Advanced Nanobiocomposite Hydrogels Incorporating Organofunctionalized LDH for Soft Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
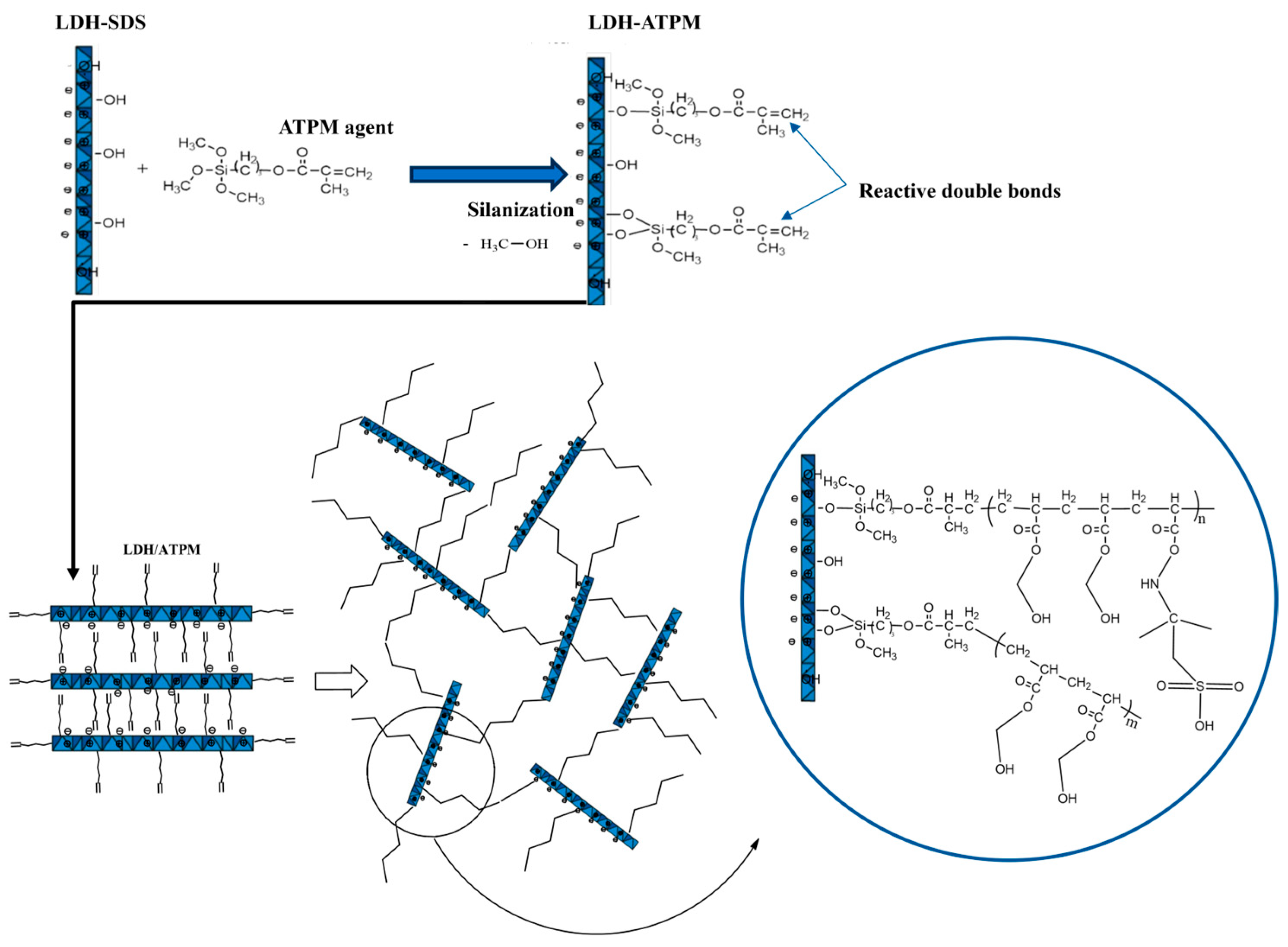
2.1. Synthesis of Modified Layered Double Hydroxide (LDH-ATPM)
2.2. Preparation of HEMA-AMPSA/LDH-ATPM Nanocomposite Hydrogels
2.3. Preparation of Lyophilized Nanocomposite Samples
2.4. Fourier Transform Infrared-Attenuated Total Reflectance (FTIR-ATR) Analysis
2.5. X-Ray Diffraction (XRD) Analysis
2.6. Evaluation of Clay Modification via Titration
2.7. Swelling Behavior and Kinetics
2.8. Thermogravimetric Analysis (TGA)
2.9. Morphological Characterization
2.10. Mechanical Investigation of the Nanocomposite Hydrogels
2.11. Rheological Measurements
2.12. Biocompatibility Assessment of the Materials
3. Results and Discussion
3.1. Evaluation of Clay Modification by Titration
3.2. Swelling Degree and 3D Nanocomposite Network Design
3.3. FTIR Investigation
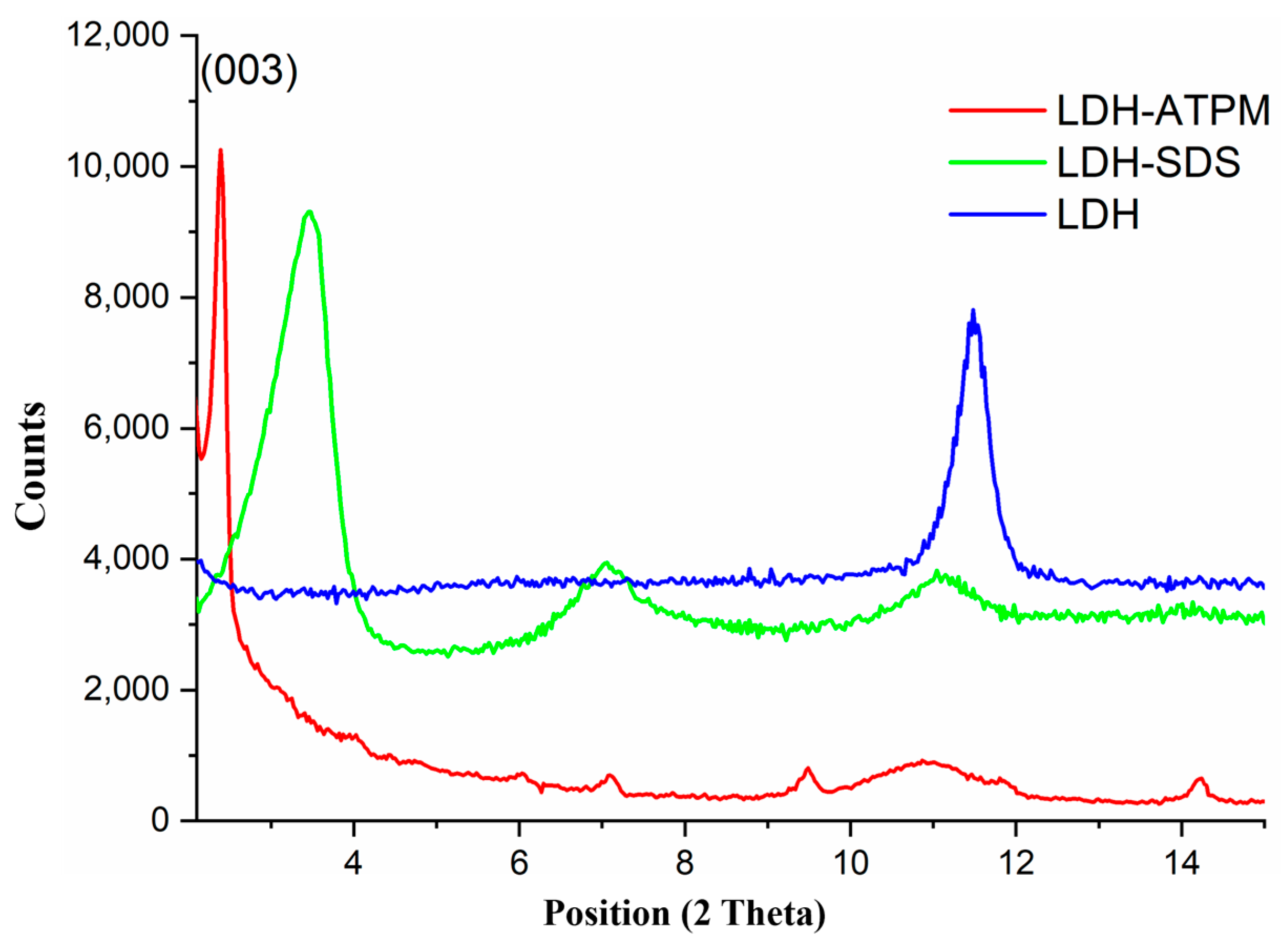
3.4. X-Ray Diffraction (XRD) Investigation
3.5. Thermogravimetric Analysis (TGA)
3.6. Mechanical Testing
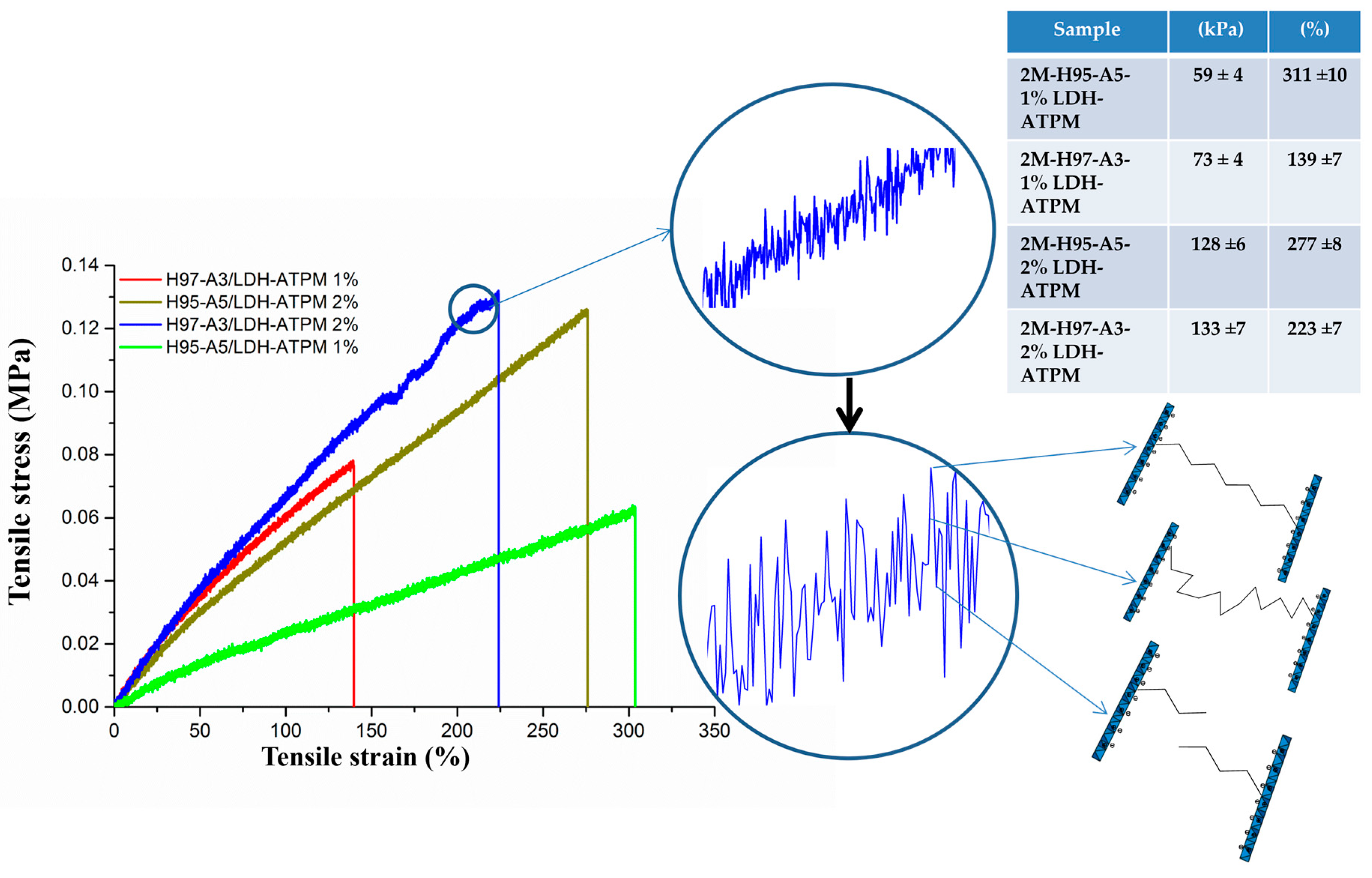
3.6.1. Tensile Test
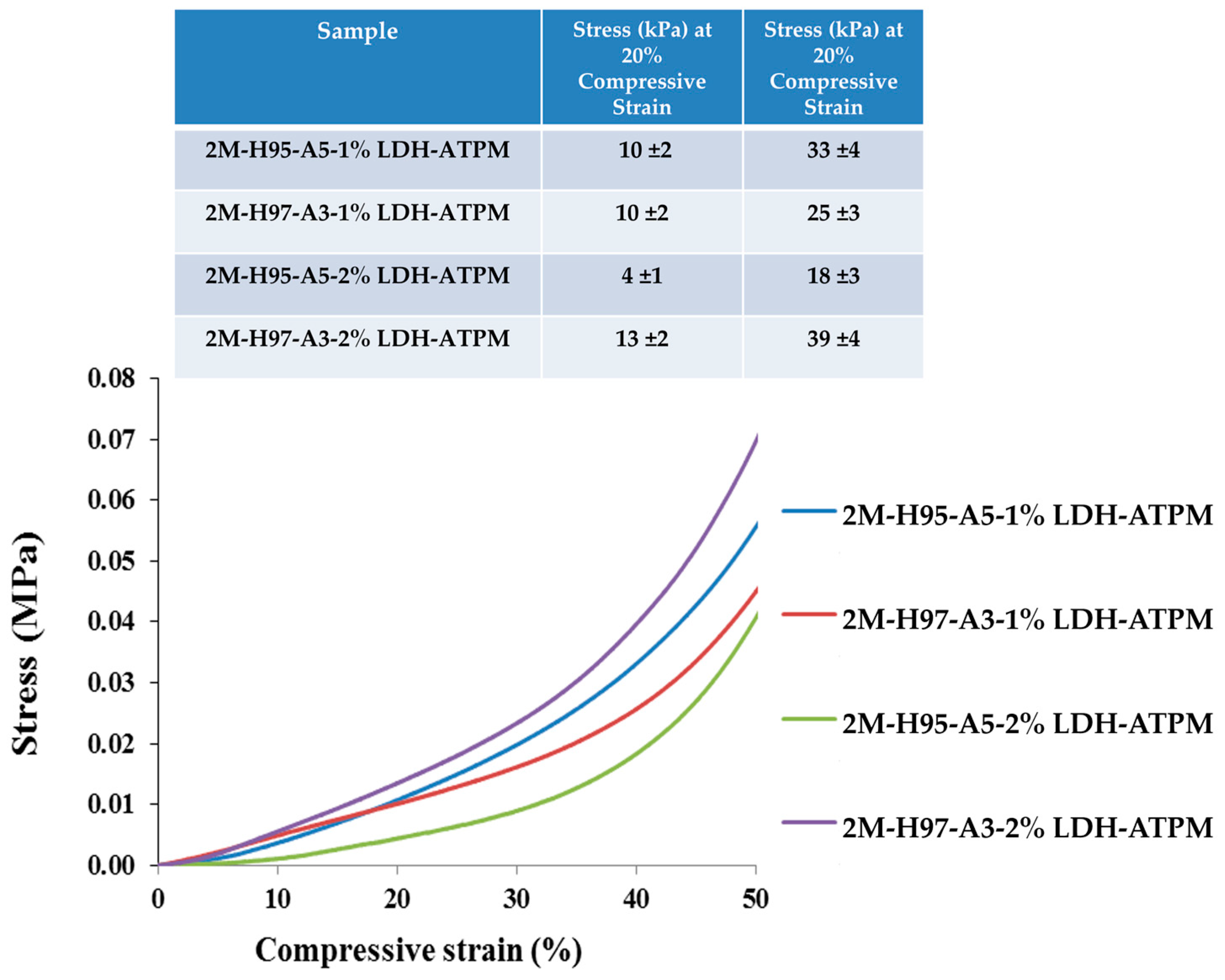
3.6.2. Compressive Test
3.7. Rheological Measurements
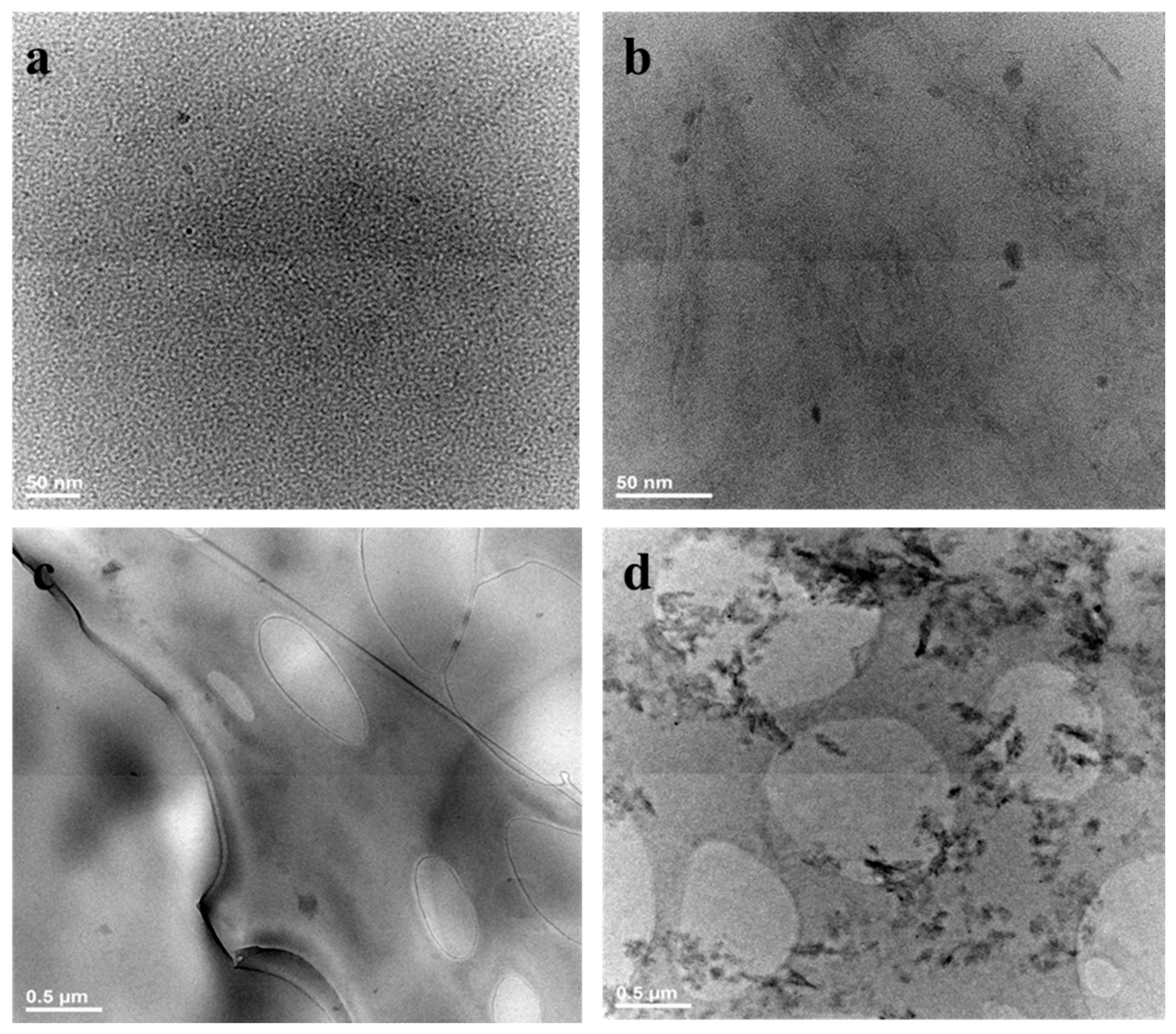
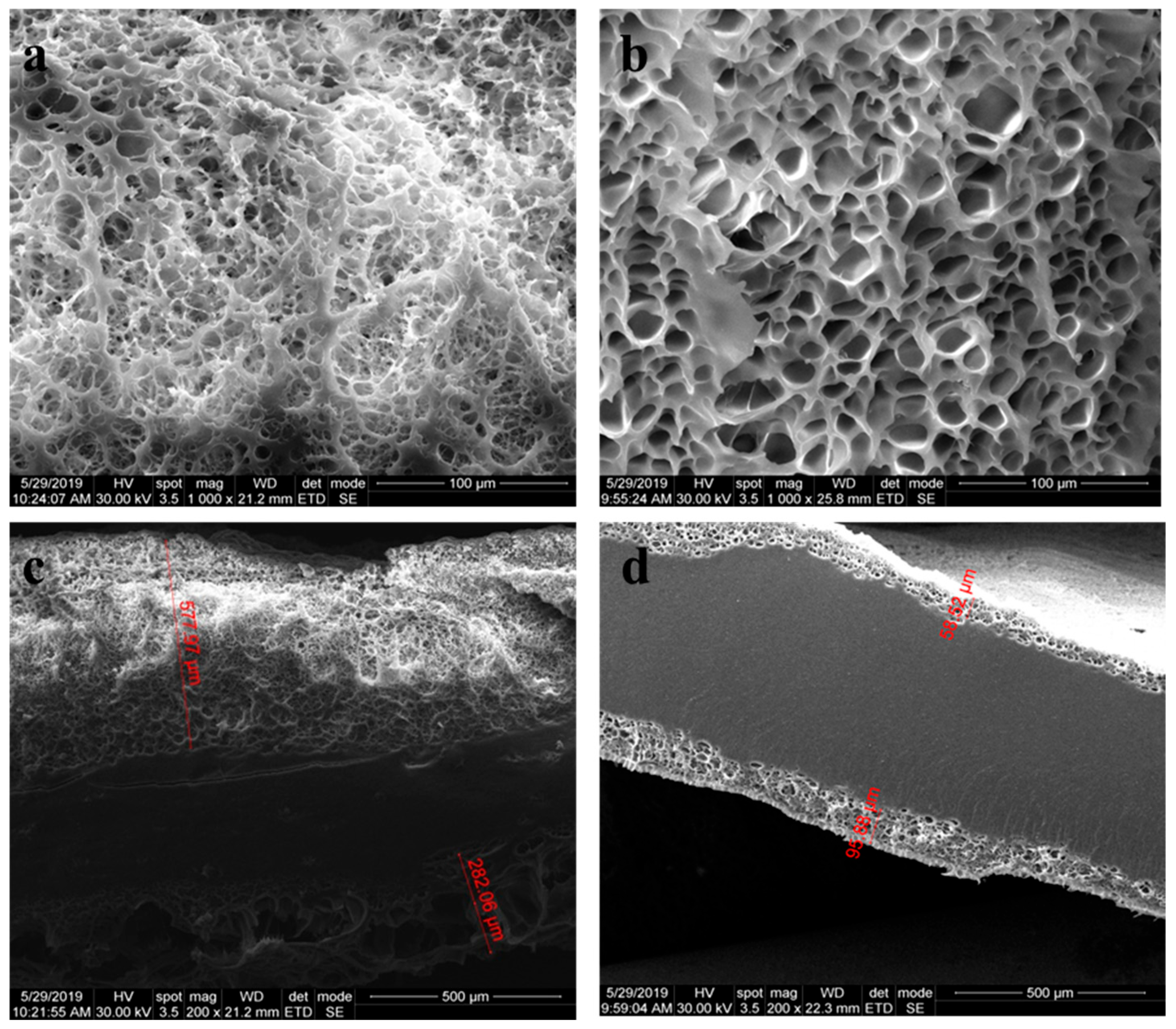
3.8. Morphological Investigation
3.9. Biocompatibility Assessment of the Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saegusa, T. Organic/inorganic polymer hybrids. Macromol. Symp. 1995, 98, 719–729. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer Layered Silicate Nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Mark, J.E. New developments and directions in the area of elastomers and rubberlike elasticity. Macromol. Symp. 2003, 201, 77–84. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Haraguchi, K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223–241. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G. Novel Nanocomposite Hydrogels Consisting of Layered Double Hydroxide with Ultrahigh Tensibility and Hierarchical Porous Structure at Low Inorganic Content. Adv. Mater. 2014, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, S.; Feng, S.; Yu, L.; Wang, J.; Liu, Y. Tough dual nanocomposite hydrogels with inorganic hybrid crosslinking. Soft Matter 2016, 12, 1649–1654. [Google Scholar] [CrossRef]
