Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bio-Based Waterborne Polyurethane (BWPU) Dispersions
2.3. Characterization
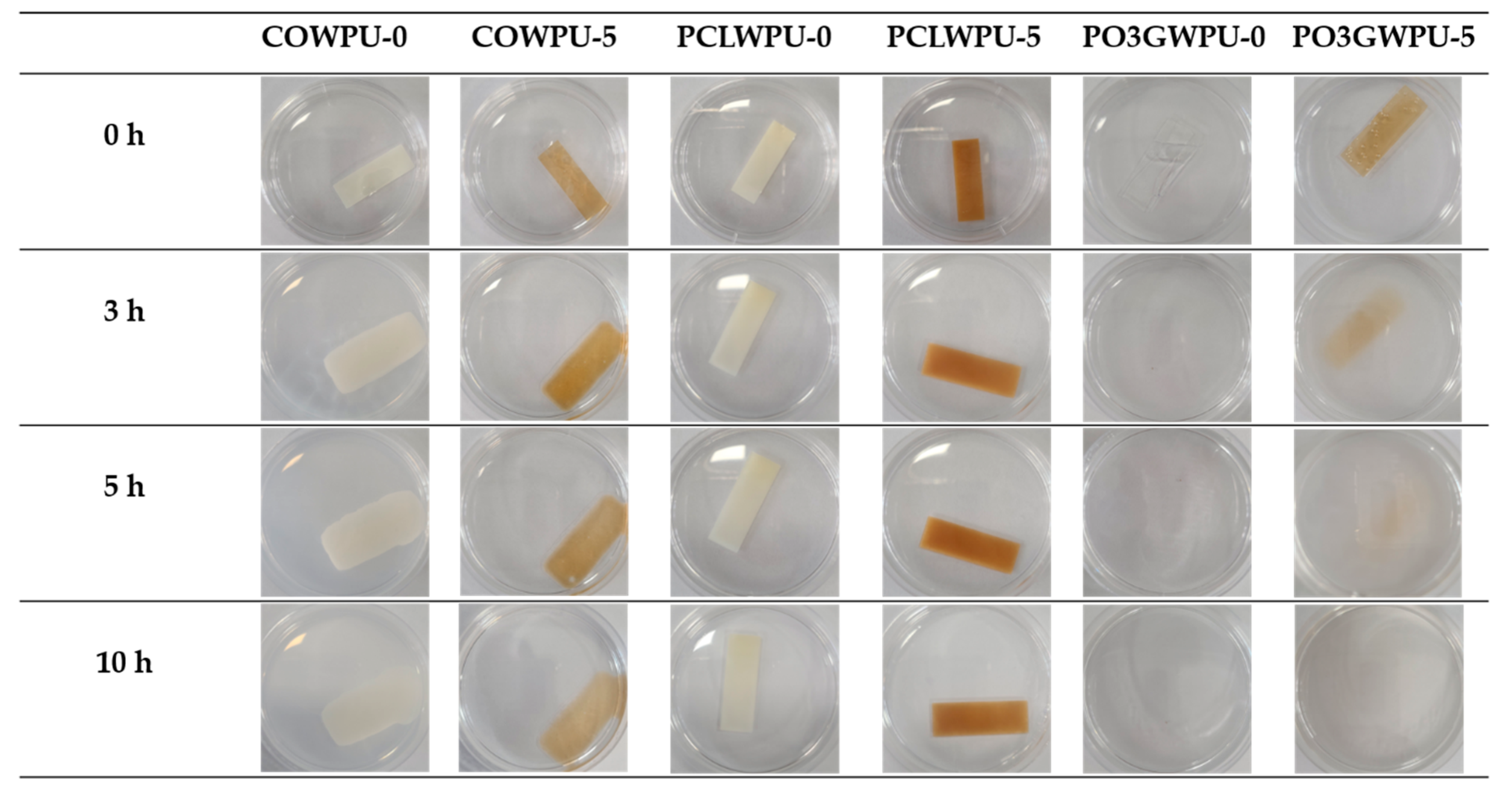
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhari, A.; Kuwar, A.; Mahulikar, P.; Hundiwale, D.; Kulkarni, R.; Gite, V. Development of anticorrosive two pack polyurethane coatings based on modified fatty amide of Juss oil cured at room temperature-a sustainable resource. RSC Adv. 2014, 4, 17866–17872. [Google Scholar] [CrossRef]
- Shi, M.; Wang, X.; Yang, J. Development of lignin-based waterborne polyurethane materials for flame retardant leather application. Polym. Bull. 2022, 80, 5553–5571. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G. Graft-copolymer-based approach to clear, durable, and anti-smudge polyurethane coatings. Angew. Chem. Int. Ed. Engl. 2015, 54, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Peng, H.K.; Wang, X.X.; Li, T.T.; Huang, S.Y.; Lin, Q.; Shiu, B.C.; Lou, C.W.; Lin, J.H. Effects of hydrotalcite on rigid polyurethane foam composites containing a fire retarding agent: Compressive stress, combustion resistance, sound absorption, and electromagnetic shielding effectiveness. RSC Adv. 2018, 8, 33542–33550. [Google Scholar] [CrossRef]
- Czlonka, S.; Strakowska, A.; Strzelec, K.; Kairyte, A.; Kremensas, A. Bio-Based Polyurethane Composite Foams with Improved Mechanical, Thermal, and Antibacterial Properties. Mater 2020, 13, 1108. