 b |
| PLA/TEC/15 AC | 2881.7 ± 683.5 d,c | 50.7 ± 7.6 d,b | 2.9 ± 0.5 b |
| PLA/TEC/5CV@AC | 2003.5 ± 279.3 d,c | 34.6 ± 9.7 d,b,c | 15.9 ± 24.7 b |
| PLA/TEC/10CV@AC | 1736.7 ± 467.7 d,c | 30.2 ± 4.7 e,d,b,c | 171.3 ± 58.0 a,c |
| PLA/TEC/15CV@AC | 625.0 ± 267.0 a | 8.8 ± 1.9 a | 367.7 ± 34.4 d,a |
| Thickness (mm) | WVTR (g·cm−2·s−1) × 10−6 | Dwv (cm2·s−1) × 10−4 | Thickness (mm) | OTR (mL·m−2·day−1) | PeO2 (cm2·s−1) × 10−9 | |
|---|---|---|---|---|---|---|
| PLA/TEC | 0.14 ± 0.01 | 1.8 ± 0.67 | 5.71 ± 1.69 a | 0.08 ± 0.01 | 284.6 ± 56.2 | 2.63 ± 0.55 a |
| PLA/TEC/5 AC | 0.11 ± 0.02 | 0.83 ± 0.21 | 1.57 ± 0.75 b | 0.12 ± 0.04 | 151.5 ± 27.6 | 2.12 ± 0.31 a |
| PLA/TEC/10 AC | 0.13 ± 0.01 | 0.37 ± 0.05 | 1.13 ± 0.05 b | 0.15 ± 0.01 | 121.0 ± 14.1 | 2.04 ± 0.03 b,a |
| PLA/TEC/15 AC | 0.12 ± 0.02 | 0.58 ± 0.19 | 1.56 ± 0.17 c,b | 0.13 ± 0.01 | 159.3 ± 55.1 | 2.39 ± 1.20 a |
| PLA/TEC/5CV@AC | 0.10 ± 0.02 | 0.54 ± 0.28 | 1.14 ± 0.54 b | 0.14 ± 0.02 | 137.0 ± 39.6 | 2.23 ± 0.35 a |
| PLA/TEC/10CV@AC | 0.10 ± 0.03 | 0.94 ± 0.28 | 1.60 ± 0.92 b | 0.09 ± 0.01 | 268.0 ± 152.7 | 2.79 ± 1.14 a |
| PLA/TEC/15CV@AC | 0.13 ± 0.01 | 0.65 ± 0.22 | 1.46 ± 0.52 b | 0.08 ± 0.01 | 361.5 ± 87.0 | 3.43 ± 1.37 a |
| Sample | EC50 (mg/L) |
|---|---|
| PLA/TEC/5CV@AC | 14.39 ± 0.60 |
| PLA/TEC/10CV@AC | 6.72 ± 1.23 |
| PLA/TEC/15CV@AC | 10.47 ± 0.43 |
| k2 (s−1) | qe | |
|---|---|---|
| PLA/TEC/5CV@AC | 0.000238 ± 0.000055 | 0.78 ± 0.08 |
| PLA/TEC/10CV@AC | 0.0000441 ± 0.0000032 | 0.99 ± 0.01 |
| PLA/TEC/15CV@AC | 0.000117 ± 0.000011 | 0.97 ± 0.01 |
| Sample | S. aureus | S. typhimurium |
|---|---|---|
| No. of 6 replicates with growth in the contact area of the sample | No. of 6 replicates with growth in the contact area of the sample | |
| PLA/TEC/5CV@AC | 0/6 a | 0/6 a |
| PLA/TEC/10CV@AC | 0/6 a | 0/6 a |
| PLA/TEC/15CV@AC | 0/6 a | 0/6 a |
| Sample | DAYS | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| odor | ||||||
| CONTROL | 5.00 ± 0.00 a | 4.45 ± 0.10 b | 3.90 ± 0.20 d | 3.20 ± 0.10 g | 2.85 ± 0.10 j | 2.50 ± 0.10 m |
| PLA/TEC | 5.00 ± 0.00 a | 4.55 ± 0.10 b | 4.25 ± 0.10 e | 3.60 ± 0.20 h | 3.25 ± 0.18 k | 2.80 ± 0.20 m |
| PLA/TEC/10CV@AC | 5.00 ± 0.00 a | 4.68 ± 0.03 c,b | 4.51 ± 0.09 f | 4.12 ± 0.19 i | 3.91 ± 0.07 l | 3.16 ± 0.19 n,m |
| color | ||||||
| CONTROL | 5.00 ± 0.00 a | 4.30 ± 0.20 b | 3.98 ± 0.29 d | 2.85 ± 0.15 e | 2.70 ± 0.15 h | 2.55 ± 0.12 j |
| PLA/TEC | 5.00 ± 0.00 a | 4.50 ± 0.10 b | 4.35 ± 0.27 d | 3.45 ± 0.16 f | 2.85 ± 0.11 h | 2.70 ± 0.16 j |
| PLA/TEC/10CV@AC | 5.00 ± 0.00 a | 4.69 ± 0.05 c | 4.48 ± 0.21 d | 4.19 ± 0.12 g | 3.78 ± 0.06 i | 3.44 ± 0.11 k |
| texture | ||||||
| CONTROL | 5.00 ± 0.00 a | 4.48 ± 0.29 b | 3.80 ± 0.20 c | 3.32 ± 0.11 e | 2.84 ± 0.08 g | 2.54 ± 0.10 j |
| PLA/TEC | 5.00 ± 0.00 a | 4.40 ± 0.20 b | 4.22 ± 0.15 d | 3.70 ± 0.10 f | 3.22 ± 0.18 h | 2.75 ± 0.10 k |
| PLA/TEC/10CV@AC | 5.00 ± 0.00 a | 4.72 ± 0.18 b | 4.44 ± 0.15 d | 3.94 ± 0.18 f | 3.64 ± 0.16 i | 2.98 ± 0.21 k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, V.K.; Giannakas, A.E.; Leontiou, A.A.; Karydis-Messinis, A.; Moschovas, D.; Andritsos, N.D.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films. Polymers 2025, 17, 605. https://doi.org/10.3390/polym17050605
Karabagias VK, Giannakas AE, Leontiou AA, Karydis-Messinis A, Moschovas D, Andritsos ND, Avgeropoulos A, Zafeiropoulos NE, Proestos C, Salmas CE. Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films. Polymers. 2025; 17(5):605. https://doi.org/10.3390/polym17050605
Chicago/Turabian StyleKarabagias, Vassilios K., Aris E. Giannakas, Areti A. Leontiou, Andreas Karydis-Messinis, Dimitrios Moschovas, Nikolaos D. Andritsos, Apostolos Avgeropoulos, Nikolaos E. Zafeiropoulos, Charalampos Proestos, and Constantinos E. Salmas. 2025. "Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films" Polymers 17, no. 5: 605. https://doi.org/10.3390/polym17050605
APA StyleKarabagias, V. K., Giannakas, A. E., Leontiou, A. A., Karydis-Messinis, A., Moschovas, D., Andritsos, N. D., Avgeropoulos, A., Zafeiropoulos, N. E., Proestos, C., & Salmas, C. E. (2025). Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films. Polymers, 17(5), 605. https://doi.org/10.3390/polym17050605