- Siti Nurasikin, A.; Norhayati, H.; Yusri, N.H.; Md Isa, I.; Kamari, A.; Mohamed, A.; Mohd Damanhuri, M.I. Synthesis and Characterization of Layered-Double Hydroxide 3-(4-Hydroxyphenyl) Propionate Nanocomposite. Nano Hybrids 2014, 7, 53–67. [Google Scholar] [CrossRef]
- Liu, R.; Liang, S.; Tang, X.-Z.; Yan, D.; Li, X.; Yu, Z.-Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012, 22, 14160–14167. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.-J. Mechanical Properties and Structure of Polymer−Clay Nanocomposite Gels with High Clay Content. Macromolecules 2006, 39, 1898–1905. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.-J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of Forming Organic/Inorganic Network Structures during In-situ Free-Radical Polymerization in PNIPA−Clay Nanocomposite Hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, K.; Zou, C.; Gao, M.; Fang, Y.; Zhao, M.; Wu, Y.; You, Q. Study on a Novel Cross-Linked Polymer Gel Strengthened with Silica Nanoparticles. Energy Fuels 2017, 31, 9152–9161. [Google Scholar] [CrossRef]
- Ignat, S.-R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile Biomaterial Platform Enriched with Graphene Oxide and Carbon Nanotubes for Multiple Tissue Engineering Applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-Swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Fan, S. Effects of Clay Content on the Properties of Nanocomposite Hydrogels Composed of Poly(N-isopropylacrylamide) and Clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Oh, J.-M.; Biswick, T.T.; Choy, J.-H. Layered nanomaterials for green materials. J. Mater. Chem. 2009, 19, 2553–2563. [Google Scholar] [CrossRef]
- Karchoubi, F.; Afshar Ghotli, R.; Pahlevani, H.; Baghban Salehi, M. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Barrett-Catton, E.; Ross, M.L.; Asuri, P. Multifunctional Hydrogel Nanocomposites for Biomedical Applications. Polymers 2021, 13, 856. [Google Scholar] [CrossRef]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Fratoddi, I.; Chronopoulou, L.; Palocci, C. Peptide-Hydrogel Nanocomposites for Anti-Cancer Drug Delivery. Gels 2023, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Suberlyak, S.; Petrina, R.; Grytsenko, O.; Baran, N.; Komar, A.; Berezhnyy, B. Investigation of the Sorption Capacity of Polyvinylpyrrolidone Copolymers As the Basis of Hydrogel Cosmetic Masks with Plant Biomass Extracts. Chem. Chem. Technol. 2022, 16, 555–563. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Chen, K.; Wu, X.; Zong, T.; Feng, C.; Zhang, D. Anisotropic hydrogels with enhanced mechanical and tribological performance by magnetically oriented nanohybrids. Chem. Eng. J. 2022, 430, 133036. [Google Scholar] [CrossRef]