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chen, Y.-C. Preparation and optimization of WPU dispersion from polyether/polyester polyols for film and coating applications. J. Taiwan Inst. Chem. Eng. 2023, 145, 104832–104842. [Google Scholar] [CrossRef]
- Wang, T.-L.; Hsieh, T.-H. Effect of polyol structure and molecular weight on the thermal stability of segmented poly (urethaneureas). Polym. Degrad. Stab. 1997, 55, 95–102. [Google Scholar] [CrossRef]
- Dieterich, D. Aqueous emulsions, dispersions and solutions of polyurethanes; synthesis and properties. Prog. Org. Coat. 1981, 9, 281–340. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S.; Osman Al Edrus, S.S. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Stankus, J.J.; Guan, J.; Wagner, W.R. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J. Biomed. Mater. Res. A 2004, 70, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Sun, L.; Wei, W.; Taylor, D.K.; Su, S.; Yu, H. Structure and performance control of high-damping bio-based thermoplastic polyurethane. J. Appl. Polym. Sci. 2021, 139, 52059–52069. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Insight on Castor Oil Based Polyurethane and Nanocomposites: Recent Trends and Development. Polym.-Plast. Technol. Eng. 2017, 56, 1556–1585. [Google Scholar] [CrossRef]
- Ionescu, M. 8. Polyester Polyols for Elastic Polyurethanes. In Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited, ed.; iSmithers Rapra Publishing: Shropshire, UK, 2005; Volume 2, p. 263. [Google Scholar]
