Effect of Interfacial Compatibility on Mechanical Property of Polyamide 6 Modified by Polyborosiloxane
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PA6/PBS Blends
2.3. Characterization
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscope (SEM)
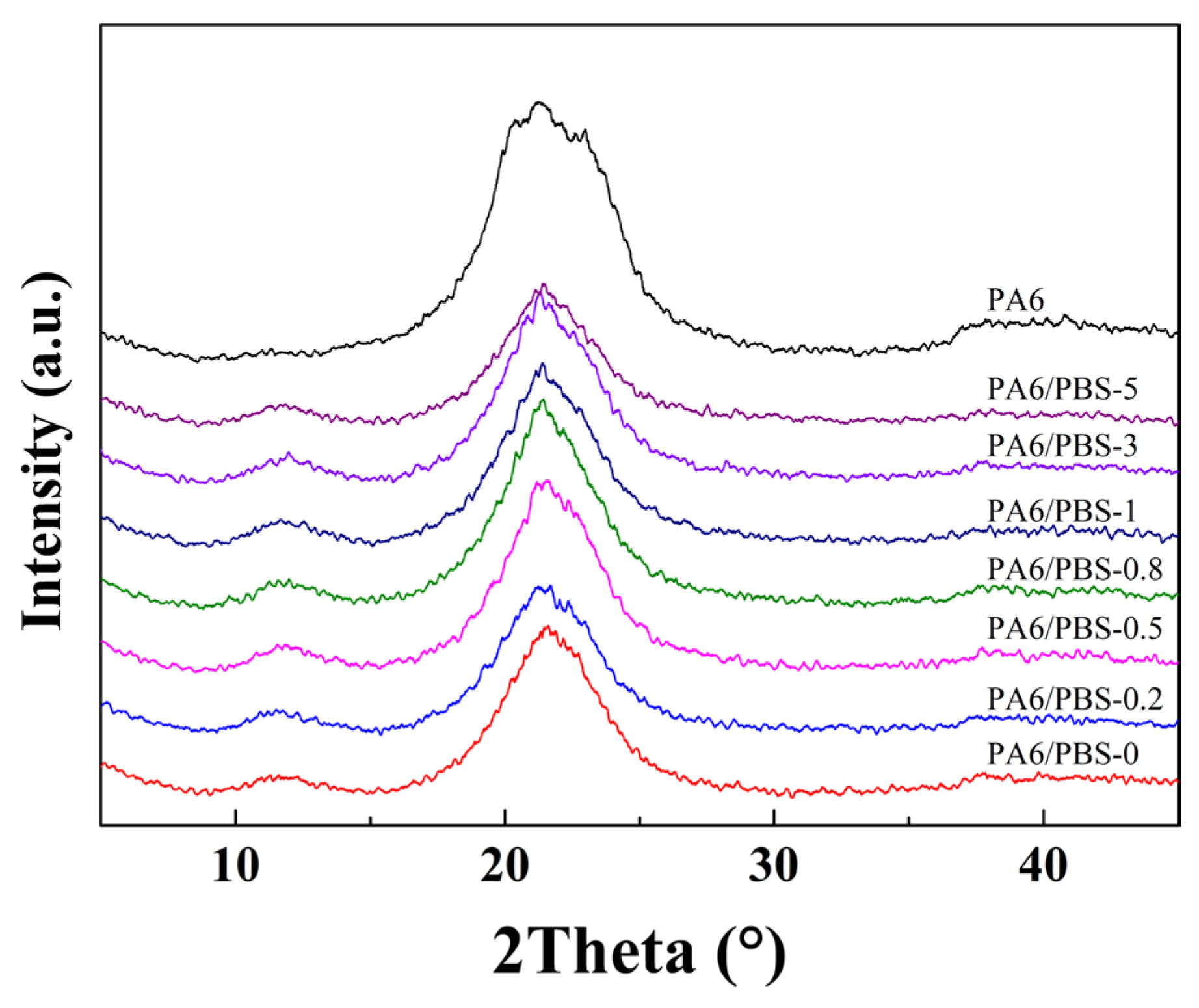
2.3.3. X-Ray Diffraction (XRD)
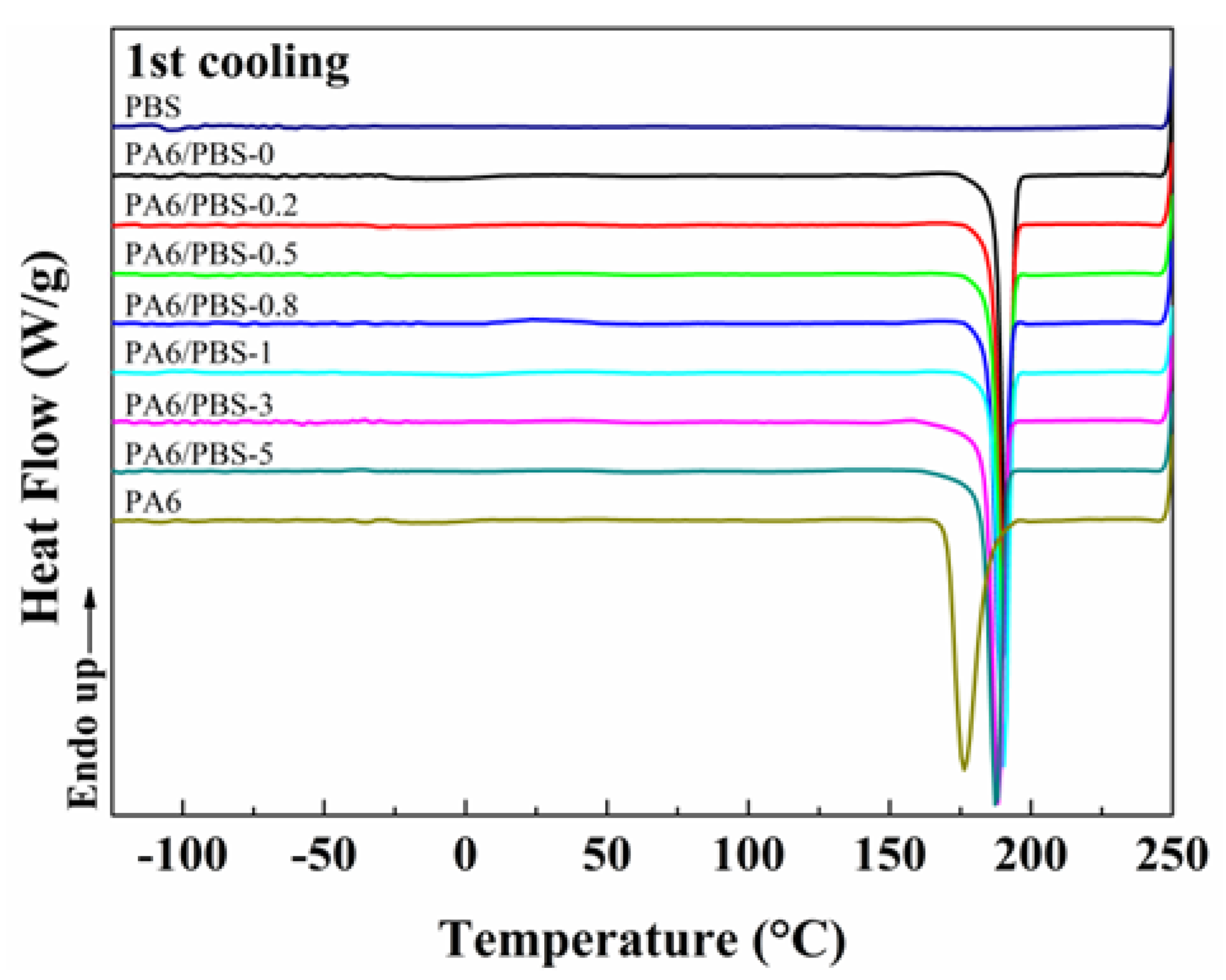
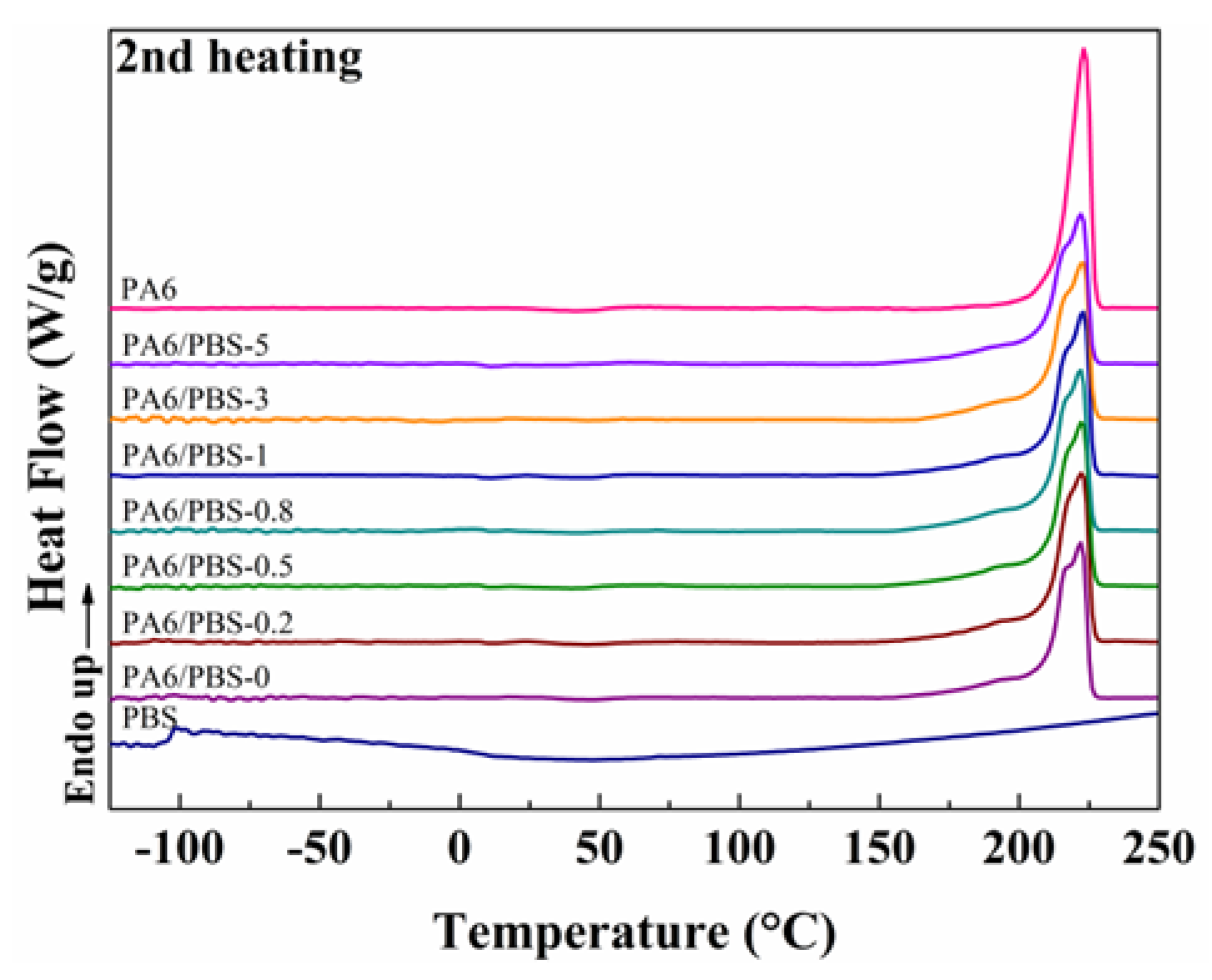
2.3.4. Differential Scanning Calorimetry (DSC)
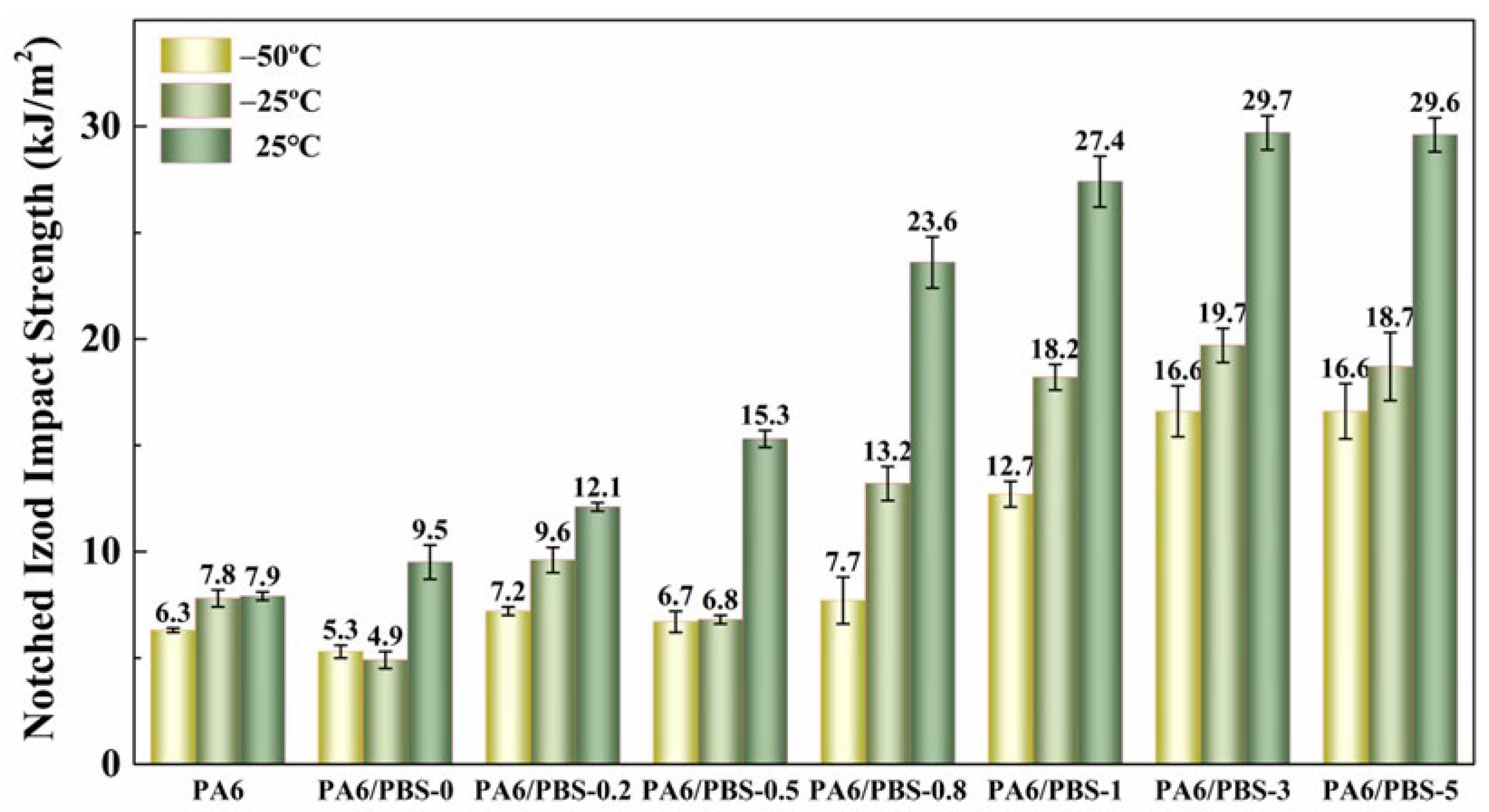
2.3.5. Mechanical Testing
2.4. Therory and Model
3. Results and Discussion
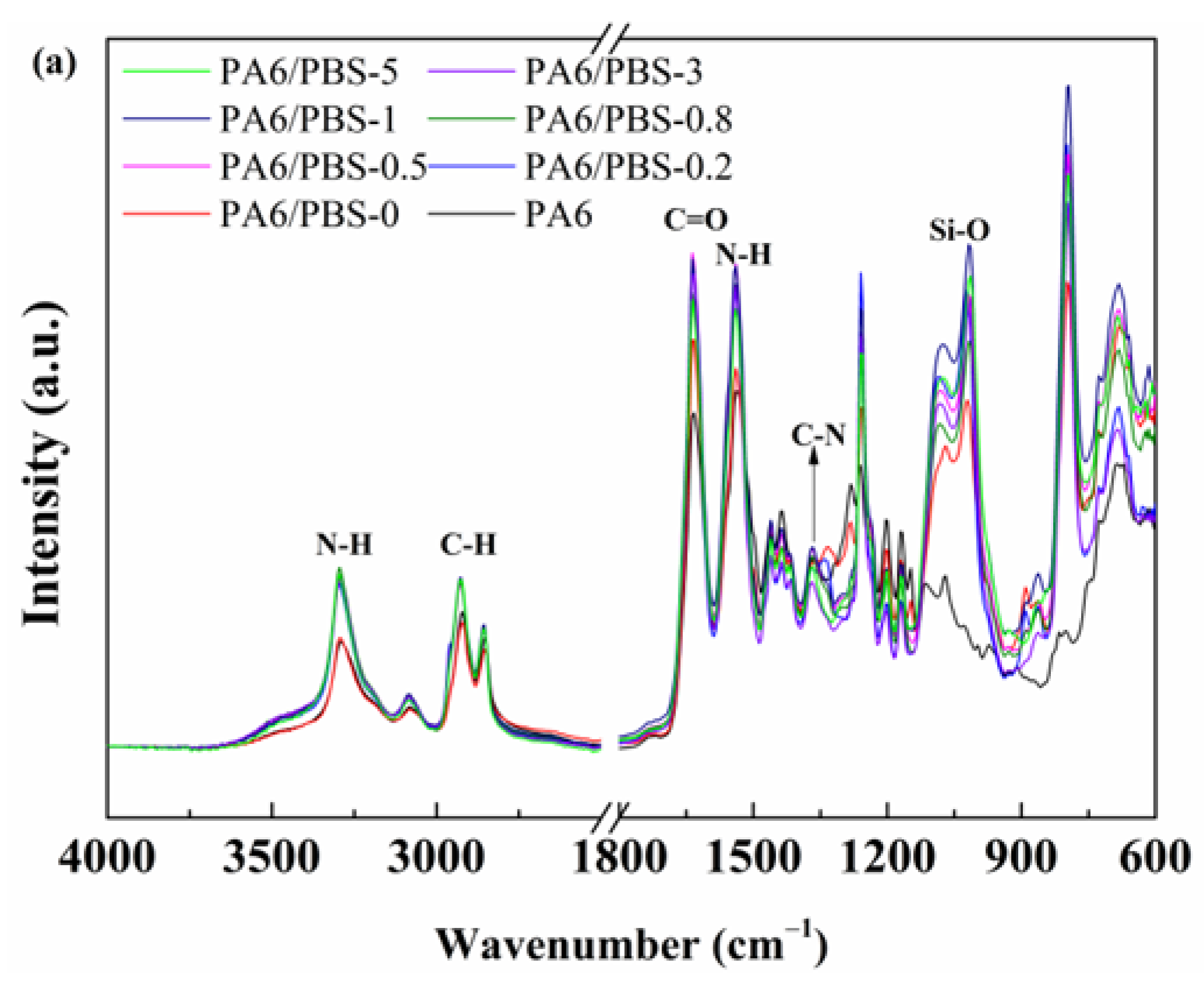
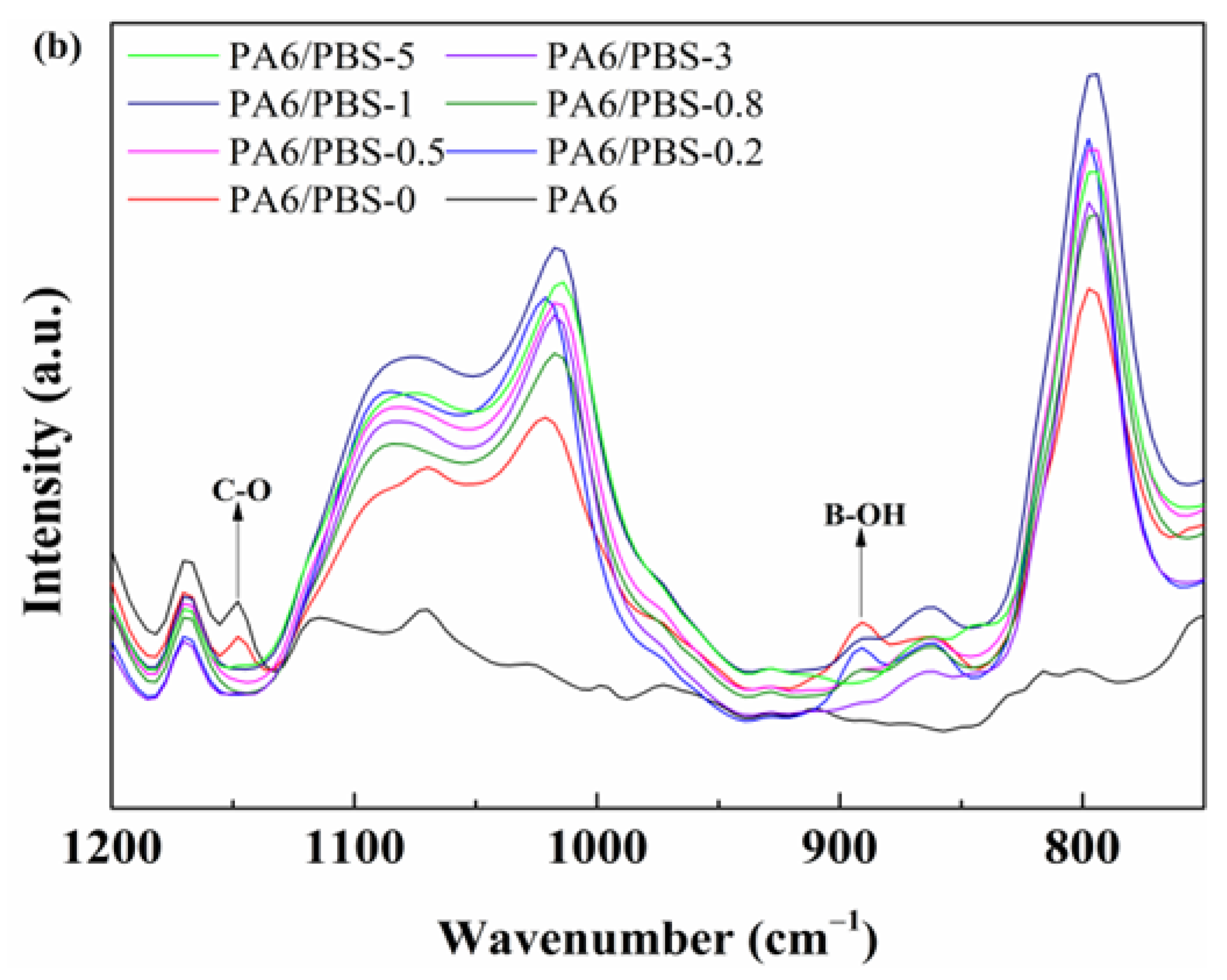
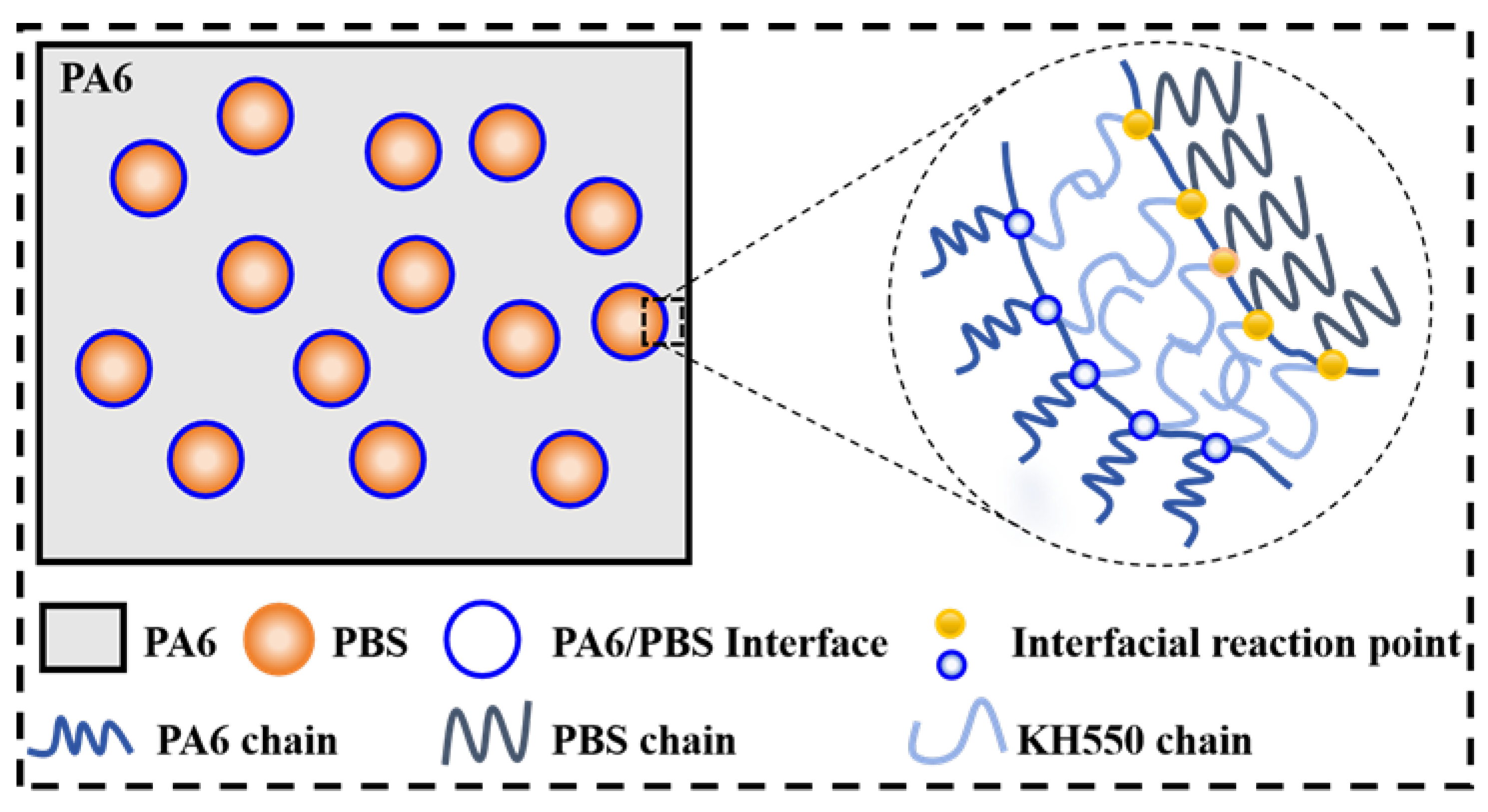
3.1. Structural Characterization
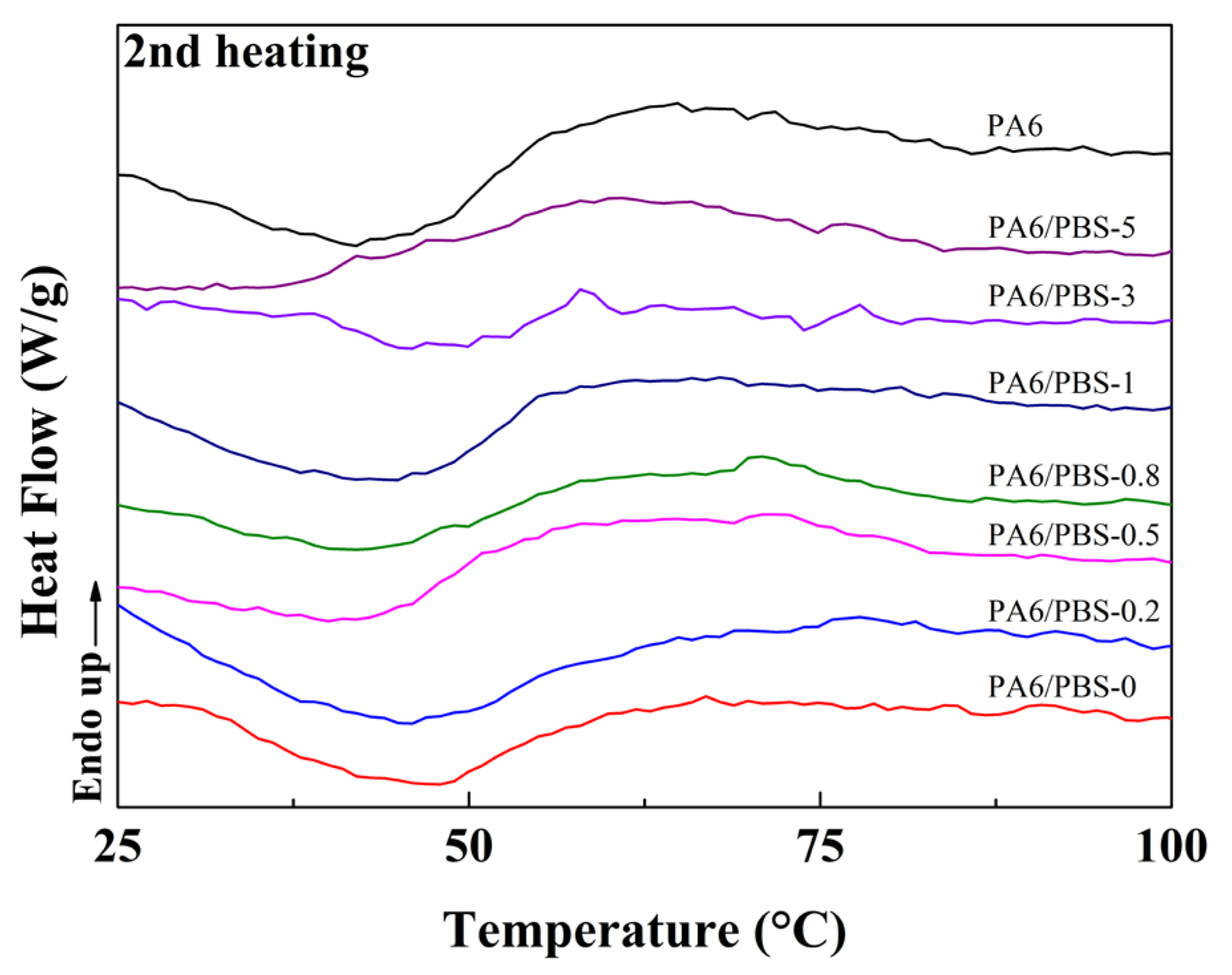
3.2. Melting and Crystallization Behaviors
3.3. SEM Analysis
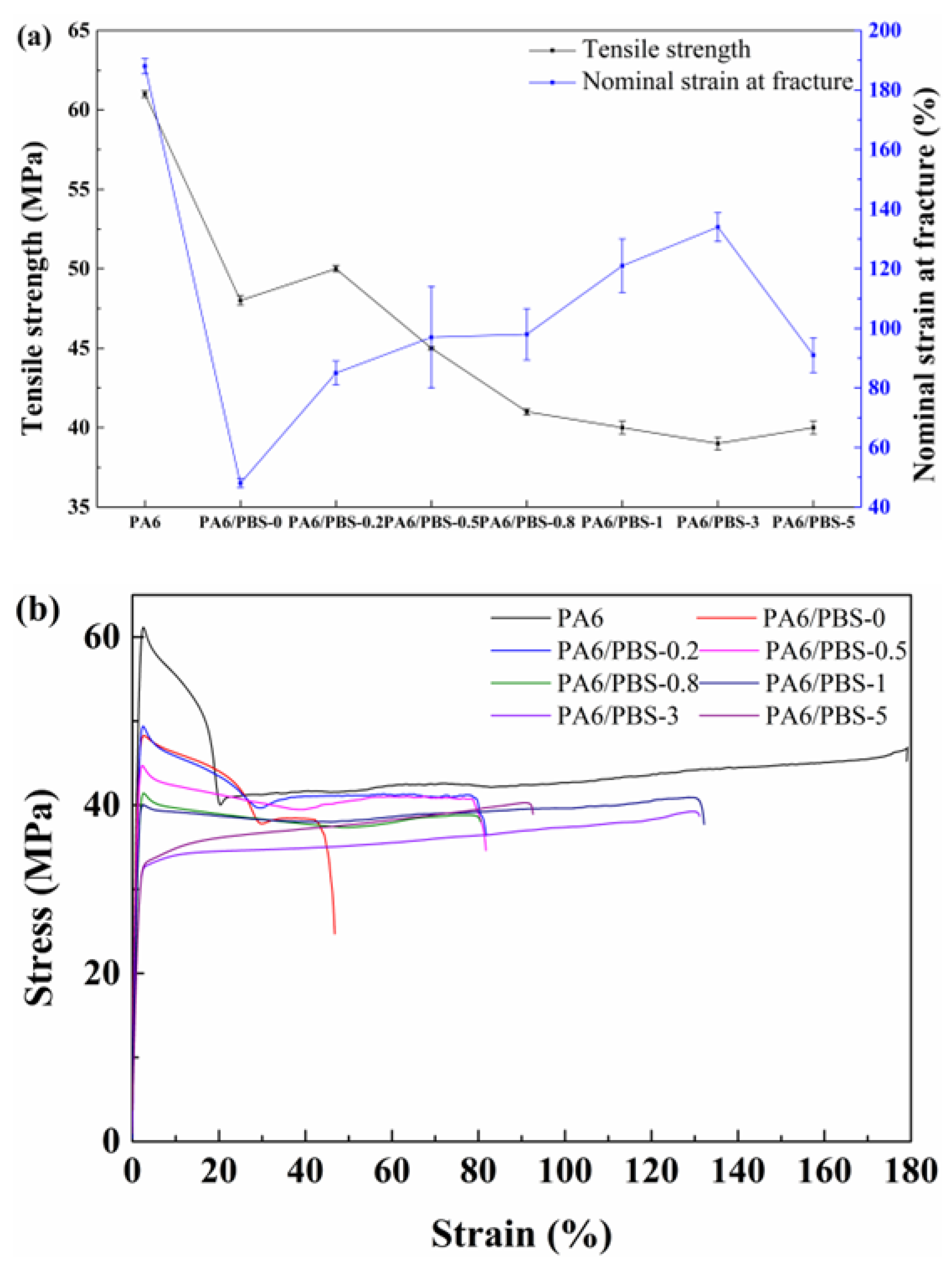