- Fakhruddin, K.; Hassan, R.; Khan, M.U.A.; Allisha, S.N.; Razak, S.I.A.; Zreaqat, M.H.; Latip, H.F.M.; Jamaludin, M.N.; Hassan, A. Halloysite nanotubes and halloysite-based composites for biomedical applications. Arab. J. Chem. 2021, 14, 103294. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Borges, J.P.; Costa, L.C.; Silva, J.C.; Soares, P.I.P. Clay-Based Nanocomposite Hydrogels for Biomedical Applications: A Review. Nanomaterials 2022, 12, 3308. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Nanoclay-Composite Hydrogels for Bone Tissue Engineering. Gels 2024, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, M.; Stetsyshyn, Y.; Harhay, K.; Melnyk, Y.; Donchak, V.; Gubriy, Z.; Kracalik, M. Impact of the functionalized clay nanofillers on the properties of the recycled polyethylene terephthalate nanocomposites. J. Appl. Polym. Sci. 2024, 141, e55543. [Google Scholar] [CrossRef]
- Fu, J. Strong and tough hydrogels crosslinked by multi-functional polymer colloids. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1336–1350. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef]
- Șerban, M.V.; Nazarie, S.-R.; Dinescu, S.; Radu, I.-C.; Zaharia, C.; Istrătoiu, E.-A.; Tănasă, E.; Herman, H.; Gharbia, S.; Baltă, C.; et al. Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering. Nanomaterials 2022, 12, 503. [Google Scholar] [CrossRef]
- Vasile, E.; Radu, I.-C.; Galateanu, B.; Rapa, M.; Hudita, A.; Jianu, D.; Stanescu, P.-O.; Cioflan, H.; Zaharia, C. Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials 2020, 13, 4488. [Google Scholar] [CrossRef] [PubMed]
- Kameliya, J.; Verma, A.; Dutta, P.; Arora, C.; Vyas, S.; Varma, R.S. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics 2023, 11, 121. [Google Scholar] [CrossRef]
- Li, L.; Soyhan, I.; Warszawik, E.; van Rijn, P. Layered Double Hydroxides: Recent Progress and Promising Perspectives Toward Biomedical Applications. Adv. Sci. 2024, 11, e2306035. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Liu, J. Tunable photoluminescence of europium-doped layered double hydroxides intercalated by coumarin-3-carboxylate. RSC Adv. 2014, 4, 7991–7997. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Z.; Chen, G.; Shen, J. Photoluminescence of colloids of pristine MgAl layered double hydroxides. RSC Adv. 2014, 4, 19218–19220. [Google Scholar] [CrossRef]
