Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing
Abstract
1. Introduction
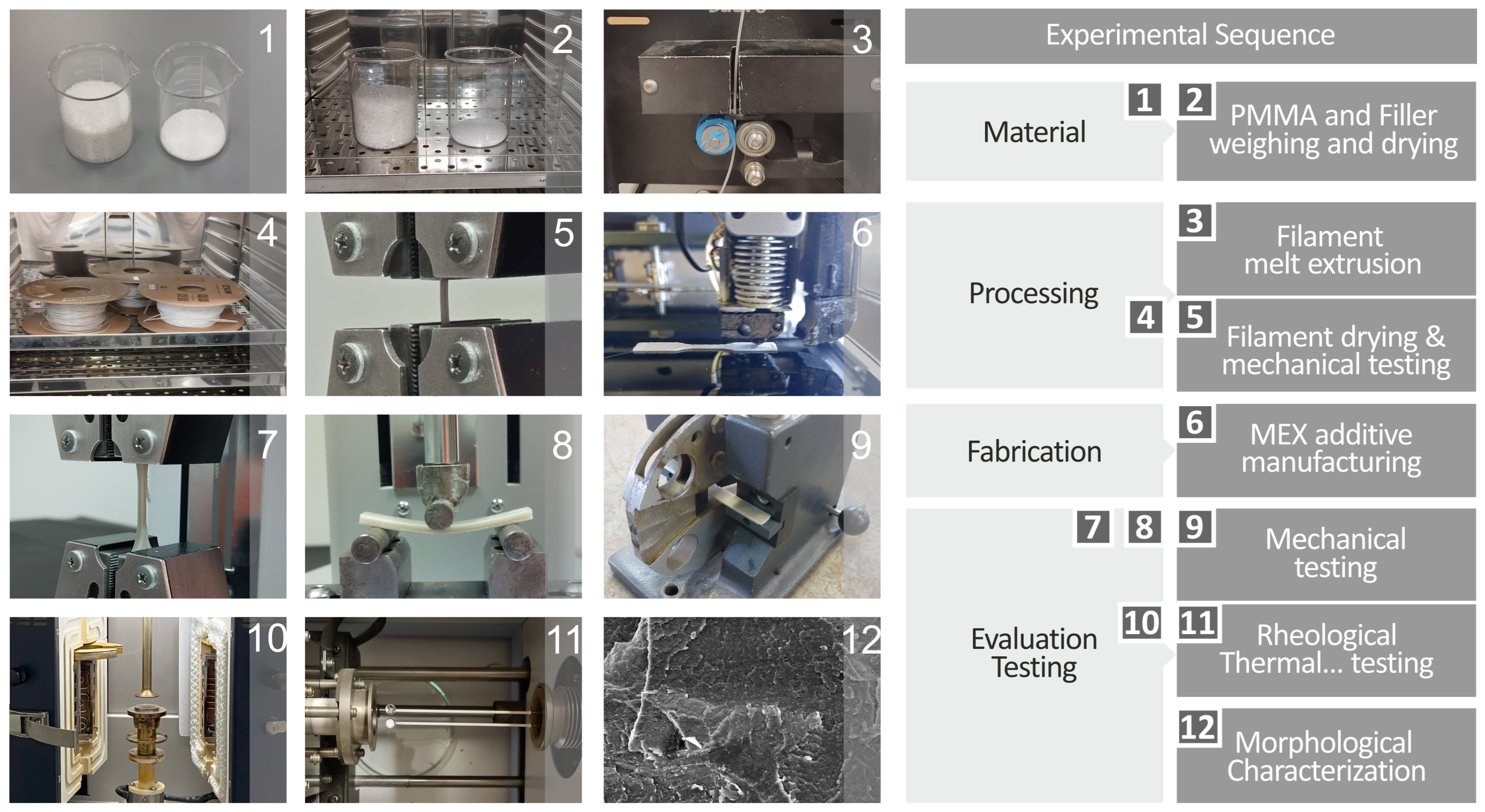
2. Materials and Methods
2.1. PMMA and Antibacterial Nanopowder Materials
2.2. PMMA Nanocomposites and Corresponding Filaments Preparation
2.3. 3DP of the PMMA—Antibacterial Nanocomposites
2.4. Spectroscopic and Thermophysical Characterization of the PMMA—Antibacterial Nanocomposites
2.5. Rheology Analysis of the PMMA—Antibacterial Nanocomposites
2.6. Mechanical Response of the PMMA—Antibacterial Nanocomposites
2.7. Evaluation of the Morphological Characteristics and the Chemical Composition of the PMMA—Antibacterial Nanocomposites
2.8. Quality Metrics Evaluation of the PMMA—Antibacterial Nanocomposites
2.9. Evaluation of the Biocidal Effect of the PMMA—Antibacterial Nanocomposites
3. Results
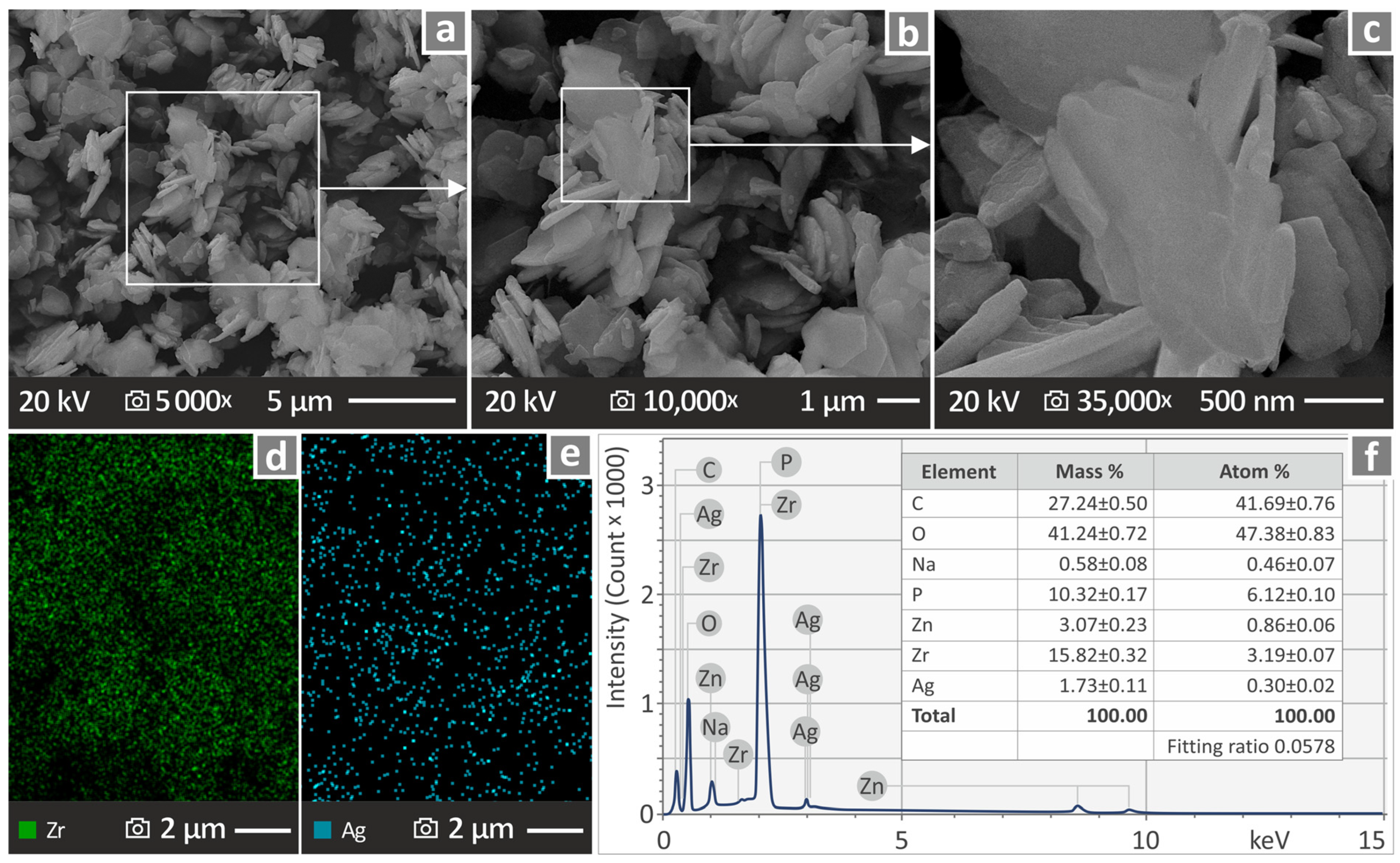
3.1. Morphological and Chemical Assessment of the Antibacterial Nanopowder
3.2. Vibrational Spectroscopy Investigation of the PMMA—Antibacterial Nanocomposites
3.3. Rheometry and Melt Flow Rate Characteristics of the PMMA—Antibacterial Nanocomposites
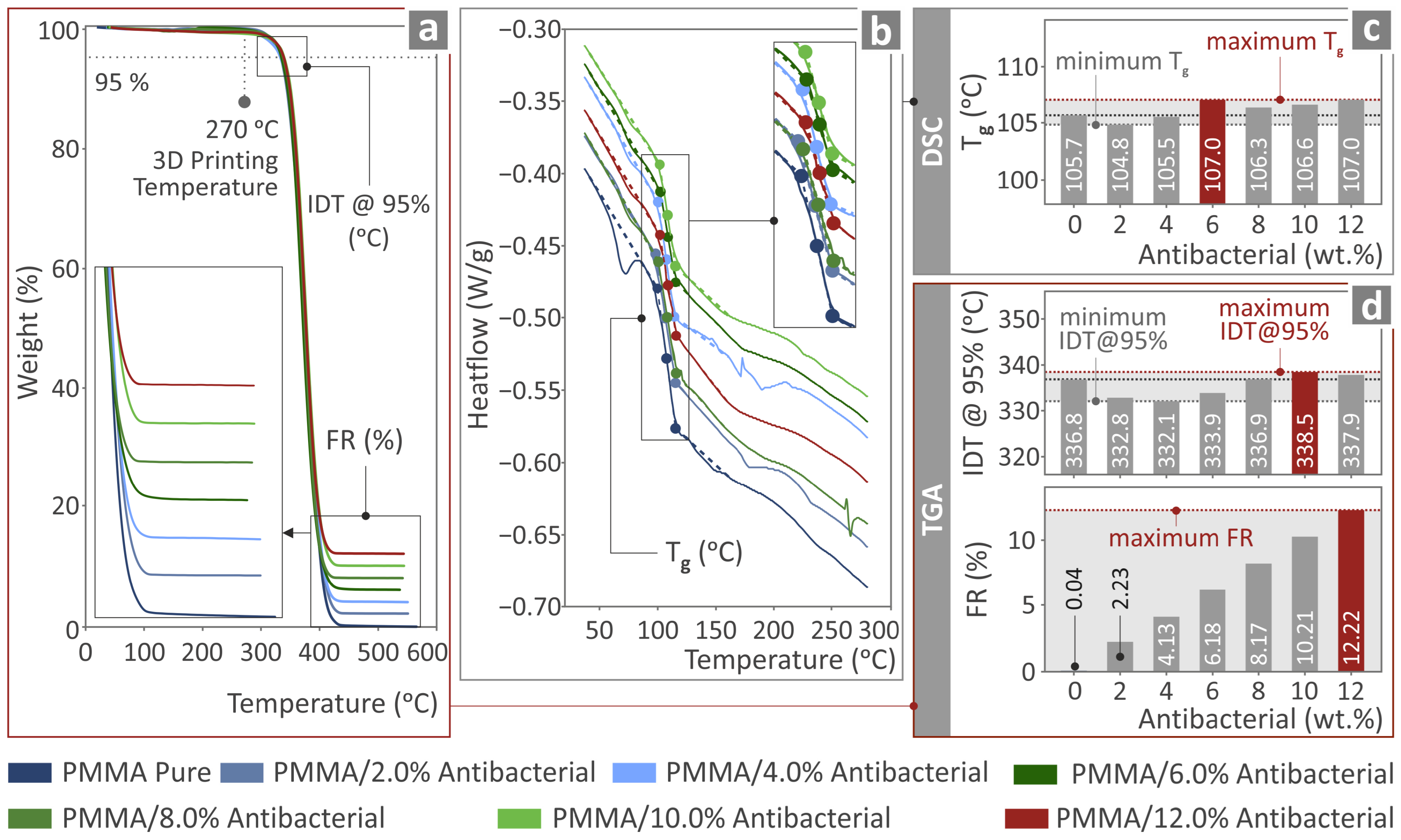
3.4. Thermochemical Investigation of the PMMA—Antibacterial Nanocomposites
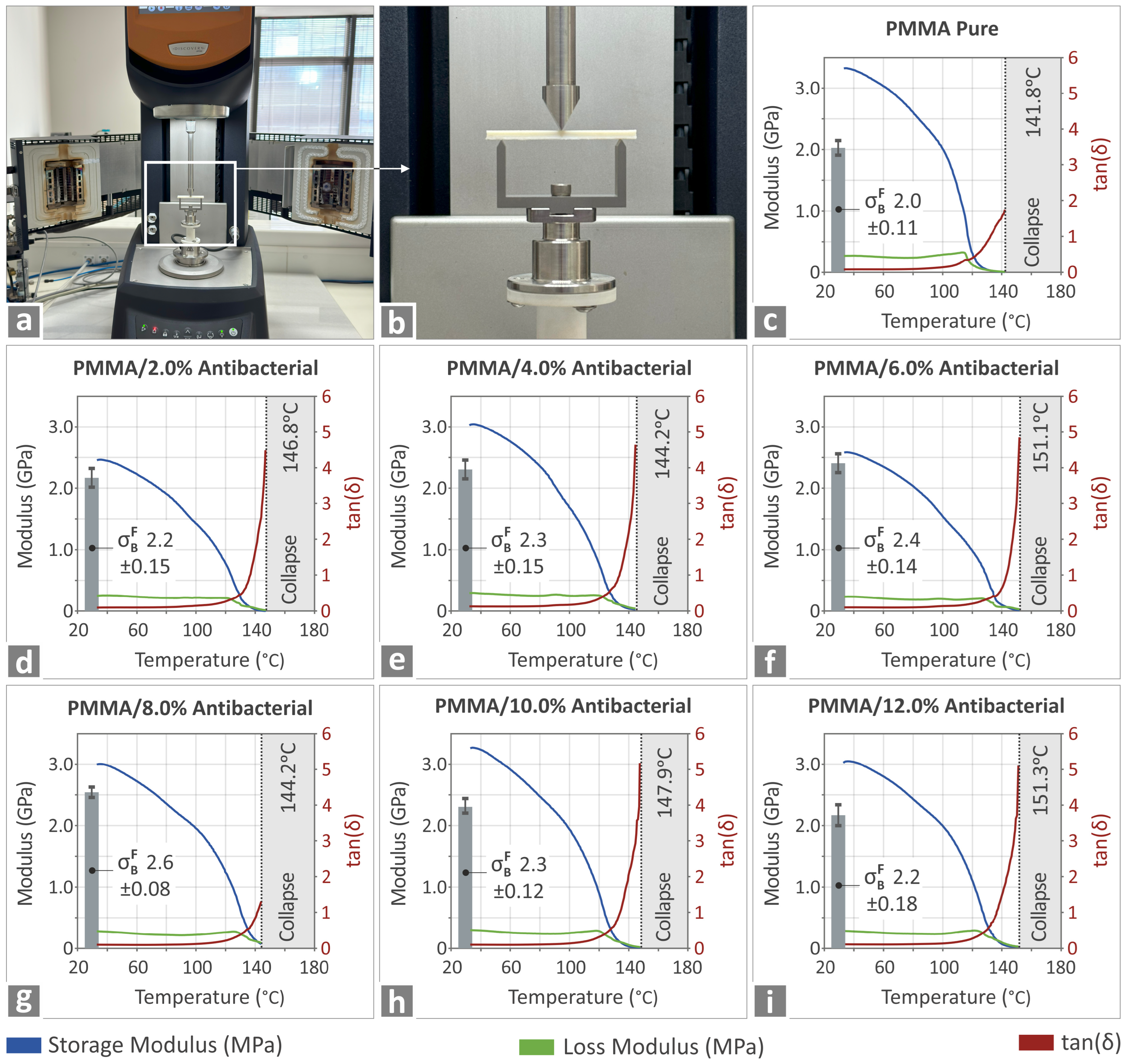
3.5. Investigation of the Thermomechanical Properties and Behavior of the PMMA—Antibacterial Nanocomposites
3.6. Quality Assurance of the Prepared PMMA—Antibacterial Nanocomposites Produced Filaments
3.7. Evaluating the Mechanical Response of the PMMA—Antibacterial Nanocompounds
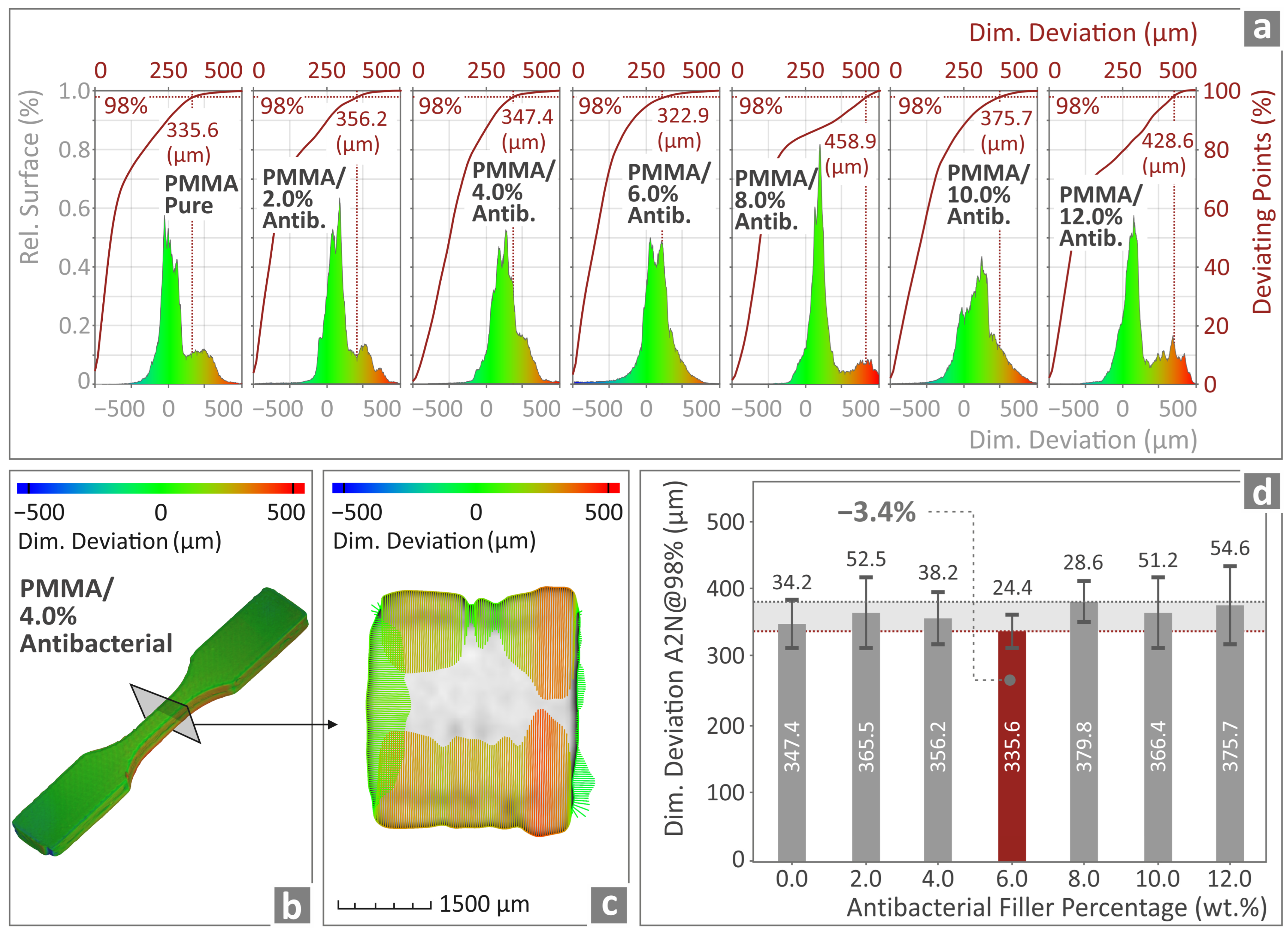
3.8. Examination of the Overall Morphology and Dimensional Characteristics of the PMMA—Antibacterial Nanocomposites Through μ-Computed High-Resolution Tomography
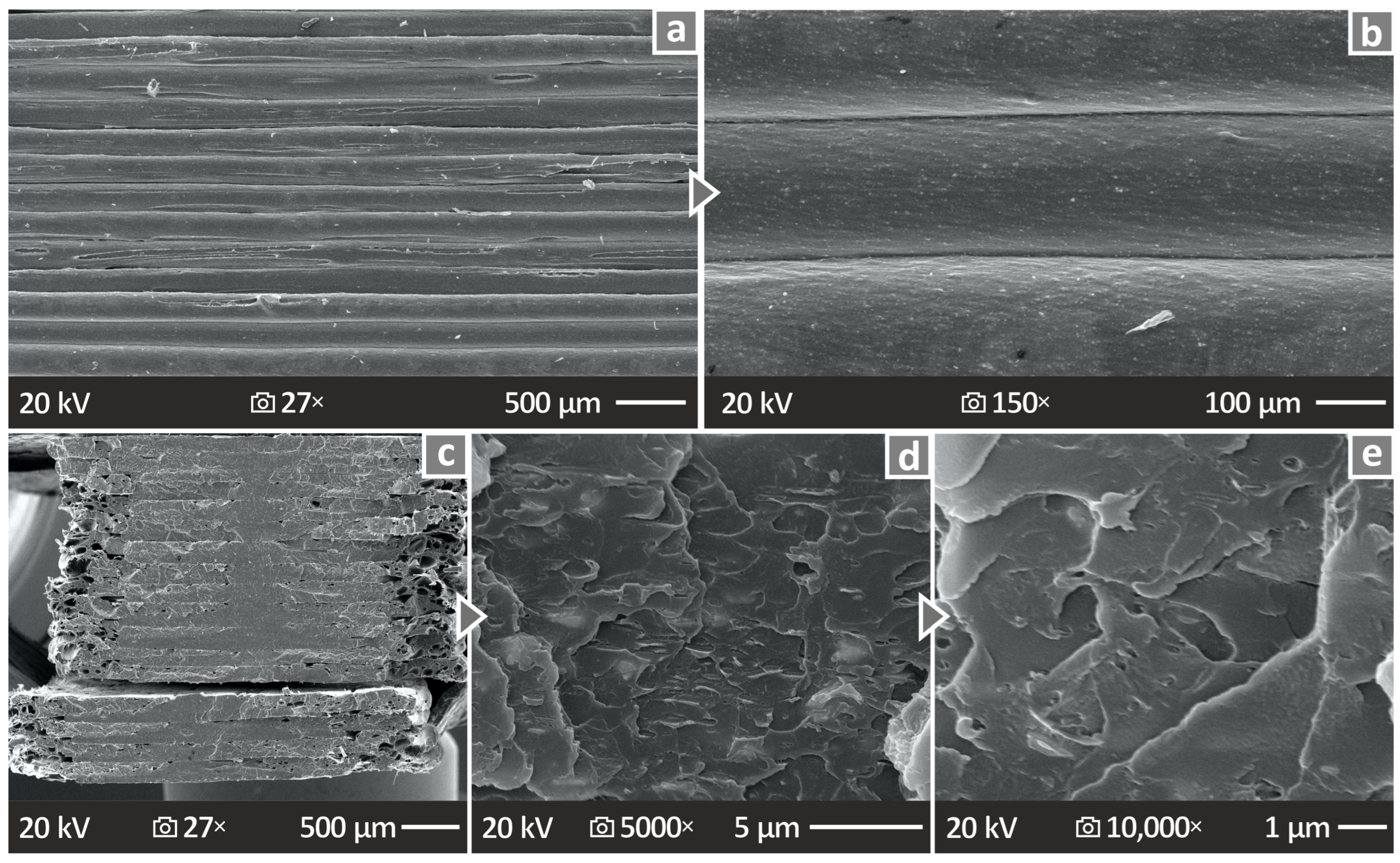
3.9. SEM Investigation of the Morphological Characteristics of the PMMA—Antibacterial Nanocomposites
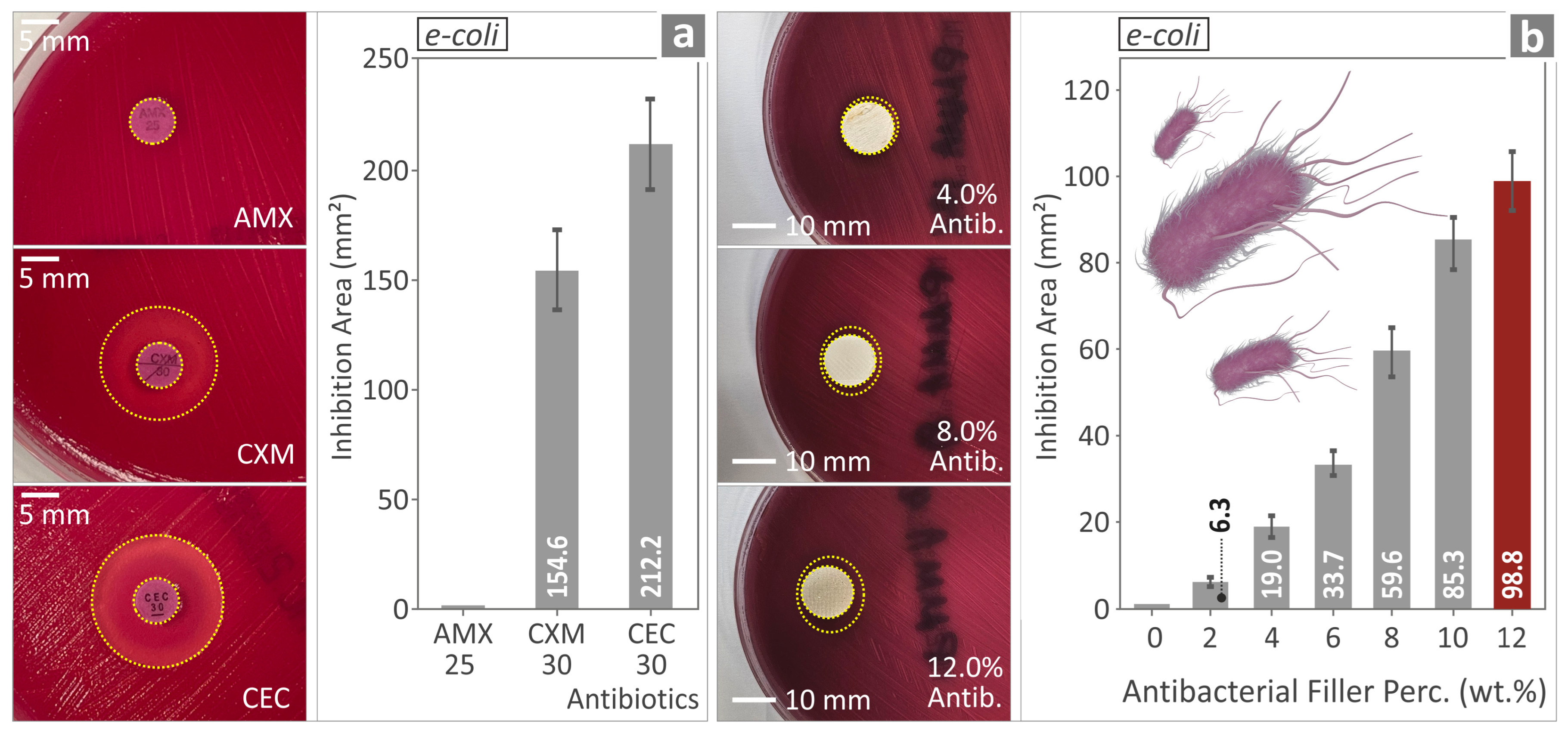
3.10. Evaluating the Biocidal Capabilities of the PMMA—Antibacterial Nanocompounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dittenber, D.B.; GangaRao, H.V.S. Critical Review of Recent Publications on Use of Natural Composites in Infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Cruickshank, C.; Foged, S.; Thorsen, J.; Krebs, F.C. Business, Market and Intellectual Property Analysis of Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1553–1571. [Google Scholar] [CrossRef]
- Cheah, C.M.; Chua, C.K.; Lee, C.W.; Feng, C.; Totong, K. Rapid Prototyping and Tooling Techniques: A Review of Applications for Rapid Investment Casting. Int. J. Adv. Manuf. Technol. 2005, 25, 308–320. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of Selective Laser Melting: Materials and Applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Parandoush, P.; Lin, D. A Review on Additive Manufacturing of Polymer-Fiber Composites. Compos. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Lambert-Perlade, A.; Gourgues, A.F.; Besson, J.; Sturel, T.; Pineau, A. Mechanisms and Modeling of Cleavage Fracture in Simulated Heat-Affected Zone Microstructures of a High-Strength Low Alloy Steel. Metall. Mater. Trans. A 2004, 35, 1039–1053. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Pei, Q.; Zhang, J.-X.; Bi, Z.-Y.; Li, P.-C. Study on the Microstructure and Mechanical Properties of Explosive Welded 2205/X65 Bimetallic Sheet. Mater. Des. 2014, 64, 462–476. [Google Scholar] [CrossRef]
- Angrish, A. A Critical Analysis of Additive Manufacturing Technologies for Aerospace Applications. In Proceedings of the 2014 IEEE Aerospace Conference, Big Sky, MT, USA, 1–8 March 2014; pp. 1–6. [Google Scholar]
- Weyhrich, C.W.; Long, T.E. Additive Manufacturing of High-Performance Engineering Polymers: Present and Future. Polym. Int. 2022, 71, 532–536. [Google Scholar] [CrossRef]