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kausar, A. Nanocarbon and Macrocarbonaceous Filler–Reinforced Epoxy/Polyamide: A Review. J. Thermoplast. Compos. Mater. 2022, 35, 2620–2640. [Google Scholar] [CrossRef]
- Faridirad, F.; Ahmadi, S.; Barmar, M. Polyamide/Carbon Nanoparticles Nanocomposites: A Review. Polym. Eng. Sci. 2017, 57, 475–494. [Google Scholar] [CrossRef]
- Seguela, R. Overview and Critical Survey of Polyamide6 Structural Habits: Misconceptions and Controversies. J. Polym. Sci. 2020, 58, 2971–3003. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, Q.; Jiang, P.; Li, Y.; Li, J. Ionic Liquids Incorporating Polyamide 6: Miscibility and Physical Properties. Polymers 2018, 10, 562. [Google Scholar] [CrossRef]
- Parodi, E.; Govaert, L.E.; Peters, G.W.M. Glass Transition Temperature versus Structure of Polyamide 6: A Flash-DSC Study. Thermochim. Acta 2017, 657, 110–122. [Google Scholar] [CrossRef]
- Mahmud, M.B.; Anstey, A.; Shaayegan, V.; Lee, P.C.; Park, C.B. Enhancing the Mechanical Performance of PA6 Based Composites by Altering Their Crystallization and Rheological Behavior via In-Situ Generated PPS Nanofibrils. Compos. Part B Eng. 2020, 195, 108067. [Google Scholar] [CrossRef]
- Ghanta, T.S.; Aparna, S.; Verma, N.; Purnima, D. Review on Nano-and Microfiller-based Polyamide 6 Hybrid Composite: Effect on Mechanical Properties and Morphology. Polym. Eng. Sci. 2020, 60, 1717–1759. [Google Scholar] [CrossRef]
- Tjong, S.C.; Bao, S.P. Impact Fracture Toughness of Polyamide-6/Montmorillonite Nanocomposites Toughened with a Maleated Styrene/Ethylene Butylene/Styrene Elastomer. J. Polym. Sci. B Polym. Phys. 2005, 43, 585–595. [Google Scholar] [CrossRef]
- Wang, Y.; Ghita, O.; Kavanagh, D.; Chandler, D. A Study of Interface Adhesion between Polyamide 6 (PA6) and Nitrile Rubber (NBR): A Study of Interface Adhesion between PA6 and NBR. Surf. Interface Anal. 2014, 46, 1000–1004. [Google Scholar] [CrossRef]