- Tadanaga, K.; Furukawa, Y.; Hayashi, A.; Tatsumisago, M. Direct Ethanol Fuel Cell Using Hydrotalcite Clay as a Hydroxide Ion Conductive Electrolyte. Adv. Mater. 2010, 22, 4401–4404. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A New Concept of Ecological, Bioinspired, and Functional Hybrid Materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Y.; Luo, J.; Zhong, Z.; Borgna, A.; Guo, Z.; O’Hare, D. Synthesis of nano-sized spherical Mg3Al–CO3 layered double hydroxide as a high-temperature CO2 adsorbent. RSC Adv. 2013, 3, 3414–3420. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H. Synthesis and characterization of carboxymethyl cellulose/layered double hydroxide nanocomposites. J. Nanopart. Res. 2013, 15, 1563. [Google Scholar] [CrossRef]
- Ying, Z.; Xianggao, L.; Bin, C.; Fei, C.; Jing, F. Highly exfoliated epoxy/clay nanocomposites: Mechanism of exfoliation and thermal/mechanical properties. Compos. Struct. 2015, 132, 44–49. [Google Scholar] [CrossRef]
- Matei, A.; Birjega, R.; Vlad, A.A.; Mitu, B.; Baciu, D.D.; Dinescu, M.; Zavoianu, R. Chapter 10—LDH-interlayered nanostructures for biomedical and environmental applications. In Functional Nanostructured Interfaces for Environmental and Biomedical Applications; Dinca, V., Suchea, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–284. [Google Scholar]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Dong, L.-e.; Gou, G.; Jiao, L. Characterization of a dextran–coated layered double hydroxide acetylsalicylic acid delivery system and its pharmacokinetics in rabbit. Acta Pharm. Sin. B 2013, 3, 400–407. [Google Scholar] [CrossRef][Green Version]
- O’Halloran, N.A.; Dolan, E.B.; Kerin, M.J.; Lowery, A.J.; Duffy, G.P. Hydrogels in adipose tissue engineering—Potential application in post-mastectomy breast regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 2234–2247. [Google Scholar] [CrossRef]
- Bertozzi, N.; Simonacci, F.; Grieco, M.P.; Grignaffini, E.; Raposio, E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann. Med. Surg. 2017, 20, 41–48. [Google Scholar] [CrossRef]