- Bird, D.T.; Ravindra, N.M. Additive Manufacturing of Sensors for Military Monitoring Applications. Polymers 2021, 13, 1455. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, N.; Petousis, M.; Moutsopoulou, A.; Argyros, A.; Ntintakis, I.; Papadakis, V.; Nasikas, N.K.; Vidakis, N. Engineering Response of Biomedical Grade Isotactic Polypropylene Reinforced with Titanium Nitride Nanoparticles for Material Extrusion Three-Dimensional Printing. Eur. J. Mater. 2024, 4, 2340944. [Google Scholar] [CrossRef]
- Nasikas, N.K.; Petousis, M.; Papadakis, V.; Argyros, A.; Valsamos, J.; Gkagkanatsiou, K.; Sagris, D.; David, C.; Michailidis, N.; Maravelakis, E.; et al. A Comprehensive Optimization Course of Antimony Tin Oxide Nanofiller Loading in Polyamide 12: Printability, Quality Assessment, and Engineering Response in Additive Manufacturing. Nanomaterials 2024, 14, 1285. [Google Scholar] [CrossRef] [PubMed]
- Petousis, M.; Michailidis, N.; Saltas, V.; Papadakis, V.; Spiridaki, M.; Mountakis, N.; Argyros, A.; Valsamos, J.; Nasikas, N.K.; Vidakis, N. Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing. Nanomaterials 2024, 14, 761. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Charou, C.; Moutsopoulou, A. Polylactic Acid/Silicon Nitride Biodegradable and Biomedical Nanocomposites with Optimized Rheological and Thermomechanical Response for Material Extrusion Additive Manufacturing. Biomed. Eng. Adv. 2023, 6, 100103. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Nasikas, N.; Papadakis, V.; Argyros, A.; Mountakis, N.; Charou, C.; Moutsopoulou, A. Optimizing Titanium Carbide (TiC) Ceramic Nanofiller Loading in Isotactic Polypropylene for MEX Additive Manufacturing: Mechano-Thermal and Rheology Aspects. Mater. Today Commun. 2023, 37, 107368. [Google Scholar] [CrossRef]
- Michailidis, N.; Petousis, M.; Saltas, V.; Papadakis, V.; Spiridaki, M.; Mountakis, N.; Argyros, A.; Valsamos, J.; Nasikas, N.K.; Vidakis, N. Investigation of the Effectiveness of Silicon Nitride as a Reinforcement Agent for Polyethylene Terephthalate Glycol in Material Extrusion 3D Printing. Polymers 2024, 16, 1043. [Google Scholar] [CrossRef] [PubMed]
- Petousis, M.; Michailidis, N.; Papadakis, V.; Argyros, A.; Spiridaki, M.; Mountakis, N.; Valsamos, J.; Nasikas, N.K.; Moutsopoulou, A.; Vidakis, N. Enhanced Engineering and Biocidal Polypropylene Filaments Enabling Melt Reduction of AgNO3 through PVP Agent: A Scalable Process for the Defense Industry with MEX Additive Manufacturing. Def. Technol. 2024, in press. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; David, C.; Nasikas, N.K.; Sagris, D.; Mountakis, N.; Spiridaki, M.; Moutsopoulou, A.; Stratakis, E. Critical Quality Indicators of High-Performance Polyetherimide (ULTEM) over the MEX 3D Printing Key Generic Control Parameters: Prospects for Personalized Equipment in the Defense Industry. Def. Technol. 2024, 43, 150–167. [Google Scholar] [CrossRef]
- Vidakis, N.; Michailidis, N.; Petousis, M.; Nasikas, N.K.; Saltas, V.; Papadakis, V.; Mountakis, N.; Argyros, A.; Spiridaki, M.; Valsamos, I. Multifunctional HDPE/Cu Biocidal Nanocomposites for MEX Additive Manufactured Parts: Perspectives for the Defense Industry. Def. Technol. 2024, 38, 16–32. [Google Scholar] [CrossRef]
- Xue, J.; Hou, Y.; Chu, W.; Zhang, Z.; Dong, Z.; Zhang, L.; Wen, G. 3D Printed B4C-Based Honeycomb Ceramic Composite and Its Potential Application in Three-Dimensional Armor Structure. Chem. Eng. J. 2024, 493, 152607. [Google Scholar] [CrossRef]
- Du, Z.; Chen, C.; Wang, X. Study on the Mechanism and Performance of 3D-Printed PLA/Epoxy Composite for Stab Resistance. Rapid Prototyp. J. 2024, 30, 239–252. [Google Scholar] [CrossRef]