- Tanrattanakul, V.; Sungthong, N.; Raksa, P. Rubber Toughening of Nylon 6 with Epoxidized Natural Rubber. Polym. Test. 2008, 27, 794–800. [Google Scholar] [CrossRef]
- Zou, X.; Liu, B.; Wen, J.; Zeng, W.; Jing, B.; Dai, W. Improvement of Polyamide/Thermoplastic Polyurethane Blends with Polyamide 6-polyurethane Copolymer Prepared via Suspension Polymerization as Compatibilizer. J. Appl. Polym. Sci. 2020, 137, 49155. [Google Scholar] [CrossRef]
- Mehrabi-Mazidi, M.; Razavi-Aghjeh, M.K. Microscopic Deformation Behavior and Crack Resistance Mechanism of Core–Shell Structures in Highly-Toughened PP/PA6/EPDM-g-MA Ternary Blends. Macromol. Mater. Eng. 2021, 306, 2100174. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Vahidifar, A.; Shojaie, S.; Naderi, G.; Kalaei, M.R.; Mekonnen, T.H. Tailoring the Properties of PA6 into High-Performance Thermoplastic Elastomer: Simultaneous Reinforcement and Impact Property Modification. Mater. Today Commun. 2021, 26, 102027. [Google Scholar] [CrossRef]