- Kolaparthy, L.K.; Sanivarapu, S.; Moogla, S.; Kutcham, R.S. Adipose Tissue—Adequate, Accessible Regenerative Material. Int. J. Stem Cells 2015, 8, 121–127. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Ignat, S.-R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Fukuda, K.; Sato, Y.; Hattori, H. Biomaterials as cell carriers for augmentation of adipose tissue-derived stromal cell transplantation. Bio-Med. Mater. Eng. 2018, 29, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, B.H.; Yang, C.; Jeong, S.; Shim, J.H.; Park, M.H.; Choy, Y.B.; Heo, C.Y.; Lee, K. Development of Poly(HEMA-Am) Polymer Hydrogel Filler for Soft Tissue Reconstruction by Facile Polymerization. Polymers 2018, 10, 772. [Google Scholar] [CrossRef]
- Choudhury, P.; Kumar, S.; Singh, A.; Kumar, A.; Kaur, N.; Sanyasi, S.; Chawla, S.; Goswami, C.; Goswami, L. Hydroxyethyl methacrylate grafted carboxy methyl tamarind (CMT-g-HEMA) polysaccharide based matrix as a suitable scaffold for skin tissue engineering. Carbohydr. Polym. 2018, 189, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Vasile, E.; Pandele, A.M.; Andronescu, C.; Selaru, A.; Dinescu, S.; Costache, M.; Hanganu, A.; Raicopol, M.D.; Teodorescu, M. Hema-Functionalized Graphene Oxide: A Versatile Nanofiller for Poly(Propylene Fumarate)-Based Hybrid Materials. Sci. Rep. 2019, 9, 18685. [Google Scholar] [CrossRef] [PubMed]
- Harata, M.; Watanabe, M.; Nagata, S.; Ko, E.C.; Ohba, S.; Takato, T.; Hikita, A.; Hoshi, K. Improving chondrocyte harvests with poly(2-hydroxyethyl methacrylate) coated materials in the preparation for cartilage tissue engineering. Regen. Ther. 2017, 7, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Fabrication of Salecan/poly(AMPS-co-HMAA) semi-IPN hydrogels for cell adhesion. Carbohydr. Polym. 2017, 174, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, J.; Li, H.; Du, N.; Song, S.; Hou, W. Synthesis of layered double hydroxide/poly(N-isopropylacrylamide) nanocomposite hydrogels with excellent mechanical and thermoresponsive performances. Soft Matter 2018, 14, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Xu, Z.; Chen, Y.; Ke, Q.; Zhang, C.; Guo, Y. Ag-loaded MgSrFe-layered double hydroxide/chitosan composite scaffold with enhanced osteogenic and antibacterial property for bone engineering tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, F.; Solati-Hashjin, M.; Keshtkar, A.; Shokrgozar, M.A.; Dehghan, M.M.; Larijani, B. Novel layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffolds: Fabrication, characterization, and in vivo study. Mater. Sci. Eng. C 2017, 76, 701–714. [Google Scholar] [CrossRef]