- Sitotaw, D.B.; Muenks, D.; Kebash, A.K. 3D Printing Applications on Textiles: Measurement of Air Permeability for Potential Use in Stab-Proof Vests. J. Eng. Fiber Fabr. 2024, 19, 15589250241232152. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Saltas, V.; Sagris, D.; Spiridaki, M.; Argyros, A.; Mountakis, N.; Papadakis, V. Interpretation of the Optimization Course of Silicon Nitride Nano-Powder Content in Biomedical Resins for Vat Photopolymerization Additive Manufacturing. Ceram. Int. 2024, 50, 14919–14935. [Google Scholar] [CrossRef]
- Aktitiz, İ.; Aydın, K.; Darıcık, F.; Topcu, A. Production of Different Metal Oxide Nanoparticle Embedded Polymer Matrix Composite Structures by the Additive Manufacturing Technology and Investigation of Their Properties. Polym. Compos. 2022, 43, 7826–7835. [Google Scholar] [CrossRef]
- Cano, S.; Gooneie, A.; Kukla, C.; Rieß, G.; Holzer, C.; Gonzalez-Gutierrez, J. Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing. Appl. Sci. 2020, 10, 1471. [Google Scholar] [CrossRef]
- Billings, C.; Cai, C.; Liu, Y. Utilization of Antibacterial Nanoparticles in Photocurable Additive Manufacturing of Advanced Composites for Improved Public Health. Polymers 2021, 13, 2616. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; David, C.N.; Sagris, D.; Mountakis, N. Biomedical Resin Reinforced with Cellulose Nanofibers (CNF) in VAT Photopolymerization (VPP) Additive Manufacturing (AM): The Effect of Filler Loading and Process Control Parameters on Critical Quality Indicators (CQIs). J. Manuf. Process. 2023, 101, 755–769. [Google Scholar] [CrossRef]
- Kwon, H.; Park, S.; Kwon, S.; Lee, H.-T. Effect of the Cellulose Nanofiber on Ultraviolet Curable Resin for Additive Manufacturing: Mechanical Properties and Printability. Addit. Manuf. 2023, 78, 103879. [Google Scholar] [CrossRef]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Gauss, C.; Pickering, K.L. A New Method for Producing Polylactic Acid Biocomposites for 3D Printing with Improved Tensile and Thermo-Mechanical Performance Using Grafted Nanofibrillated Cellulose. Addit. Manuf. 2023, 61, 103346. [Google Scholar] [CrossRef]
- Dong, J.; Mei, C.; Han, J.; Lee, S.; Wu, Q. 3D Printed Poly(Lactic Acid) Composites with Grafted Cellulose Nanofibers: Effect of Nanofiber and Post-Fabrication Annealing Treatment on Composite Flexural Properties. Addit. Manuf. 2019, 28, 621–628. [Google Scholar] [CrossRef]
- Yao, K.; Hong, G.; Yuan, X.; Kong, W.; Xia, P.; Li, Y.; Chen, Y.; Liu, N.; He, J.; Shi, J.; et al. 3D Printing of Tough Hydrogel Scaffolds with Functional Surface Structures for Tissue Regeneration. Nano-Micro Lett. 2024, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, Z.; Chen, X.; Zhu, W.; Yang, M.; Yu, M.; Sun, J.; Zuo, Y.; He, J.; Pan, H.; et al. High Mechanical Performance and Multifunctional Degraded Fucoidan-Derived Bioink for 3D Bioprinting. Carbohydr. Polym. 2025, 348, 122805. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K. PLA Based Biodegradable Bionanocomposite Filaments Reinforced with Nanocellulose: Development and Analysis of Properties. Sci. Rep. 2024, 14, 23819. [Google Scholar] [CrossRef]
- Mirzavandi, Z.; Poursamar, S.A.; Amiri, F.; Bigham, A.; Rafienia, M. 3D Printed Polycaprolactone/Gelatin/Ordered Mesoporous Calcium Magnesium Silicate Nanocomposite Scaffold for Bone Tissue Regeneration. J. Mater. Sci. Mater. Med. 2024, 35, 58. [Google Scholar] [CrossRef]
- Kaul, N.; Cachelin, S. Non-Lethal Weapons and the Sensory Repression of Dissent in Democracies. Secur. Dialogue 2024, 55, 368–385. [Google Scholar] [CrossRef]