- Liu, R.; Li, S.; Yao, N.; Xia, J.; Li, M.; Ding, H.; Xu, L.; Yang, X. Castor oil-based polyurethane networks containing diselenide bonds: Self-healing, shape memory, and high flexibility. Prog. Org. Coat. 2022, 163, 106615–106623. [Google Scholar] [CrossRef]
- Honarkar, H. Waterborne polyurethanes: A review. J. Dispers. Sci. Technol. 2017, 39, 507–516. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Formulation optimization of unreinforced and lignin nanoparticle-reinforced phenolic foams using an analysis of variance approach. Compos. Sci. Technol. 2012, 72, 667–674. [Google Scholar] [CrossRef]
- Kim, B.M.; Choi, J.S.; Jang, S.; Park, H.; Lee, S.Y.; Jung, J.; Park, J. Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics. Polymers 2023, 15, 3987. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Zhang, S.; Yin, Y.; Wang, C. UV-resistant transparent lignin-based polyurethane elastomer with repeatable processing performance. Eur. Polym. J. 2021, 159, 110763–110774. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, J.V.; Mishra, D.; Sinha, V.K. Synthesis and Characterization of Low Volatile Content Polyurethane Dispersion from Depolymerised Polyethylene Terphthalate. J. Polym. Environ. 2007, 15, 97–105. [Google Scholar] [CrossRef]