- Radu, I.-C.; Vasile, E.; Damian, C.M.; Iovu, H.; Stanescu, P.O.; Zaharia, C. Influence of the Double Bond LDH Clay on the Exfoliation/Intercalation Mechanism of Polyacrylamide Nanocomposite Hydrogels. Mater. Plast. 2018, 55, 263–268. [Google Scholar] [CrossRef]
- Galateanu, B.; Radu, I.-C.; Vasile, E.; Hudita, A.; Serban, M.; Costache, M.; Iovu, H.; Zaharia, C. Fabrication of Novel Silk Fibroin—LDHs Composite Arhitectures for Potential Bone Tissue Engineering. Mater. Plast. 2017, 54, 659–665. [Google Scholar] [CrossRef]
- Mahkam, M.; Doostie, L. The relation between swelling properties and cross-linking of hydrogels designed for colon-specific drug delivery. Drug Deliv. 2005, 12, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ganji, F.; Vasheghani Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Ren, W.; Peng, Z.; Zhang, Y.; Zhang, Y. Preparation and Properties of a Water-Swelling Rubber by in situ Formed Lithium Acrylate in Nitrile Rubber. Polym. Polym. Compos. 2005, 13, 181–190. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; De Kee, D. Mass transport through swelling membranes. Int. J. Eng. Sci. 2005, 43, 1464–1470. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Vrentas, C.M. Steady viscoelastic diffusion. J. Appl. Polym. Sci. 2003, 88, 3256–3263. [Google Scholar] [CrossRef]
- El Afif, A.; Grmela, M. Non-Fickian Mass Transport in Polymers. J. Rheol. 2002, 46, 591–628. [Google Scholar] [CrossRef]
- Rajagopal, K.R. Diffusion through polymeric solids undergoing large deformations. Mater. Sci. Technol. 2003, 19, 1175–1180. [Google Scholar] [CrossRef]
- Shoukat, H.; Noreen, S.; Asghar, S. Hydrogels as Controlled Drug Delivery System: A Brief Review of Properties Classification and Synthesis. Glob. Pharm. Sci. Rev. 2020, V, 40–49. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Rodríguez-Rego, J.M.; Macías-García, A.; Mendoza-Cerezo, L.; Díaz-Parralejo, A. Relationship between shear-thinning rheological properties of bioinks and bioprinting parameters. Int. J. Bioprint. 2023, 9, 422–431. [Google Scholar] [CrossRef]
- Zhong, D.; Xiang, Y.; Wang, Z.; Chen, Z.; Liu, J.; Wu, Z.L.; Xiao, R.; Qu, S.; Yang, W. A visco-hyperelastic model for hydrogels with tunable water content. J. Mech. Phys. Solids 2023, 173, 105206. [Google Scholar] [CrossRef]