- Robbe, C.; Papy, A.; Nsiampa, N.; Drapela, P.; Bir, C. NATO Standardized Method for Assessing the Thoracic Impact of Kinetic Energy Non-Lethal Weapons. Hum. Factors Mech. Eng. Def. Saf. 2023, 7, 7. [Google Scholar] [CrossRef]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced Polymer-Based Composites and Structures for Biomedical Applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; Rehan, Z.A.; Noreen, S.; Zahid, M. Recent Developments for Antimicrobial Applications of Graphene-Based Polymeric Composites: A Review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.-Q.; Tan, P.-F.; Gu, Z.-P.; Liu, Z.-M.; Tan, L. Polymeric Antibacterial Materials: Design, Platforms and Applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Gupta, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Conducting Polymers and Their Composites. Macromol 2022, 2, 78–99. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Guney, M.; Yildiran Avcu, Y.; Ur Rehman, M.A.; Boccaccini, A.R. Biodegradable Polymer Matrix Composites Containing Graphene-Related Materials for Antibacterial Applications: A Critical Review. Acta Biomater. 2022, 151, 1–44. [Google Scholar] [CrossRef]
- Satanovsky, A.; Gilor, Y.; Benov, A.; Chen, J.; Shlaifer, A.; Talmy, T.; Radomislensky, I.; Siman-Tov, M.; Peleg, K.; Weil, Y.A.; et al. Combat Injury Profile in Urban Warfare. Mil. Med. 2024, 189, 973–979. [Google Scholar] [CrossRef]
- Sanyal, A.; Roy, S.; Ghosh, A.; Chakraborty, M.; Ghosh, A.; Mandal, D. The next Frontier in Hemorrhagic Management: A Comprehensive Review on Development of Natural Polymer-Based Injectable Hydrogels as Promising Hemostatic Dressings. Chem. Eng. J. 2024, 497, 155033. [Google Scholar] [CrossRef]
- Pasquier, P.; David, M.; Petit, L.; Chery, M.; Habas, S.; Patey, E.; Conort, S.; Zeller, N.; Gelmann, M.-O.; Peyrefitte, S.; et al. Irregular Warfare Must Combine Good Medicine, with Both Good Tactics and Good Strategies: Position Paper by the French Special Operations Forces Medical Command. J. Trauma Acute Care Surg. 2024, 97, S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ramakrishna, S.; Singh, R. Material Issues in Additive Manufacturing: A Review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive Manufacturing Methods and Modelling Approaches: A Critical Review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef]
- Yadav, S.; Liu, S.; Singh, R.K.; Sharma, A.K.; Rawat, P. A State-of-Art Review on Functionally Graded Materials (FGMs) Manufactured by 3D Printing Techniques: Advantages, Existing Challenges, and Future Scope. J. Manuf. Process. 2024, 131, 2051–2072. [Google Scholar] [CrossRef]
- Boretti, A. A Techno-Economic Perspective on 3D Printing for Aerospace Propulsion. J. Manuf. Process. 2024, 109, 607–614. [Google Scholar] [CrossRef]
- Atalie, D.; Guo, Z.-S.; Berihun, D.; Tadesse, M.; Ma, P.-C. 20—Role of Additive Manufacturing in Defense Technologies: Emerging Trends and Future Scope. In Additive Manufacturing Materials and Technology; Rangappa, S.M., Ayyappan, V., Siengchin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 501–521. ISBN 978-0-443-18462-8. [Google Scholar]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-Based Crystalline Nanoparticles, Microbially Fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, D.; Santo, L.; Quadrini, F.; Hassiba, M.; Bader, N.; Chowdhury, S.H.; Hassan, M.K.; Zughaier, S.M. Cytotoxicity and Antibiofilm Activity of Silver-Polypropylene Nanocomposites. Antibiotics 2023, 12, 924. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R. Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Salam, O.A.; Hamad, H.A.; Eltokhy, M.A.R.; Ali, A.I.; Son, J.Y.; Ramzy, G.H. A Comparative Study of PMMA/PEG Polymer Nanocomposites Doped with Different Oxides Nanoparticles for Potential Optoelectronic Applications. Sci. Rep. 2024, 14, 19295. [Google Scholar] [CrossRef]
- Kim, S.-I.; Moon, J.-Y.; Hyeong, S.-K.; Ghods, S.; Kim, J.-S.; Choi, J.-H.; Park, D.S.; Bae, S.; Cho, S.H.; Lee, S.-K.; et al. Float-Stacked Graphene–PMMA Laminate. Nat. Commun. 2024, 15, 2172. [Google Scholar] [CrossRef]
- Ahn, C.H.; Zhang, G.; Suo, Z. Ductility of a Nanocomposite of Glassy and Rubbery Polymers. J. Mech. Phys. Solids 2024, 191, 105760. [Google Scholar] [CrossRef]
