Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Feedstock, Media, and Working Microorganisms
2.3. Microbial Cultivations in Erlenmeyer Flasks
2.4. Microbial Cultivations in the Bubble Column and Stirred-Tank Bioreactor
2.5. Analytical Methods
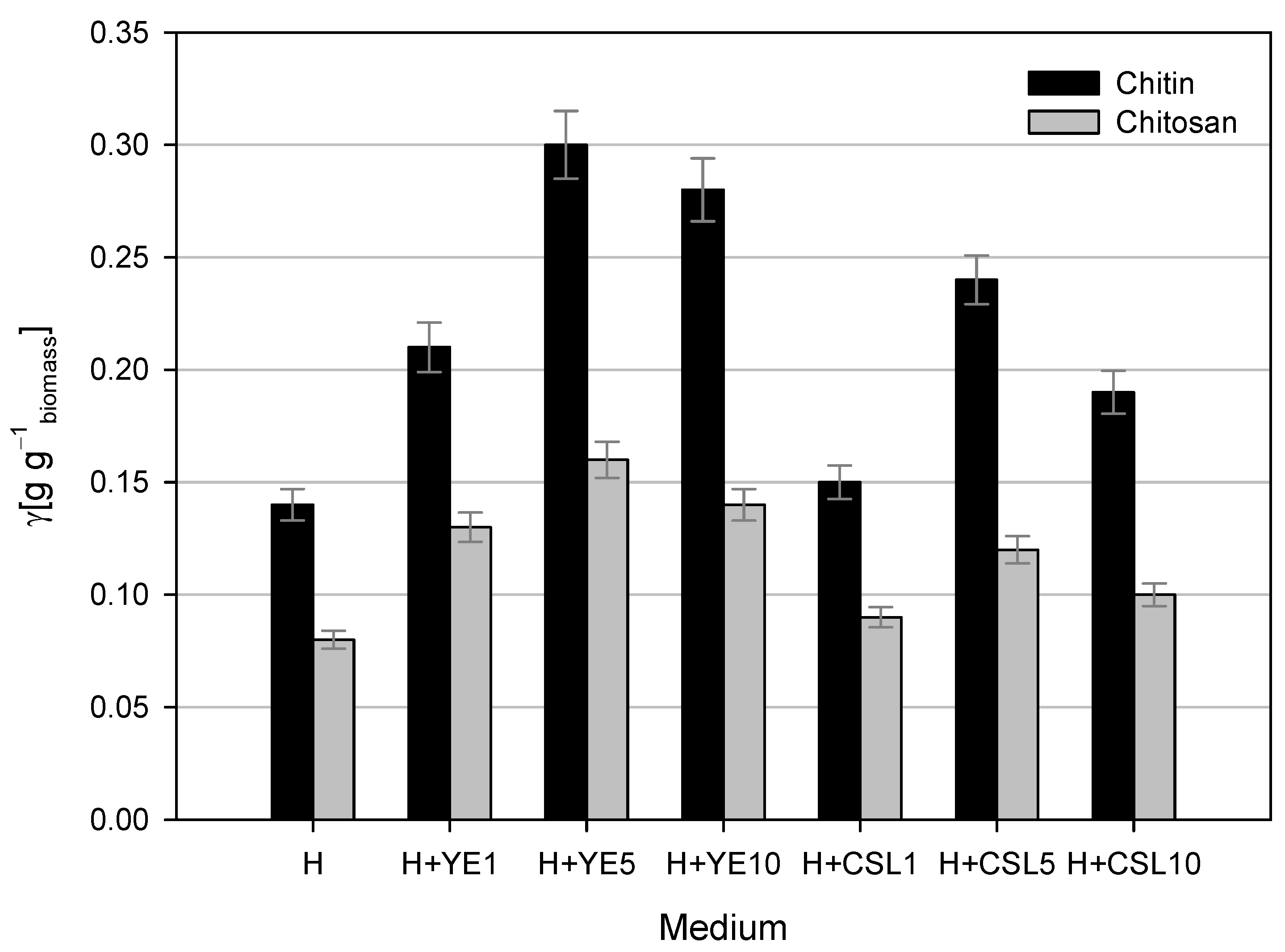
2.5.1. Isolation of Chitin and Chitosan
2.5.2. UPLC Analysis
2.5.3. Optical Density Determination and Gravimetric Analysis
2.5.4. GC-FID Analysis
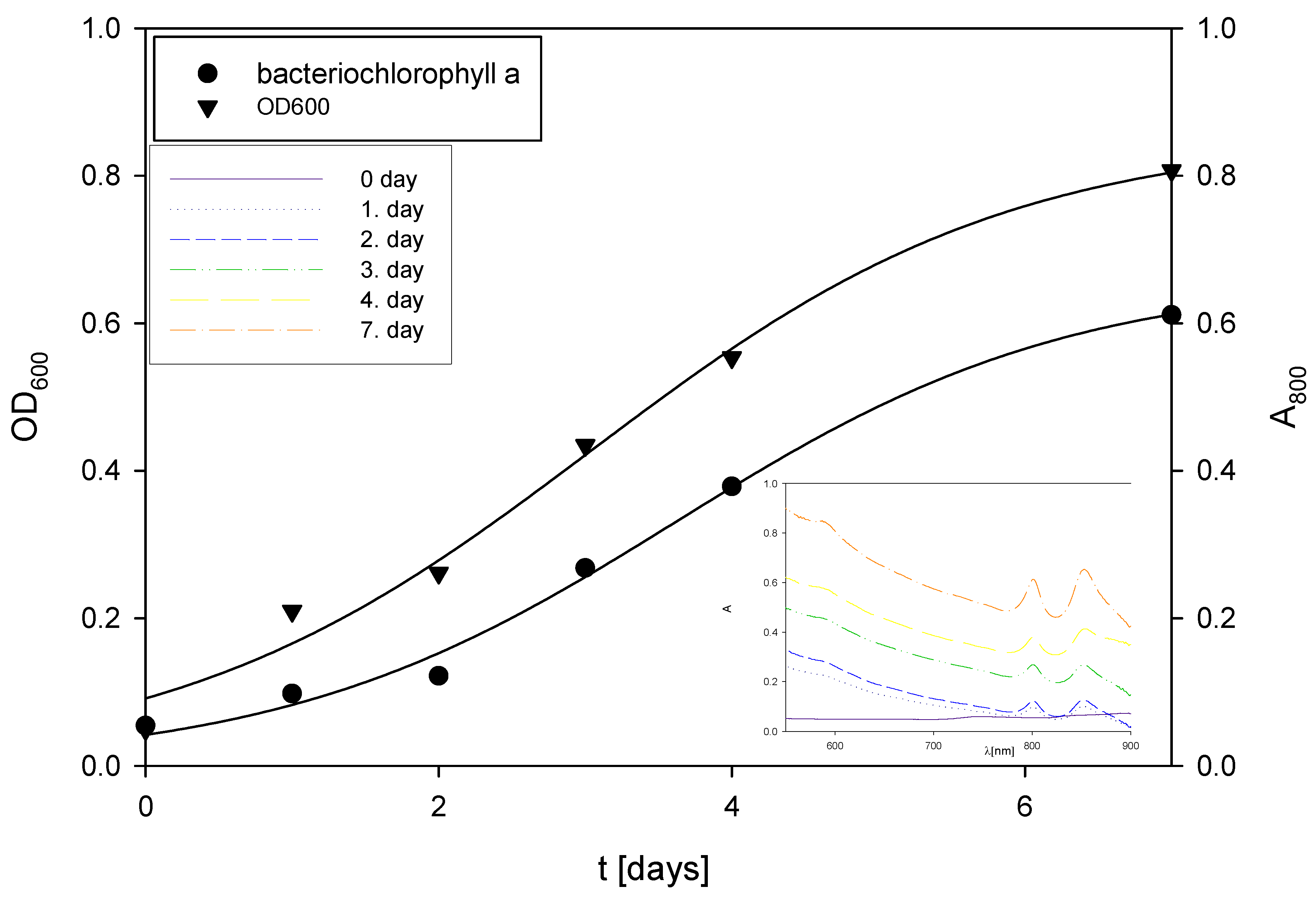
2.5.5. Extraction of Total Pigments and Spectrophotometric Determination of Bacteriochlorophyll-a
2.5.6. Statistical Analysis
2.5.7. Calculation of Bioprocess Efficiency Parameters
3. Results and Discussion
3.1. Cultivations of Mucor Indicus DSM 2185
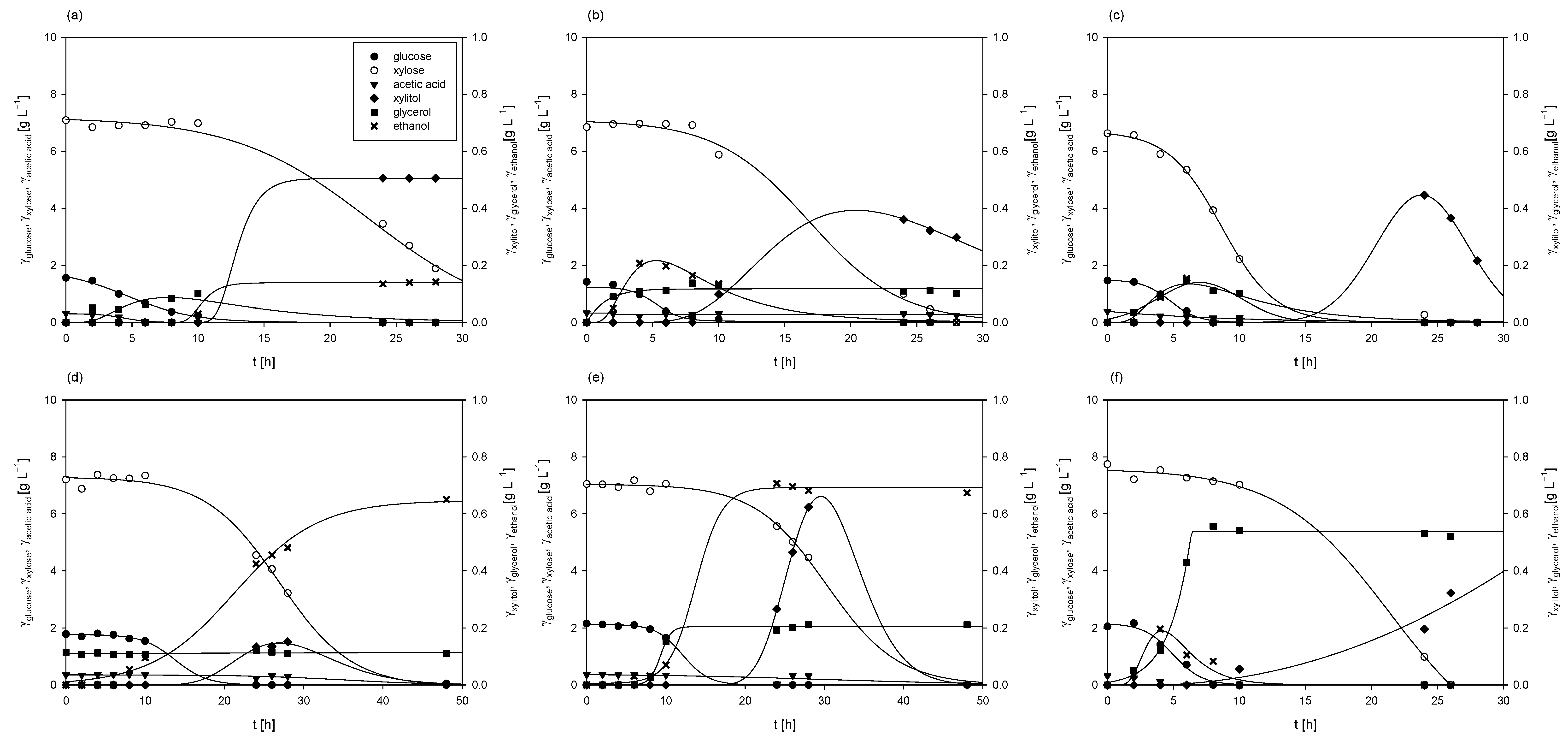
3.1.1. Cultivations of Mucor indicus DSM 2185 on Media Containing Various Sugars Derived from Grass Hydrolysates
3.1.2. Cultivations of Mucor indicus DSM 2185 in Erlenmeyer Flasks on Media Containing Liquid Phase of Grass Hydrolysates (LGH)