- Kwei, T.K.; Wang, T.T. Diffusion in Glassy Polymers. In Permeability of Plastic Films and Coatings: To Gases, Vapors, and Liquids; Hopfenberg, H.B., Ed.; Springer: Boston, MA, USA, 1974; pp. 63–71. [Google Scholar]
- Arya, R.K.; Thapliyal, D.; Sharma, J.; Verros, G.D. Glassy Polymers—Diffusion, Sorption, Ageing and Applications. Coatings 2021, 11, 1049. [Google Scholar] [CrossRef]
- Perova, T.S.; Vij, J.K.; Xu, H. Fourier transform infrared study of poly (2-hydroxyethyl methacrylate) PHEMA. Colloid Polym. Sci. 1997, 275, 323–332. [Google Scholar] [CrossRef]
- Tümay Özer, E.; Göçenoğlu Sarıkaya, A.; Osman, B. Adsorption and removal of diethyl phthalate from aqueous media with poly(hydroxyethyl methacrylate) nanobeads. Desalination Water Treat. 2016, 57, 28864–28874. [Google Scholar] [CrossRef]
- Morita, S. Hydrogen-bonds structure in poly(2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy. Front. Chem. 2014, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, R.; Delibaş, A. Removal of methylene blue from aqueous solutions by poly(2-acrylamido-2-methylpropane sulfonic acid-co-itaconic acid) hydrogels. Polym. Bull. 2012, 68, 1889–1903. [Google Scholar] [CrossRef]
- Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Vimala, K.; Raju, K.M. Synthesis and Characterizations of Macroporous Poly(acrylamide-2-acrylamido-2-methyl-1-propanesulfonic acid) Hydrogels for In Vitro Drug Release of Ranitidine Hydrochloride. Int. J. Polym. Mater. Polym. Biomater. 2011, 60, 490–503. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Anamul Haque, M.D.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z. A constitutive model of nanocomposite hydrogels with nanoparticle crosslinkers. J. Mech. Phys. Solids 2016, 94, 127–147. [Google Scholar] [CrossRef]






| Sample | Monomer Concentration; 5 mol/L LDH-ATPM Concentration (w/v) 1% | Monomer Concentration; 5 mol/L LDH-ATPM Concentration (w/v) 2% | Monomer Concentration; 2 mol/L LDH-ATPM Concentration (w/v) 1% | Monomer Concentration; 2 mol/L LDH-ATPM Concentration (w/v) 2% |
|---|---|---|---|---|
| Monomer ratio (mol/mol): 100–0 | 5M-H100-A0-1% LDH-ATPM | 5M-H100-A0-2% LDH-ATPM | No crosslinking | No crosslinking |
| Monomer ratio (mol/mol): 97–3 | 5M-H97-A3-1% LDH-ATPM | 5M-H97-A3-2% LDH-ATPM | 2M-H97-A3-1% LDH-ATPM | 2M-H97-A3-2% LDH-ATPM |