- Attar, S.; Chen, B.; Cicala, G.; Catalanotti, G.; Scalici, T.; Falzon, B.G. On the Mechanical Properties of Melt-Blended Nylon 6/Ethylene-Octene Copolymer/Graphene Nanoplatelet Nanocomposites. Polymer 2022, 243, 124619. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Son, Y. Toughening of Nylon 6 with a Ethylene–Octene Copolymer Grafted with Maleic Anhydride and Styrene. Mater. Lett. 2014, 126, 43–47. [Google Scholar] [CrossRef]
- Rösch, J.; Mülhaupt, R. Mechanical and Morphological Properties of Elastomer-Modified Polypropylene/Polyamide-6 Blends. J. Appl. Polym. Sci. 1995, 56, 1599–1605. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Li, Y.; Zhang, Q.; Zhang, S.; Zhang, L. Enhanced Compatibility, Morphology, and Low-Temperature Toughness of Polyamide 6/Poly(Dimethylsiloxane)/Epoxidized Silicone Rubber Composites. Ind. Eng. Chem. Res. 2023, 62, 9694–9702. [Google Scholar] [CrossRef]
- Lin, H.; Yan, H.; Liu, B.; Wei, L.; Xu, B. The Influence of KH-550 on Properties of Ammonium Polyphosphate and Polypropylene Flame Retardant Composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Yoo, Y.; Cui, L.; Yoon, P.J.; Paul, D.R. Morphology and Mechanical Properties of Rubber Toughened Amorphous Polyamide/MMT Nanocomposites. Macromolecules 2010, 43, 615–624. [Google Scholar] [CrossRef]
- Tedesco, A.; Barbosa, R.V.; Nachtigall, S.M.B.; Mauler, R.S. Comparative Study of PP-MA and PP-GMA as Compatibilizing Agents on Polypropylene/Nylon 6 Blends. Polym. Test. 2002, 21, 11–15. [Google Scholar] [CrossRef]
- Mirzaee, R.; Aref-Azar, A. Modeling and Optimizing Toughness and Rigidity of PA6/SBR: Using Compatibilizer and Response Surface Methodology. Polym. Test. 2020, 83, 106346. [Google Scholar] [CrossRef]
- Ishigami, A.; Nishitsuji, S.; Kurose, T.; Ito, H. Evaluation of Toughness and Failure Mode of PA6 mSEBS PS Ternary Blends with an Oil-Extended Viscoelastic Controlled Interface. Polymer 2019, 177, 57–64. [Google Scholar] [CrossRef]
- Belkhiri, A.; Virgilio, N.; Santanach-Carreras, E.; Esvan, J.; Nassiet, V.; Welemane, H.; De Almeida, O.; Chabert, F. Influence of Silane Interfacial Chemistry on the Curing Process of Anionic Polyamide 6 in Glass Reinforced Composites. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132183. [Google Scholar] [CrossRef]
- Miri, V.; Persyn, O.; Seguela, R.; Lefebvre, J.M. On the Deformation Induced Order–Disorder Transitions in the Crystalline Phase of Polyamide 6. Eur. Polym. J. 2011, 47, 88–97. [Google Scholar] [CrossRef]
- Odrobina, M.; Deák, T.; Székely, L.; Mankovits, T.; Keresztes, R.Z.; Kalácska, G. The Effect of Crystallinity on the Toughness of Cast Polyamide 6 Rods with Different Diameters. Polymers 2020, 12, 293. [Google Scholar] [CrossRef]
- GB/T 1843-2008; Plastics—Determination of Izod Impact Strength. Standards Press of China: Beijing, China, 2008.
- GB/T1040-2018; Plastics—Determination of Tensile Properties. Standards Press of China: Beijing, China, 2018.