3.2. Cultivations of Bacterium Rhodovulum adriaticum DSM 2781 in Erlenmeyer Flasks
3.3. Cultivations of Mucor indicus DSM 2185 and Rhodovulum Adriaticum in Bioreactors
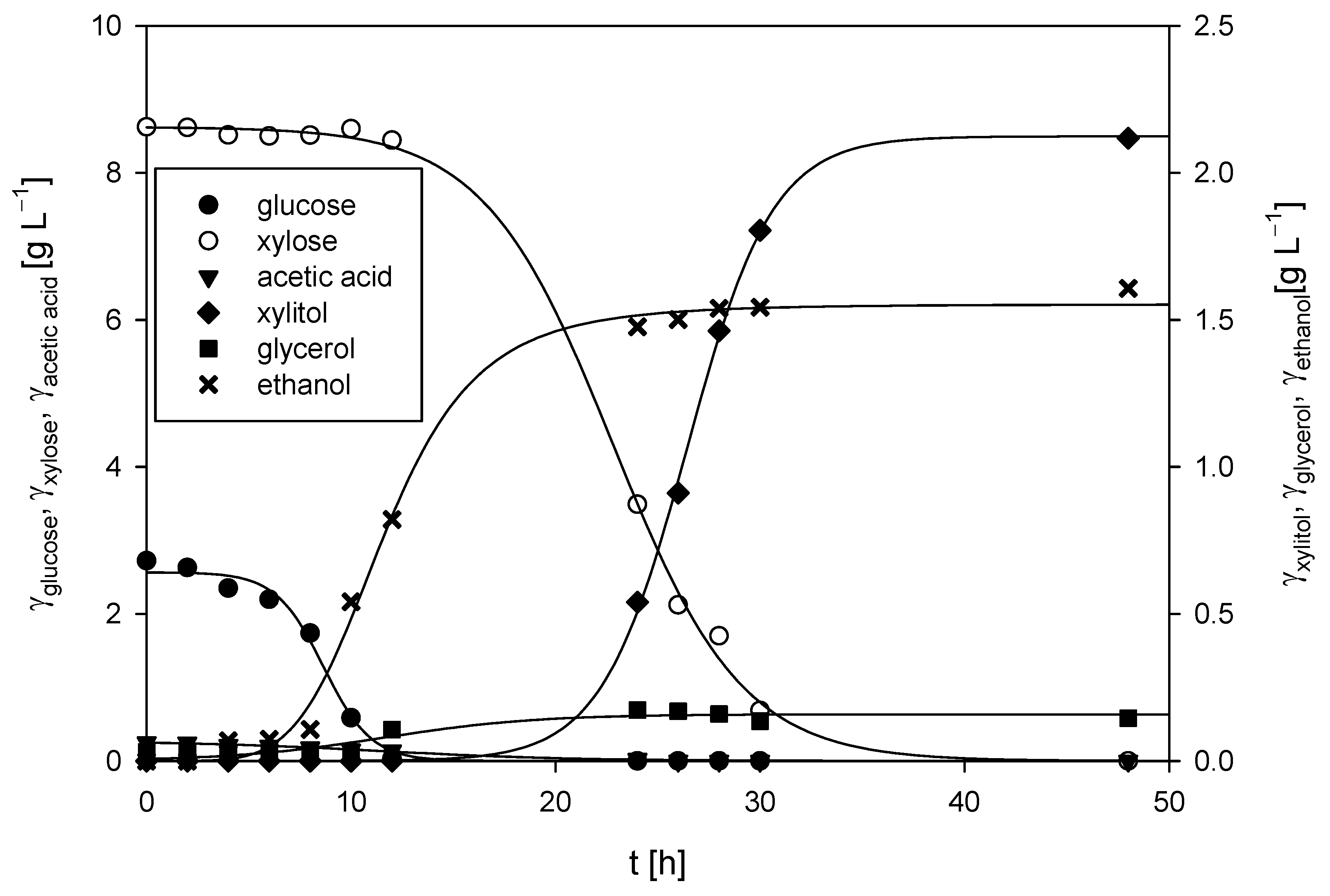
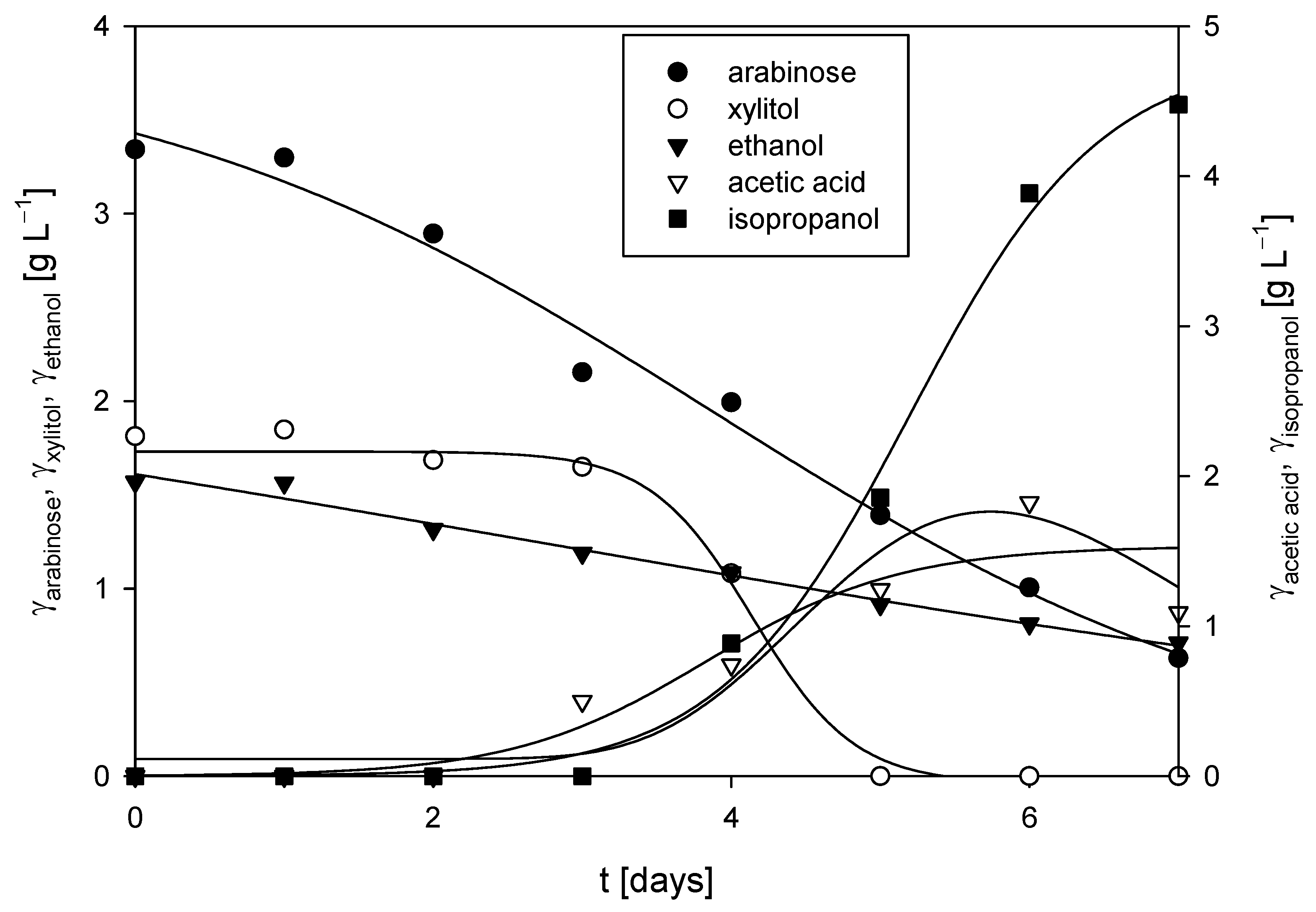
3.3.1. Cultivation of M. indicus DSM 2185 in a Bubble Column Bioreactor
3.3.2. Cultivation of R. adriaticum DSM 2781 in a Stirred-Tank Bioreactor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novak, M.; Marđetko, N.; Trontel, A.; Pavlečić, M.; Kelemen, Z.; Perković, L.; Petravić Tominac, V.; Šantek, B. Development of an Integrated Bioprocess System for Bioethanol and Arabitol Production from Sugar Beet Cossettes. Food Technol. Biotechnol. 2024, 62, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Marđetko, N.; Trontel, A.; Novak, N.; Pavlečić, M.; Didak Ljubas, B.; Grubišić, M.; Petravić Tominac, V.; Ludwig, R.; Šantek, B. Screening of Lignocellulolytic Enzyme Activities in Fungal Species and Sequential Solid-State and Submerged Cultivation for the Production of Enzyme Cocktails. Polymers 2021, 13, 3736. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, H.F.; Becer, C.R. Lignocellulosic Biomas: A Sustainable Platform for Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Palonen, H. Role of Lignin in the Enzymatic Hydrolysis of Lignocellulose; ESPOO 2004; VTT Technical Research Centre of Finland: Otaniemi, Finland, 2004. [Google Scholar]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: London, UK, 2014; pp. 1–185. [Google Scholar]
- Howard, R.L.; Abotsi, E.; Jansen van Rensburg, R.L.; Howars, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 12, 602–619. [Google Scholar] [CrossRef]
- Johannes, L.P.; Xuan, T.D. Comparative Analysis of Acidic and Alkaline Pretreatment Techniques for Bioethanol Production from Perennial Grasses. Energies 2024, 17, 1048. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Kostylev, M.; Wilson, D. Synergistic interactions in cellulose hydrolysis. Biofuels 2012, 3, 61–70. [Google Scholar] [CrossRef]
- Antonopoulou, G. Designing Efficient Processes for Sustainable Bioethanol and Bio-Hydrogen Production from Grass Lawn Waste. Molecules 2020, 25, 2889. [Google Scholar] [CrossRef]
- Gupte, A.; Prajapati, D.; Bhatt, A.; Pandya, S.; Raghunathan, M.; Gupte, S. Agro-industrial Residues: An Eco-friendly and Inexpensive Substrate for Fungi in the Development of White Biotechnology. In Fungi and Fungal Products in Human Welfare and Biotechnology; Satyanarayana, T., Deshmukh, S.K., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Amode, N.S.; Jeetah, P. Paper Production from Mauritian Hemp Fibres. Waste Biomass Valor. 2021, 12, 1781–1802. [Google Scholar] [CrossRef]
- European Environment Agency. Climate Change, Impacts and Vulnerability in Europe 2016. Available online: https://www.eea.europa.eu/publications/climate-change-impacts-and-vulnerability-2016 (accessed on 5 September 2024).