- Zong, Y.; Wu, K.; Fang, W.; Yu, J.; Jiang, C.; Xu, G. Modified Intrascleral Fixation for Repositioning the Dislocated Single-Piece, Rigid PMMA Intraocular Lens. RETINA 2023, 43, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.M.; Jurczak, K.M.; Lynn, N.S.; Mulder, J.-P.S.H.; Verpoorte, E.M.J.; Nagelkerke, A. Rapid Prototyping of PMMA-Based Microfluidic Spheroid-on-a-Chip Models Using Micromilling and Vapour-Assisted Thermal Bonding. Sci. Rep. 2024, 14, 2831. [Google Scholar] [CrossRef]
- Obaeed, N.H.; Hamdan, W.K. Reconstruction and Evaluation of 3D Printing PMMA Cranioplasty Implants. Int. J. Interact. Des. Manuf. (IJIDeM) 2024, 18, 4233–4245. [Google Scholar] [CrossRef]
- Durkin, A.J.; Catena, D.; Woltjen, N.; Boyle, K.; Polling, M.; Weng, J.; Chim, J.H. Surgical Management of Polymethylmethacrylate-Collagen Gel Complications in the Lower Eyelid: A Case Series. Ann. Plast. Surg. 2023, 90, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mishra, R.K.; Parveen, S.; Avinashi, S.K.; Hussain, A.; Kumar, S.; Banerjee, M.; Rao, J.; Kumar, R.; Gautam, R.K.; et al. Fabrication, Structural, and Enhanced Mechanical Behavior of MgO Substituted PMMA Composites for Dental Applications. Sci. Rep. 2024, 14, 2128. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Abbasi-Firouzjah, M.; Shokri, B. Investigation of Antibacterial and Wettability Behaviours of Plasma-Modified PMMA Films for Application in Ophthalmology. J. Phys. D Appl. Phys. 2014, 47, 085401. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural Strength, Biocompatibility, and Antimicrobial Activity of a Polymethyl Methacrylate Denture Resin Enhanced with Graphene and Silver Nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary Focus on the Mechanical and Antibacterial Activity of a PMMA-Based Bone Cement Loaded with Gold Nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Su, W.; Wang, S.; Wang, X.; Fu, X.; Weng, J. Plasma Pre-Treatment and TiO2 Coating of PMMA for the Improvement of Antibacterial Properties. Surf. Coat. Technol. 2010, 205, 465–469. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Zanocco, M.; Honma, T.; Zhu, W.; Pezzotti, G. Explorative Study on the Antibacterial Effects of 3D-Printed PMMA/Nitrides Composites. Mater. Des. 2021, 206, 109788. [Google Scholar] [CrossRef]
- An, J.; Ding, N.; Zhang, Z. Mechanical and Antibacterial Properties of Polymethyl Methacrylate Modified with Zinc Dimethacrylate. J. Prosthet. Dent. 2022, 128, 100.e1–100.e8. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Moutsopoulou, A.; Karapidakis, E. Energy Consumption vs. Tensile Strength of Poly [Methyl Methacrylate] in Material Extrusion 3D Printing: The Impact of Six Control Settings. Polymers 2023, 15, 845. [Google Scholar] [CrossRef]
- Song, S.; Lin, X.; Ming, X.; Lu, D.; Sun, M.; Wang, B.; Bao, C.; Lu, B.; Ma, E. PMMA Light Channels Facilitate the Additive Manufacturing of Complex-Structured SiC Reflector Mirrors. J. Eur. Ceram. Soc. 2025, 45, 116970. [Google Scholar] [CrossRef]
- Mikhalchan, A.; Tay, T.E.; Banas, A.M.; Banas, K.; Breese, M.B.H.; Borkowska, A.M.; Nowakowski, M.; Kwiatek, W.M.; Paluszkiewicz, C. Development of Continuous CNT Fibre-Reinforced PMMA Filaments for Additive Manufacturing: A Case Study by AFM-IR Nanoscale Imaging. Mater. Lett. 2020, 262, 127182. [Google Scholar] [CrossRef]
- Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Development of Dental Poly(Methyl Methacrylate)-Based Resin for Stereolithography Additive Manufacturing. Polymers 2021, 13, 4435. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Petersmann, S.; Hentschel, L.; Gonzalez-Gutierrez, J.; Tödtling, M.; Schäfer, U.; Arbeiter, F.; Üçal, M. The Effects of Washing and Formaldehyde Sterilization on the Mechanical Performance of Poly(Methyl Methacrylate) (PMMA) Parts Produced by Material Extrusion-Based Additive Manufacturing or Material Jetting. Adv. Eng. Mater. 2023, 25, 2200225. [Google Scholar] [CrossRef]
- Puppi, D.; Morelli, A.; Bello, F.; Valentini, S.; Chiellini, F. Additive Manufacturing of Poly(Methyl Methacrylate) Biomedical Implants with Dual-Scale Porosity. Macromol. Mater. Eng. 2018, 303, 1800247. [Google Scholar] [CrossRef]