- Wu, S. Phase Structure and Adhesion in Polymer Blends: A Criterion for Rubber Toughening. Polymer 1985, 26, 1855–1863. [Google Scholar] [CrossRef]
- Borggreve, R.J.M.; Gaymans, R.J.; Schuijer, J.; Housz, J.F.I. Brittle-Tough Transition in Nylon-Rubber Blends: Effect of Rubber Concentration and Particle Size. Polymer 1987, 28, 1489–1496. [Google Scholar] [CrossRef]
- Li, F.; Gao, Y.; Zhang, C.; Jin, J.; Ji, X.; Zhang, Y.; Zhang, X.; Jiang, W. Design of High Impact Thermal Plastic Polymer Composites with Balanced Toughness and Rigidity: Effect of Matrix Polymer Molecular Weight. Polymer 2020, 208, 122957. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Yu, L.; Li, X.; Zhang, Z. Correlation between Interfacial Interactions and Mechanical Properties of PA-6 Doped with Surface-Capped Nano-Silica. Appl. Surf. Sci. 2009, 255, 7871–7877. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Milenin, S.A.; Gorodov, V.V.; Demchenko, N.V.; Buzin, M.I.; Muzafarov, A.M. Crosslinked Polymers Based on Polyborosiloxanes: Synthesis and Properties. J. Organomet. Chem. 2019, 891, 72–77. [Google Scholar] [CrossRef]
- Kurkin, A.; Lipik, V.; Tan, K.B.L.; Seah, G.L.; Zhang, X.; Tok, A.I.Y. Correlations Between Precursor Molecular Weight and Dynamic Mechanical Properties of Polyborosiloxane (PBS). Macromol. Mater. Eng. 2021, 306, 2100360. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, H.; Jiang, B. Interparticle Distance-Temperature-Strain Rate Equivalence for the Brittle-Tough Transition in Polymer Blends. Polymer 1998, 39, 4437–4442. [Google Scholar] [CrossRef]
- Seguela, R.; Rietsch, F. Double Yield Point in Polyethylene under Tensile Loading. J. Mater. Sci. Lett. 1990, 9, 46–47. [Google Scholar] [CrossRef]
- Xiao, Y.; Mu, X.; Wang, B.; Hu, W.; Wang, J.; Zhou, F.; Ma, C.; Hu, Y.; Song, L. A Novel Phosphorous-Containing Polymeric Compatibilizer: Effective Reinforcement and Flame Retardancy in Glass Fiber Reinforced Polyamide 6 Composites. Compos. Part B Eng. 2021, 205, 108536. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Gao, Y.; Bai, H. Effects of Coupling Agents on the Impact Fracture Behaviors of T-ZnOw/PA6 Composites. Compos. Sci. Technol. 2008, 68, 1338–1347. [Google Scholar] [CrossRef]



| Samples | PA6 (wt%) | PBS (wt%) | KH550 (wt%) | Antioxidant (wt%) |
|---|---|---|---|---|
| PA6/PBS-0 | 80 | 20 | 0 | 0.2 |
| PA6/PBS-0.2 | 80 | 20 | 0.2 | 0.2 |
| PA6/PBS-0.5 | 80 | 20 | 0.5 | 0.2 |
| PA6/PBS-0.8 | 80 | 20 | 0.8 | 0.2 |
| PA6/PBS-1 | 80 | 20 | 1 | 0.2 |
| PA6/PBS-3 | 80 | 20 | 3 | 0.2 |
| PA6/PBS-5 | 80 | 20 | 5 | 0.2 |
| Samples | Tm (°C) | ΔHm (J/g) | Tg (°C) | Tc (°C) | Tc,onset (°C) | ΔHc (J/g) | χc (%) |
|---|---|---|---|---|---|---|---|
| PA6 | 223.3 | 52.57 | 52.4 | 176.3 | 195.2 | 58.63 | 22.9 |
| PA6/PBS-0 | 222.1 | 32.51 | 53.2 | 191.4 | 196.2 | 43.39 | 17.7 |
| PA6/PBS-0.2 | 222.4 | 37.03 | 53.5 | 190.8 | 196.2 | 47.02 | 20.2 |
| PA6/PBS-0.5 | 222.4 | 34.88 | 49.2 | 190.4 | 195.2 | 44.82 | 19.1 |
| PA6/PBS-0.8 | 221.9 | 33.63 | 51.9 | 189.3 | 195.2 | 44.46 | 18.4 |
| PA6/PBS-1 | 222.8 | 35.33 | 51.4 | 190.4 | 195.2 | 44.41 | 19.5 |
| PA6/PBS-3 | 222.8 | 36.19 | 52.8 | 188.4 | 193.2 | 44.84 | 20.3 |
| PA6/PBS-5 | 222.2 | 31.33 | 46 | 187.7 | 193.2 | 41.91 | 17.9 |
| KH550 (Mass Fraction) | 0 wt% | 0.2 wt% | 0.5 wt% | 0.8 wt% | 1 wt% | 3 wt% |
|---|---|---|---|---|---|---|
| dw (μm) | 3.63 | 0.68 | 0.62 | 0.52 | 0.47 | 0.39 |
| ID (μm) | 1.21 | 0.23 | 0.21 | 0.18 | 0.16 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, F.; Zhai, Z.; Li, S.; Cai, Y.; Li, Q. Effect of Interfacial Compatibility on Mechanical Property of Polyamide 6 Modified by Polyborosiloxane. Polymers 2025, 17, 392. https://doi.org/10.3390/polym17030392
Chen Q, Li F, Zhai Z, Li S, Cai Y, Li Q. Effect of Interfacial Compatibility on Mechanical Property of Polyamide 6 Modified by Polyborosiloxane. Polymers. 2025; 17(3):392. https://doi.org/10.3390/polym17030392
Chicago/Turabian StyleChen, Qian, Feng Li, Zhe Zhai, Shufeng Li, Yongfei Cai, and Qiang Li. 2025. "Effect of Interfacial Compatibility on Mechanical Property of Polyamide 6 Modified by Polyborosiloxane" Polymers 17, no. 3: 392. https://doi.org/10.3390/polym17030392
APA StyleChen, Q., Li, F., Zhai, Z., Li, S., Cai, Y., & Li, Q. (2025). Effect of Interfacial Compatibility on Mechanical Property of Polyamide 6 Modified by Polyborosiloxane. Polymers, 17(3), 392. https://doi.org/10.3390/polym17030392