- Morrow, W.R.; Griffin, W.M.; Matthews, H.S. Modeling Switchgrass Derived Cellulosic Ethanol Distribution in the United States. Environ. Sci. Technol. 2006, 40, 2877–2886. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P.; Zając, G.; Wassen, M.J. Grass from Road Verges as a Substrate for Biogas Production. Energies 2023, 16, 4488. [Google Scholar] [CrossRef]
- Bedoić, R.; Čuček, L.; Ćosić, B.; Krajnc, D.; Smoljanić, G.; Kravanja, Z.; Ljubas, D.; Pukšec, T.; Duić, N. Green Biomass to Biogas—A Study on Anaerobic Digestion of Residue Grass. J. Clean. Prod. 2019, 213, 700–709. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Patterson, T.; Massanet-Nicolau, J.; Jones, R.; Boldrin, A.; Valentino, F.; Dinsdale, R.; Guwy, A. Utilizing grass for the biological production of polyhydroxyalkanoates (PHAs) via green biorefining: Material and energy flows. J. Ind. Ecol. 2021, 25, 802–815. [Google Scholar] [CrossRef]
- Varriale, L.; Hengsbach, J.-N.; Guo, T.; Kuka, K.; Tippkötter, N.; Ulber, R. Sustainable Production of Lactic Acid Using a Perennial Ryegrass as Feedstock—A Comparative Study of Fermentation at the Bench- and Reactor-Scale, and Ensiling. Sustainability 2024, 16, 8054. [Google Scholar] [CrossRef]
- Vargas, A.C.G.; Dresch, A.P.; Schmidt, A.R.; Tadioto, V.; Giehl, A.; Fogolari, O.; Mibielli, G.M.; Alves, S.L.; Bender, J.P. Batch Fermentation of Lignocellulosic Elephant Grass Biomass for 2G Ethanol and Xylitol Production. Bioenerg. Res. 2023, 16, 2219–2228. [Google Scholar] [CrossRef]
- Piepenschneider, M.; Bühle, L.; Hensgen, F.; Wachendorf, M. Energy Recovery from Grass of Urban Roadside Verges by Anaerobic Digestion and Combustion after Pre-Processing. Biomass Bioenerg. 2016, 85, 278–287. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Prabhu, G.; Bhat, D.; Bhat, R.M.; Selvaraj, S. A Critical Look at Bioproducts Co-cultured Under Solid State Fermentation and Their Challenges and Industrial Applications. Waste Biomass Valor. 2022, 13, 3095–3111. [Google Scholar] [CrossRef]
- Zhao, J.; Jing, Z.D.; Yin, X.J.; Li, J.F.; Dong, Z.H.; Wang, S.R.; Shao, T. Sustainable utilization of residual grass: Effect of anaerobic storage days on chemical composition, fermentation performance, microbial community, and functional profiles of Pennisetum giganteum. Environ. Sci. Pollut. Res. 2024, 31, 38866–38877. [Google Scholar] [CrossRef] [PubMed]
- Myrsini, S.; Brecht, D.; Ntagia, E.; Chatzigiannidou, I.; Gabet, X.; Ganigué, R.; Rabaey, K. Production of microbial protein from fermented grass. Chem. Eng. J. 2022, 433, 133631. [Google Scholar] [CrossRef]
- Sun, J.; Wang, T.; Huang, F.; Liu, Y.; Shi, W.; Ma, C.; Zhong, J. Silage Fermentation: A Potential Microbial Approach for the Forage Utilization of Cyperus esculentus L. By-Product. Fermentation 2021, 7, 273. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Ellilä, S.; Poikkimäki, S.; Nappa, M.; Rinne, M.; Lantto, R.; Siika-aho, M. Comparison of pretreatments and cost-optimization of enzymatic hydrolysis for production of single cell protein from grass silage fibre. Bioresour. Technol. Rep. 2020, 9, 100357. [Google Scholar] [CrossRef]
- Ecker, J.; Schaffenberger, M.; Koschuh, W.; Mandl, M.; Böchzelt, H.G.; Schnitzer, H.; Harasek, M.; Steinmüller, H. Green Biorefinery Upper Austria—Pilot Plant operation. Sep. Purif. Technol. 2012, 96, 237–247. [Google Scholar] [CrossRef]
- IMarc. Transforming Ideas into Impact. Available online: https://www.imarcgroup.com/amino-acid-technical-material-market-report (accessed on 10 January 2025).