- Zhang, M.; Hemp, S.T.; Zhang, M.; Allen, M.H.; Carmean, R.N.; Moore, R.B.; Long, T.E. Water-dispersible cationic polyurethanes containing pendant trialkylphosphoniums. Polym. Chem. 2014, 5, 3795–3803. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, S.; Park, J.; Shin, E.J. One-Shot Synthesis of Thermoplastic Polyurethane Based on Bio-Polyol (Polytrimethylene Ether Glycol) and Characterization of Micro-Phase Separation. Polymers 2022, 14, 4269. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhu, M.-F. A comparison of the surface properties of lignin and sulfonated lignins by FTIR spectroscopy and wicking technique. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 57–60. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Liang, H.; Liang, D.; Cao, H.; Liu, C.; Qian, Y.; Lu, Q.; Zhang, C. High bio-content castor oil based waterborne polyurethane/sodium lignosulfonate composites for environmental friendly UV absorption application. Ind. Crops Prod. 2019, 142, 111836–111843. [Google Scholar] [CrossRef]
- Ristic, I.; Cakic, S.; Vukic, N.; Teofilovic, V.; Tanasic, J.; Pilic, B. The Influence of Soft Segment Structure on the Properties of Polyurethanes. Polymers 2023, 15, 3755. [Google Scholar] [CrossRef]
- Sun, N.; Di, M.; Liu, Y. Lignin-containing polyurethane elastomers with enhanced mechanical properties via hydrogen bond interactions. Int. J. Biol. Macromol. 2021, 184, 1–8. [Google Scholar] [CrossRef]
- Grzelak, A.W.; Boinard, P.; Liggat, J.J. The influence of diol chain extender on morphology and properties of thermally-triggered UV-stable self-healing polyurethane coatings. Prog. Org. Coat. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, J.; Jiang, G. Synthesis, Characterization and Properties of Soybean Oil-Based Polyurethane. Polymers 2022, 14, 2201. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Larock, R.C. Castor-Oil-Based Waterborne Polyurethane Dispersions Cured with an Aziridine-Based Crosslinker. Macromol. Mater. Eng. 2011, 296, 703–709. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, S.J.; Kim, B.K. Shape memory polyurethane foams. Express Polym. Lett. 2012, 6, 63–69. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Liu, W.; Fang, C.; Wang, S.; Huang, J.; Qiu, X. High-Performance Lignin-Containing Polyurethane Elastomers with Dynamic Covalent Polymer Networks. Macromolecules 2019, 52, 6474–6484. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Y.; Chen, R.; Huh, S.; Johnston, P.A.; Kessler, M.R. Soy-castor oil based polyols prepared using a solvent-free and catalyst-free method and polyurethanes therefrom. Green. Chem. 2013, 15, 1477–1484. [Google Scholar] [CrossRef]
- Hazarika, D.; Gupta, K.; Mandal, M.; Karak, N. High-Performing Biodegradable Waterborne Polyester/Functionalized Graphene Oxide Nanocomposites as an Eco-Friendly Material. ACS Omega 2018, 3, 2292–2303. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Li, T.; Zheng, T.; Han, J.; Liu, Z.; Guo, Z.X.; Zhuang, Z.; Xu, J.; Guo, A.B. Effects of Diisocyanate Structure and Disulfide Chain Extender on Hard Segmental Packing and Self-Healing Property of Polyurea Elastomers. Polymers 2019, 11, 838. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, Y.; He, J.; Li, T.; Li, Y.; Li, M.; Wang, M. Synthesis of epoxy resin-based aqueous polyurethane and application to polyester-cotton fabric finishing. Text. Res. J. 2022, 93, 2590–2603. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Mihailescu, A.; Socol, M.; Zgura, I.; Chiritoiu, M.; Elena Sima, L.; Antohe, F.; Ivan, L.; et al. Long-Term Evaluation of Dip-Coated PCL-Blend-PEG Coatings in Simulated Conditions. Polymers 2020, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, K.; Liu, H.; Cai, H.; Su, J.; Fu, Z.; Guo, Y.; Chen, M. Preparation and properties of waterborne polyurethanes with natural dimer fatty acids based polyester polyol as soft segment. Prog. Org. Coat. 2011, 72, 612–620. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Xing, Q.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.-J. Biodegradable and High-Performance Poly(butylene adipate-co-terephthalate)–Lignin UV-Blocking Films. ACS Sustain. Chem. Eng. 2017, 5, 10342–10351. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]

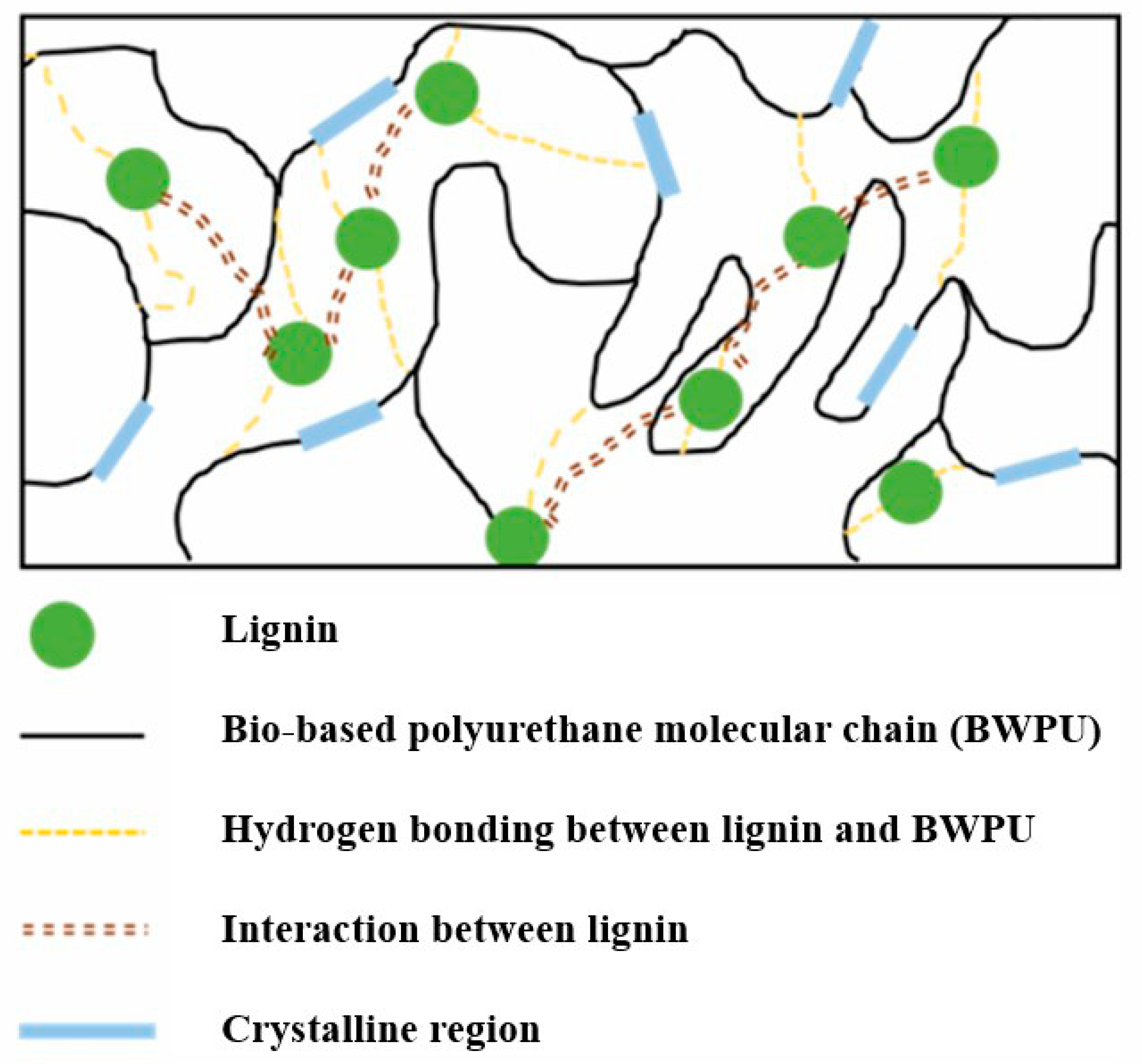
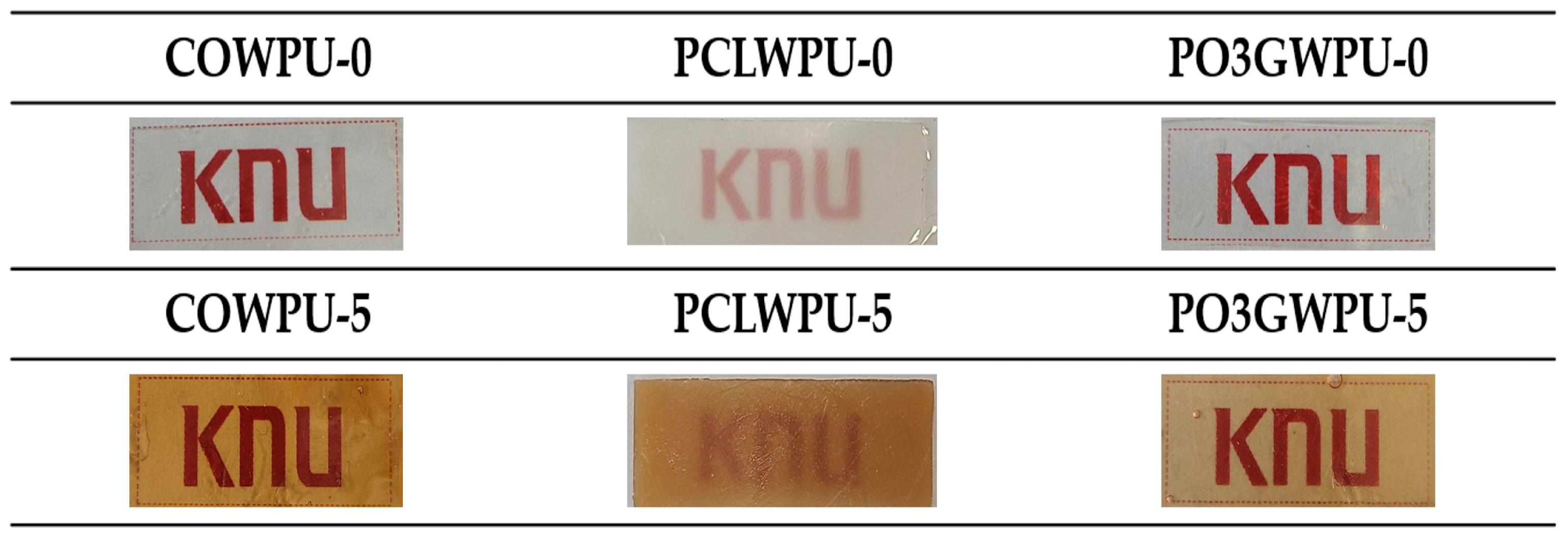
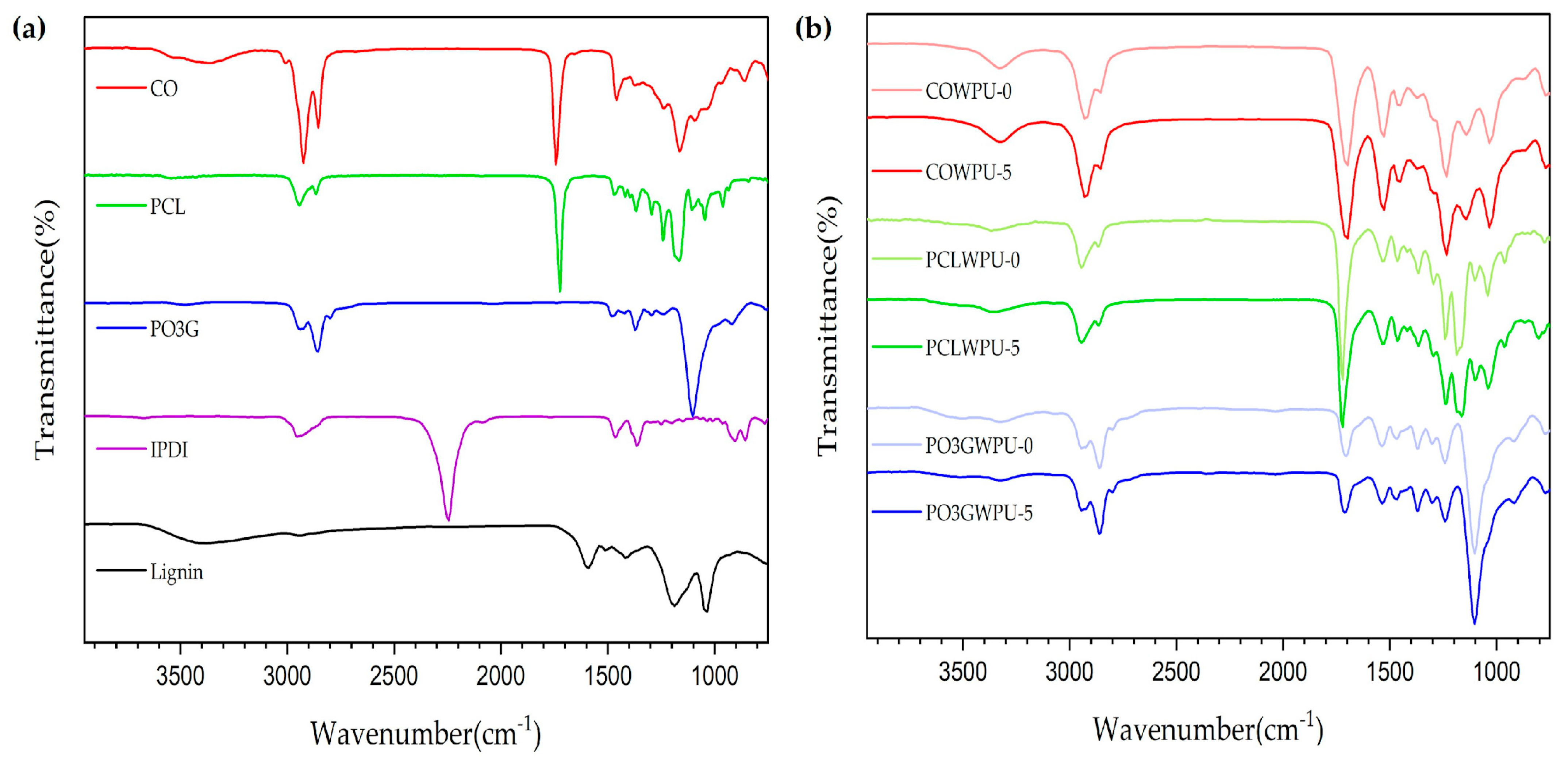



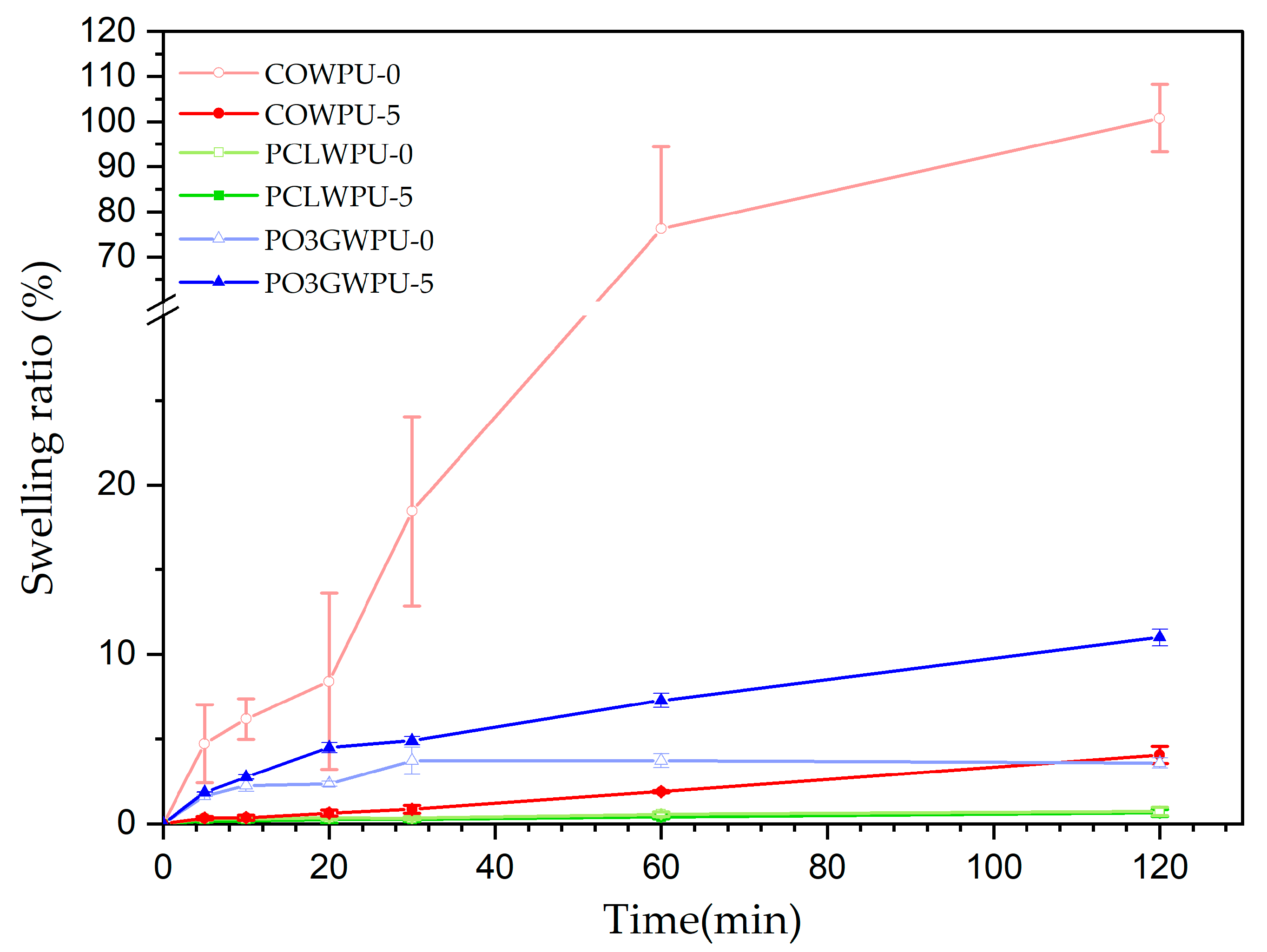

| Samples | Polyol (Func. mol) | IPDI (Func. mol) | Chain Extender (Func. mol) | Lignin (wt%) | Bio-Based Component (wt%) | |
|---|---|---|---|---|---|---|
| MDEA | DEA | |||||
| COWPU-0 | 1.00 | 2.75 | 0.600 | 0.100 | 0.00 | 45.0% |
| COWPU-5 | 5.00 | 47.6% | ||||