| Monomer ratio (mol/mol): 95–5 | 5M-H95-A5-1% LDH-ATPM | 5M-H95-A5-2% LDH-ATPM | 2M-H95-A5-1% LDH-ATPM | 2M-H95-A5-2% LDH-ATPM |
| Sample | LDH-ATPM | MBA Equivalent | Sample | LDH-ATPM | MBA Equivalent |
|---|---|---|---|---|---|
| 2M-H95-A5 | 1% | 2.5% | 2M-H95-A5 | 2% | 1.6% |
| 2M-H97-A3 | 1% | 2.75% | 2M-H97-A3 | 2% | 2.5% |
| 2M-H100-A0 | - | - | 2M-H100-A0 | - | - |
| 5M-H95-A5 | 1% | 0.8% | 5M-H95-A5 | 2% | 0.9% |
| 5M-H97-A3 | 1% | 1.1% | 5M-H97-A3 | 2% | 1.1% |
| 5M-H100-A0 | 1% | 2.6% | 5M-H100-A0 | 2% | 2.75% |
| Sample | Time (minutes) | Swelling Degree (%) | n | Sample | Time (minute) | Swelling Degree (%) | n |
|---|---|---|---|---|---|---|---|
| 2M-H95-A5-1% LDH-ATPM | 4920 | 78 ± 4 | 0.4130 ± 0.002 | 2M-H95-A5-2% LDH-ATPM | 2140 | 86 ± 5 | 0.6103 ± 0.002 |
| 2M-H97-A3-1% LDH-ATPM | 4920 | 67 ± 5 | 0.4130 ± 0.002 | 2M-H97-A3-2% LDH-ATPM | 2020 | 78 ± 5 | 0.601 ± 0.002 |
| 2M-H100-A0-1% LDH-ATPM | - | - | - | 2M-H100-A0-2% LDH-ATPM | - | - | - |
| 5M-H95-A5-1% LDH-ATPM | 4140 | 145 ± 10 | 0.4826 ± 0.003 | 5M-H95-A5-2% LDH-ATPM | 2880 | 137 ± 8 | 0.4978 ± 0.004 |
| 5M-H97-A3-1% LDH-ATPM | 4140 | 112 ± 8 | 0.4815 ± 0.003 | 5M-H97-A3-2% LDH-ATPM | 2880 | 107 ± 6 | 0.4961 ± 0.003 |
| 5M-H100-A0-1% LDH-ATPM | 4140 | 74 ± 4 | 0.4808 ± 0.005 | 5M-H100-A0-2% LDH-ATPM | 2880 | 68 ± 5 | 0.4961 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, I.-C.; Tanasa, E.; Dinescu, S.; Vlasceanu, G.; Zaharia, C. Advanced Nanobiocomposite Hydrogels Incorporating Organofunctionalized LDH for Soft Tissue Engineering Applications. Polymers 2025, 17, 536. https://doi.org/10.3390/polym17040536
Radu I-C, Tanasa E, Dinescu S, Vlasceanu G, Zaharia C. Advanced Nanobiocomposite Hydrogels Incorporating Organofunctionalized LDH for Soft Tissue Engineering Applications. Polymers. 2025; 17(4):536. https://doi.org/10.3390/polym17040536
Chicago/Turabian StyleRadu, Ionut-Cristian, Eugenia Tanasa, Sorina Dinescu, George Vlasceanu, and Catalin Zaharia. 2025. "Advanced Nanobiocomposite Hydrogels Incorporating Organofunctionalized LDH for Soft Tissue Engineering Applications" Polymers 17, no. 4: 536. https://doi.org/10.3390/polym17040536
APA StyleRadu, I.-C., Tanasa, E., Dinescu, S., Vlasceanu, G., & Zaharia, C. (2025). Advanced Nanobiocomposite Hydrogels Incorporating Organofunctionalized LDH for Soft Tissue Engineering Applications. Polymers, 17(4), 536. https://doi.org/10.3390/polym17040536