- Saini, M.L.; Jain, P.; Joshi, U.N. Morphological characteristics and nutritive value of some grass species in an arid ecosystem. Grass Forage Sci. 2007, 62, 104–108. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P. GHG Emissions and Efficiency of Energy Generation through Anaerobic Fermentation of Wetland Biomass. Energies 2020, 13, 6497. [Google Scholar] [CrossRef]
- Renewable Fuels Association. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 10 January 2025).
- Naqvi, S.Z.; Abbas, S.A.; Ali-ul-Husnain Naqvi, M.; Batool, N.; Younas, T. Comparative Analysis of Mucor indicus against Aspergillus niger and Aspergillus fumigatus for wheat straw fermentation to produce efficient, inexpensive and eco-friendly bioethanol. Int. J. Plant Anim. Environm. Sci. 2021, 11, 221–232. [Google Scholar] [CrossRef]
- Mehta, P.; Chelike, D.K. Utilizing fungal biodegradation for valorisation of lignocellulosic waste biomass and its diverse applications. Appl. Res. 2024, 4, e202300119. [Google Scholar] [CrossRef]
- Volkmar, M.; Maus, A.L.; Weisbrodt, M.; Bohlender, J.; Langsdorf, A.; Holtmann, D.; Ulber, R. Municipal green waste as substrate for the microbial production of platform chemicals. Bioresour. Bioprocess. 2023, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, A.; Kaushik, J.; Gowda, L.; Chigadannavar, P.S.; Krishnappa, R.; Venkatesh, S.; Sivadas, S.; Sneha, J.; Ramesh, U.M.; Quadri, Z.; et al. Chapter 11—Prospect of biofuel production by fungus. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Kumar Sharma, V., Maulin, P.S., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 285–313. [Google Scholar] [CrossRef]
- Dhevagi, P.; Ramya, A.; Priyatharshini, S.; Geetha Thanuja, K.; Ambreetha, S.; Nivetha, A. Industrially Important Fungal Enzymes: Productions and Applications. In Recent Trends in Mycological Research. Fungal Biology; Yadav, A.N., Ed.; Springer: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Dzurendova, S.; Losada, C.B.; Dupuy-Galet, B.X.; Fjaer, K.; Shapaval, V. Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl. Microb. Biotechnol. 2022, 106, 101–115. [Google Scholar] [CrossRef]
- Karimi, K.; Zamani, A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef]
- Pawłowska, J.; Okrasińska, A.; Kisło, K.; Aleksandrzak-Piekarczyk, T.; Szatraj, K.; Dolatabadi, S.; Muszewska, A. Carbon assimilation profiles of mucoralean fungi show their metabolic versatility. Sci. Rep. 2019, 9, 11864. [Google Scholar] [CrossRef] [PubMed]
- Goginyan, V.; Harutyunyan, B.; Hovhannisyan, S.; Novak, M. Chapter 13—5-Aminolevulinic acid production: Strategies for microbial biosynthesis, advances, and perspective. In Developments in Applied Microbiology and Biotechnology, Microbial Essentialism; Singh, R.P., Manchanda, G., Sarsan, S., Kumar, A., Panosyan, H., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 317–338. [Google Scholar] [CrossRef]
- Novak, M.; Trontel, A.; Marđetko, N.; Matoković, V.; Sarić, M.; Pavlečić, M.; Šantek, B. Photoheterotrophic cultivation of purple non-sulphur bacterium Rhodovulum adriaticum on the media with different carbon sources. Croat. J. Food Technol. Biotechnol. Nutrit. 2020, 15, 115–123. [Google Scholar] [CrossRef]
- Rashid, N.; Onwusogh, U.; Mackey, H.R. Exploring the metabolic features of purple non-sulfur bacteria for waste carbon utilization and single-cell protein synthesis. Biomass Conv. Bioref. 2024, 14, 12653–12667. [Google Scholar] [CrossRef]
- Conners, E.M.; Rengasamy, K.; Ranaivoarisoa, T.; Bose, A. The phototrophic purple non-sulfur bacteria Rhodomicrobium spp. are novel chassis for bioplastic production. Microb. Biotechnol. 2024, 17, e14552. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Bhatia, L.; Sarangi, P.K.; Singh, A.K.; Prakash, A.; Shadangi, K.P. Lignocellulosic waste biomass for biohydrogen production: Future challenges and bio-economic perspectives. Biofuels Bioprod. Bioref. 2022, 16, 838–858. [Google Scholar] [CrossRef]
- Novak, M.; Pavlečić, M.; Harutyunyan, B.; Goginyan, V.; Horvat, P.; Šantek, B. Characteristics and selection of cultures of photosynthetic purple non-sulphur bacteria as a potential 5-aminolevulinic acid producers. Croat. J. Food Technol. Biotechnol. Nutrit. 2017, 12, 113–119. [Google Scholar]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non sulfur bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef]