- Stenzler, J.S.; Goulbourne, N.C. The Effect of Polyacrylate Microstructure on the Impact Response of PMMA/PC Multi-Laminates. Int. J. Impact Eng. 2011, 38, 567–576. [Google Scholar] [CrossRef]
- Xia, F.; Yang, L.; Dai, B.; Gao, G.; Yang, Z.; Cao, W.; Xu, L.; Geng, F.; Song, Z.; Ralchenko, V.; et al. Novel Reparation Method for Polymethyl Methacrylate Optical Windows of Aircrafts Damaged by Service Environment. Sci. China Technol. Sci. 2020, 63, 1585–1590. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Fan, Y.; Ding, C.; Pan, L.; Feng, X.; Liu, X.; Hu, J.; Chen, J.; Gao, L.; et al. Highly Efficient Broadband Solar-Blind UV Photodetector Based on Gd2O3:Eu3+–PMMA Composite Film. Adv. Mater. Interfaces 2020, 7, 2000570. [Google Scholar] [CrossRef]
- Tang, E.; Wang, D.; Li, L.; Peng, H.; Han, Y.; Chen, C.; Chang, M.; Guo, K.; He, L. Polarization Response Characteristics of 6061Al and PMMA Sheets under Impact Load. Int. J. Impact Eng. 2023, 178, 104632. [Google Scholar] [CrossRef]
- Torres, S.M.; Vorobiev, O.Y.; Robey, R.E.; Hargather, M.J. A Study of Explosive-Induced Fracture in Polymethyl Methacrylate (PMMA). J. Appl. Phys. 2023, 134, 075103. [Google Scholar] [CrossRef]
- Torres, S.M.; Hargather, M.J.; Kimberley, J.; Robey, R.E. Shock Response of Polymethyl Methacrylate (PMMA) Under Explosive Loading. J. Dyn. Behav. Mater. 2024, 10, 270–280. [Google Scholar] [CrossRef]
- Kazarinov, N.A.; Bratov, V.A.; Morozov, N.F.; Petrov, Y.V.; Balandin, V.V.; Iqbal, M.A.; Gupta, N.K. Experimental and Numerical Analysis of PMMA Impact Fracture. Int. J. Impact Eng. 2020, 143, 103597. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Chen, S.; Li, Y.; Gao, G.; Gu, W.; Sha, M. Damage Behavior and Assessment of Aeronautical PMMA Subjected to High-Velocity Water-Jet Impact. Wear 2023, 534–535, 205145. [Google Scholar] [CrossRef]
- Sadeghi Esfahlani, S. Ballistic Performance of Polycarbonate and Polymethyl Methacrylate under Normal and Inclined Dynamic Impacts. Heliyon 2021, 7, e06856. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Choi, M.H.; Lee, B.W.; Lee, J.-H.; Shin, S.; Cho, S.; Cho, K.Y.; Baek, K.-Y. Feasible Detoxification Coating Material for Chemical Warfare Agents Using Poly(Methyl Methacrylate)–Branched Poly(Ethyleneimine) Copolymer and Metal–Organic Framework Composites. ACS Appl. Mater. Interfaces 2022, 14, 50246–50255. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Belhadj, A.; Lamnawar, K.; Maazouz, A. 3D Printing of PLA and PMMA Multilayered Model Polymers: An Innovative Approach for a Better-Controlled Pellet Multi-Extrusion Process. In Proceedings of the ESAFORM 2021, 24th International Conference on Material Forming, Liège, Belgium, 14–16 April 2021. [Google Scholar]
- Song, Y.; Zheng, Q. Concepts and Conflicts in Nanoparticles Reinforcement to Polymers beyond Hydrodynamics. Prog. Mater. Sci. 2016, 84, 1–58. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of Nanoparticles Aggregation/Agglomeration on the Interfacial/Interphase and Tensile Properties of Nanocomposites. Compos. Part B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Sedlarik, V.; Galya, T.; Sedlarikova, J.; Valasek, P.; Saha, P. The Effect of Preparation Temperature on the Mechanical and Antibacterial Properties of Poly(Vinyl Alcohol)/Silver Nitrate Films. Polym. Degrad. Stab. 2010, 95, 399–404. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Grammatikos, S.A.; Tzounis, L. Multi-Functional Medical Grade Polyamide12/Carbon Black Nanocomposites in Material Extrusion 3D Printing. Compos. Struct. 2023, 311, 116788. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver Nanoparticles as an Antimicrobial Agent: A Case Study on Staphylococcus aureus and Escherichia coli as Models for Gram-Positive and Gram-Negative Bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Leber, A.L. Clinical Microbiology Procedures Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Veluthandath, A.V.; Bisht, P.B. Identification of Whispering Gallery Mode (WGM) Coupled Photoluminescence and Raman Modes in Complex Spectra of MoS2 in Polymethyl Methacrylate (PMMA) Microspheres. J. Lumin. 2017, 187, 255–259. [Google Scholar] [CrossRef]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive Characterization of the Polycarbonate—Octadecylamine Interface by Surface Enhanced Raman Spectroscopy. Polym. Test. 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature Studies of Polycarbonate Using Fourier Transform Raman Spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence Spectroscopy of Polycarbonate Matrices Irradiated with Different Energy 28Si+ Ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; He, Z.; Liang, X.; Wang, M.; Jin, G. Thermal Degradation Kinetics Study of Molten Polylactide Based on Raman Spectroscopy. Polym. Eng. Sci. 2020, 61, 201–210. [Google Scholar] [CrossRef]
- Badr, Y.A.; El-Kader, K.M.A.; Khafagy, R.M. Raman Spectroscopic Study of CdS, PVA Composite Films. J. Appl. Polym. Sci. 2004, 92, 1984–1992. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Chen, J.; Zhang, W.; Zhang, M.Q. Observation of Mutual Diffusion of Macromolecules in PS/PMMA Binary Films by Confocal Raman Microscopy. Soft Matter 2012, 8, 4780–4787. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, T.H.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of Nanoparticles on the Thermal and Mechanical Properties of Epoxy Coatings. J. Nanosci. Nanotechnol. 2016, 16, 9874–9881. [Google Scholar] [CrossRef]
- Navarro Oliva, F.S.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedoui, F. Nanoparticle Size and Surface Chemistry Effects on Mechanical and Physical Properties of Nano-Reinforced Polymers: The Case of PVDF-Fe3O4 Nano-Composites. Polym. Test. 2023, 117, 107851. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Xu, C.; Li, Y.; Liu, Q.; Wang, S.; Yan, S. Effect of Nanoparticle Size on the Mechanical Properties of Polymer Nanocomposites. Polymer 2022, 252, 124944. [Google Scholar] [CrossRef]
- Chang, A.; Babhadiashar, N.; Barrett-Catton, E.; Asuri, P. Role of Nanoparticle–Polymer Interactions on the Development of Double-Network Hydrogel Nanocomposites with High Mechanical Strength. Polymers 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharma, A.Y.; Patil, S.P.; Markert, B. Effects of Porosity on the Mechanical Properties of Additively Manufactured Components: A Critical Review. Mater. Res. Express 2020, 7, 122001. [Google Scholar] [CrossRef]
- Amza, C.G.; Zapciu, A.; Constantin, G.; Baciu, F.; Vasile, M.I. Enhancing Mechanical Properties of Polymer 3D Printed Parts. Polymers 2021, 13, 562. [Google Scholar] [CrossRef]
- Chaudhary, B.; Li, H.; Matos, H. Long-Term Mechanical Performance of 3D Printed Thermoplastics in Seawater Environments. Results Mater. 2023, 17, 100381. [Google Scholar] [CrossRef]
- Vidakis, N.; Michailidis, N.; Papadakis, V.; Argyros, A.; Spiridaki, M.; Mountakis, N.; Nasikas, N.K.; Petousis, M.; Kymakis, E. Industrially Scalable Reactive Melt Mixing of Polypropylene/Silver Nitrate/Polyethylene Glycol Nanocomposite Filaments: Antibacterial, Thermal, Rheological, and Engineering Response in MEX 3D-Printing. Mater. Des. 2024, 242, 113032. [Google Scholar] [CrossRef]
- Stern, T.; Marom, G. Fracture Mechanisms and Toughness in Polymer Nanocomposites: A Brief Review. J. Compos. Sci. 2024, 8, 395. [Google Scholar] [CrossRef]
- Chen, Q.; Gong, S.; Moll, J.; Zhao, D.; Kumar, S.K.; Colby, R.H. Mechanical Reinforcement of Polymer Nanocomposites from Percolation of a Nanoparticle Network. ACS Macro Lett. 2015, 4, 398–402. [Google Scholar] [CrossRef]