| PCLWPU-0 | 0.00 | 72.2% | ||||
| PCLWPU-5 | 5.00 | 73.5% | ||||
| PO3GWPU-0 | 0.00 | 77.1% | ||||
| PO3GWPU-5 | 5.00 | 78.2% | ||||
| Polyols | Chemical Structure |
|---|---|
| Castor oil (CO) |  |
| Polycaprolactone diol (PCL) |  |
| Polyether diol (PO3G) |  |
| Samples | Mw | Mn | PDI (Polydispersity Index) |
|---|---|---|---|
| COWPU-0 | 2.07 × 104 | 1.10 × 104 | 1.87 |
| COWPU-5 | 3.37 × 104 | 1.60 × 104 | 2.11 |
| PCLWPU-0 | 2.37 × 104 | 1.31 × 104 | 1.80 |
| PCLWPU-5 | 2.06 × 104 | 1.04 × 104 | 1.98 |
| PO3GWPU-0 | 6.50 × 104 | 3.53 × 104 | 1.84 |
| PO3GWPU-5 | 9.85 × 104 | 4.40 × 104 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Lee, J.; Jang, S.; Park, J.; Choi, J.; Lee, S.; Jung, J.; Park, J. Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers 2025, 17, 604. https://doi.org/10.3390/polym17050604
Kim B, Lee J, Jang S, Park J, Choi J, Lee S, Jung J, Park J. Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers. 2025; 17(5):604. https://doi.org/10.3390/polym17050604
Chicago/Turabian StyleKim, Bomin, Jihoon Lee, Sunjin Jang, Jaehyeon Park, Jinsil Choi, Seungyeol Lee, Joonhoo Jung, and Jaehyung Park. 2025. "Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane" Polymers 17, no. 5: 604. https://doi.org/10.3390/polym17050604
APA StyleKim, B., Lee, J., Jang, S., Park, J., Choi, J., Lee, S., Jung, J., & Park, J. (2025). Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers, 17(5), 604. https://doi.org/10.3390/polym17050604