- Sandmann, G. Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria. Biology 2023, 12, 1346. [Google Scholar] [CrossRef]
- Azka, A.; Hareem, M.; Yasir, R. Chapter 9—Purple nonsulfur bacteria: An important versatile tool in biotechnology. In Recent Advancement in Microbial Biotechnology; De Mandal, S., Kumar Passari, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 309–337. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; He, S.; Zhao, R.; Zhu, D. Purple non-sulfur bacteria technology: A promising and potential approach for wastewater treatment and bioresources recovery. World J. Microb. Biotechnol. 2021, 37, 161. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Venkateswarlu, K.; Megharaj, M. Anoxygenic phototrophic purple non-sulfur bacteria: Tool for bioremediation of hazardous environmental pollutants. World J. Microb. Biotechnol. 2023, 39, 283. [Google Scholar] [CrossRef] [PubMed]
- Siwei, Y.; Peng, L.; Xu, Y.; Song, S.; Xie, G.J.; Liu, Y.; Ni, B.J. Optimizing light sources for selective growth of purple bacteria and efficient formation of value-added products. J. Clean. Prod. 2021, 280, 124493. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; He, S.; Peng, C.; Ren, Z. Production of photosynthetic bacteria using organic wastewater in photobioreactors in lieu of a culture medium in fermenters: From lab to pilot scale. J. Clean. Prod. 2020, 259, 120871. [Google Scholar] [CrossRef]
- Riccardo, A.A.; Muzzarelli, A.; Ilari, P.; Tarsi, R.; Dubini, B.; Xia, W. Chitosan from Absidia coerulea. Carbohydr. Polym. 1994, 25, 45–50. [Google Scholar]
- Kasongo, K.J.; Tubadi, D.J.; Bampole, L.D.; Kaniki, T.A.; Kanda, N.J.M.; Lukumu, M.E. Extraction and characterization of chitin and chitosan from Termitomyces titanicus. SN Appl. Sci. 2020, 2, 406. [Google Scholar] [CrossRef]
- Van Wychen, S.; Laurens, L.M.L.; Ramirez, K. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in Situ Transesterification; Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden; CO, USA, 2015. [Google Scholar]
- Ritchie, R.J. Measurment of chlorophylls a and b and bacteriochlorophyll a in organisms from hypereutrophic auxinic waters. J. Appl. Phycol. 2018, 30, 3075–3087. [Google Scholar] [CrossRef]
- Pavlečić, M.; Novak, M.; Trontel, A.; Marđetko, N.; Grubišić, M.; Ljubas, B.D.; Tominac, V.P.; Rakovac, R.C.; Šantek, B. Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation 2022, 8, 13. [Google Scholar] [CrossRef]
- Doran, P. Presentation and Analysis of Data. In Bioprocess Engineering Principles; Doran, P., Ed.; Academic Press Limited: London, UK, 1998; pp. 27–48. [Google Scholar]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005, 40, 3693. [Google Scholar] [CrossRef]
- Shafiei Alavijeh, R.; Karimi, K.; van den Berg, C. An integrated and optimized process for cleaner production of ethanol and biodiesel from corn stover by Mucor indicus. J. Clean. Prod. 2020, 249, 119321. [Google Scholar] [CrossRef]
- Sharifyazd, S.; Karimi, K. Effects of fermentation conditions on valuable products of ethanolic fungus Mucor indicus. Electron. J. Biotechnol. 2017, 30, 77–82. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Zamani, A. Oil, chitosan, and ethanol production by dimorphic fungus Mucor indicus from different lignocelluloses. J. Chem. Technol. Biotechnol. 2016, 91, 1835–1843. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochemie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The influence of phosphorus source and the nature of nitrogen substrate on the biomass production and lipid accumulation in oleaginous Mucoromycota fungi. Appl. Microb. Biotechnol. 2020, 104, 8065–8076. [Google Scholar] [CrossRef] [PubMed]
- Alloul, A.; Wille, M.; Lucenti, P.; Bossier, P.; Van Stappen, G.; Vlaeminck, S.E. Purple bacteria as added-value protein ingredient in shrimp feed: Penaeus vannamei growth performance, and tolerance against Vibrio and ammonia stress. Aquaculture 2021, 530, 735788. [Google Scholar] [CrossRef]
- Sasikala, C.; Ramana, C.V.; Rao, R. Environmental regulation for optimal biomass yield and photoproduction of hydrogen by Rhodobacter sphaeroides O.U. 001. Int. J. Hydrogen Energy 1997, 16, 597–601. [Google Scholar] [CrossRef]