- Jamil, H.; Faizan, M.; Adeel, M.; Jesionowski, T.; Boczkaj, G.; Balčiūnaitė, A. Recent Advances in Polymer Nanocomposites: Unveiling the Frontier of Shape Memory and Self-Healing Properties—A Comprehensive Review. Molecules 2024, 29, 1267. [Google Scholar] [CrossRef]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; K N, B. Nanoparticles Filled Polymer Nanocomposites: A Technological Review. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of Untreated Zirconium Oxide Nanofiller on the Flexural Strength and Surface Hardness of Autopolymerized Interim Fixed Restoration Resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Acierno, D.; Patti, A. Fused Deposition Modelling (FDM) of Thermoplastic-Based Filaments: Process and Rheological Properties—An Overview. Materials 2023, 16, 7664. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Acierno, S.; Cicala, G.; Acierno, D. Predicting the Printability of Poly(Lactide) Acid Filaments in Fused Deposition Modeling (FDM) Technology: Rheological Measurements and Experimental Evidence. ChemEngineering 2023, 7, 1. [Google Scholar] [CrossRef]
- Chee, E.; Brown, A.C. Biomimetic Antimicrobial Material Strategies for Combating Antibiotic Resistant Bacteria. Biomater. Sci. 2020, 8, 1089–1100. [Google Scholar] [CrossRef]
- Kyung, J.W.; Cheong, K.H.; Woo, K.K.; Sook, S.; Hyun, K.S.; Ho, P.Y. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.-T.; Weng, C.-H.; Than, N.A.T.; Tzeng, J.-H.; Jacobson, A.R.; Iamsaard, K.; Dang, V.D.; Lin, Y.-T. Mode of Inactivation of Staphylococcus aureus and Escherichia coli by Heated Oyster-Shell Powder. Chem. Eng. J. 2022, 432, 134386. [Google Scholar] [CrossRef]
- Payne, M.P.; Shillaker, R.O.; Wilson, A.J. Phosphoric Acid, Phosphorus Pentoxide, Phosphorus Oxychloride, Phosphorus Pentachloride, Phosphorus Pentasulphide; U.S. Environmental Protection Agency: Washington, DC, USA, 1993. [Google Scholar]
- Hemmilä, M.; Hihkiö, M.; Kasanen, J.-P.; Turunen, M.; Järvelä, M.; Suhonen, S.; Pasanen, A.-L.; Norppa, H. Cytotoxicity and Genotoxicity in Vitro and Irritation Potency in Vivo of Two Red Phosphorus-Based Pyrotechnic Smokes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 701, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]









| 603 | Increase | Small increase in all PMMA/antibacterial samples |
| 814 | Gradual Increase | Medium gradual increase |
| 1088 | Gradual Increase | Medium gradual increase |
| 1286 | Increase | Small increase |
| 1614 | Gradual Increase | Medium gradual increase with the exception of a large increase in the 6% sample Please check beam |
| 1727 | Gradual Increase | Medium gradual increase with the exception of a large increase in the 6%sample |
| 2925 | Decrease | Medium decrease appearing in all samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petousis, M.; Nasikas, N.K.; Papadakis, V.; Valsamos, I.; Gkagkanatsiou, K.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Michailidis, N.; Vidakis, N. Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing. Polymers 2025, 17, 410. https://doi.org/10.3390/polym17030410
Petousis M, Nasikas NK, Papadakis V, Valsamos I, Gkagkanatsiou K, Mountakis N, Argyros A, Dimitriou E, Michailidis N, Vidakis N. Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing. Polymers. 2025; 17(3):410. https://doi.org/10.3390/polym17030410
Chicago/Turabian StylePetousis, Markos, Nektarios K. Nasikas, Vassilis Papadakis, Ioannis Valsamos, Katerina Gkagkanatsiou, Nikolaos Mountakis, Apostolos Argyros, Evgenia Dimitriou, Nikolaos Michailidis, and Nectarios Vidakis. 2025. "Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing" Polymers 17, no. 3: 410. https://doi.org/10.3390/polym17030410
APA StylePetousis, M., Nasikas, N. K., Papadakis, V., Valsamos, I., Gkagkanatsiou, K., Mountakis, N., Argyros, A., Dimitriou, E., Michailidis, N., & Vidakis, N. (2025). Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing. Polymers, 17(3), 410. https://doi.org/10.3390/polym17030410