- Sues, A.; Millati, R.; Edebo, L.; Taherzadeh, M.J. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 2005, 5, 669–676. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Ylitervo, P.; Larsson, C.; Edebo, L.; Taherzadeh, M.J. Growth tolerance of Zygomycetes Mucor indicus in orange peel hydrolysate without detoxification. Process Biochem. 2012, 47, 836–842. [Google Scholar] [CrossRef]



| Parameters | Conditions |
|---|---|
| Column | ZB-FAME (Zebron, Newport Beach, CA, USA), 30 m × 0.25 mm, df 0.20 μm |
| Detector | FID |
| Carrier gas/flow | Helium/1.2 mL min−1 |
| Temperature program | 10 °C/min → 140 °C 3 °C/min → 190 °C 30 °C/min do 260 °C 260 °C, 2 min |
| Injector temperature | 250 °C |
| Detector temperature | 260 °C |
| Partition coefficient | 1:15 |
| Injection volume | 2 μL |
| Medium | H | H YE1 | H YE5 | H YE10 | H CSL1 | H CSL5 | H CSL10 |
|---|---|---|---|---|---|---|---|
| FAME | FAME [mg gbiomass−1] | ||||||
| C6:0 | 1.18 ± 0.09 | 1.09 ± 0.01 | 0.95 ± 0.02 | 0.91 ± 0.01 | 0.93 ± 0.01 | 1.01 ± 0.06 | 0.97 ± 0.01 |
| C11:0 | 0.82 ± 0.07 | 0 | 0 | 1.96 ± 0.09 | 0.63 ± 0.01 | 1.20 ± 0.08 | 1.16 ± 0.09 |
| C12:0 | 0.23 ± 0.03 | 0 | 0.33 ± 0.01 | 0.21 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 |
| C14:0 | 0.24 ± 0.04 | 0.35 ± 0.1 | 0.90 ± 0.01 | 1.58 ± 0.06 | 0.52 ± 0.01 | 0.53 ± 0.01 | 0.51 ± 0.01 |
| C14:1 cis 9 | 0 | 1.02 ± 0.01 | 1.30 ± 0.05 | 1.50 ± 0.04 | 0.15 ±0.01 | 0 | 0 |
| C15:0 | 0.05 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.99 ± 0.01 | 0.29 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| C15:1 cis 10 | 0 | 0.01 | 0.23 ± 0.01 | 0 | 0 | 0 | 0 |
| C16:0 | 7.63 ± 0.65 | 5.61 ± 0.24 | 6.36 ± 0.29 | 9.27 ± 0.03 | 7.48 ± 0.32 | 7.09 ± 0.32 | 6.86 ± 0.25 |
| C16:1 cis 9 | 0.55 ± 0.01 | 0.63 ± 0.01 | 2.24 ± 0.11 | 3.49 ± 0.10 | 1.18 ± 0.10 | 3.65 ± 0.18 | 3.53 ± 0.30 |
| C17:0 | 0 | 0 | 0 | 0.47 ± 0.01 | 0 | 4.72 ± 0.29 | 4.56 ± 0.27 |
| C17:1 cis 10 | 0 | 0 | 0 | 0.60 ± 0.02 | 0 | 0 | 0 |
| C18:1 cis 9 | 22.81 ± 0.94 | 19.41 ± 0.75 | 19.97 ± 0.67 | 24.17 ± 1.24 | 20.49 ± 1.20 | 18.61 ± 0.78 | 18.01 ± 0.11 |
| C18:2 trans 9,12 | 6.99 ± | 5.14 ± 0.23 | 6.86 ± 0.53 | 10.60 ± 0.98 | 7.18 ± 0.40 | 5.45 ± 0.31 | 5.56 ± 0.08 |
| C18:2 cis 9,12 | 3.12 ± | 3.88 ± 0.16 | 6.20 ± 0.57 | 8.55 ± 0.65 | 4.22 ± 0.22 | 3.08 ± 0.05 | 2.99 ± 0.12 |
| C18:3 cis 6,9,12 | 6.82 ± | 7.58 ± 0.41 | 8.71 ± 0.70 | 10.23 ± 0.78 | 8.03 ± 0.36 | 14.19 ± 1.01 | 13.74 ± 0.79 |
| C20:1 cis 11 | 0 | 0 | 0 | 0 | 0 | 12.71 ± 0.99 | 12.30 ± 0.91 |
| C20:4 cis 5,8,11,14 | 0 | 0 | 0 | 0 | 0 | 6.89 ± 0.21 | 6.47 ± 0.36 |
| C20:5 cis 5,8,11,14,17 | 0 | 0 | 0 | 0 | 0 | 0 | 11.55 ± 0.88 |
| C22:6 cis 4,7,10,13,16,19 | 0 | 0 | 0 | 0 | 0 | 7.65 ± 0.38 | 7.40 ± 0.55 |
| C23:0 | 0 | 0 | 0 | 0 | 0 | 0.32 ± 0.01 | 0.31 ± 0.01 |
| C24:0 | 0 | 0 | 0 | 0 | 0 | 12.27 ± 0.79 | 11.88 ± 0.07 |
| C24:1 cis 15 | 0 | 0 | 0 | 0 | 0 | 9.16 ± 0.80 | 0 |
| w (FAME) in biomass [%] | 6.08 ± 0.35 | 5.66 ± 0.29 | 4.18 ± 0.17 | 4.97 ± 0.21 | 7.20 ± 0.42 | 15.76 ± 0.98 | 15.25 ± 0.83 |
| γX [g L−1] | γchitin [g g−1] | γchitosan [g g−1] | Yetanol [g L−1] | YEt/S [g g−1] | Pr [g L−1 h−1] | qS [h−1] | qP [h−1] |
|---|---|---|---|---|---|---|---|
| 5.910 | 0.310 | 0.170 | 1.610 | 0.142 | 0.034 | 0.0364 | 0.0868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marđetko, N.; Kolakušić, A.; Trontel, A.; Novak, M.; Pavlečić, M.; Dobrinčić, A.; Petravić Tominac, V.; Šantek, B. Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates. Polymers 2025, 17, 369. https://doi.org/10.3390/polym17030369
Marđetko N, Kolakušić A, Trontel A, Novak M, Pavlečić M, Dobrinčić A, Petravić Tominac V, Šantek B. Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates. Polymers. 2025; 17(3):369. https://doi.org/10.3390/polym17030369
Chicago/Turabian StyleMarđetko, Nenad, Antonio Kolakušić, Antonija Trontel, Mario Novak, Mladen Pavlečić, Ana Dobrinčić, Vlatka Petravić Tominac, and Božidar Šantek. 2025. "Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates" Polymers 17, no. 3: 369. https://doi.org/10.3390/polym17030369
APA StyleMarđetko, N., Kolakušić, A., Trontel, A., Novak, M., Pavlečić, M., Dobrinčić, A., Petravić Tominac, V., & Šantek, B. (2025). Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates. Polymers, 17(3), 369. https://doi.org/10.3390/polym17030369









