Abstract
Long-chain hyperbranched polyesters (LHBPx, x = 1, 2, 3) with varying lengths of branched chains were synthesized through a thiol-ene click reaction. Subsequently, LHBPx was incorporated into PA4 via the solution method to prepare a LHBPx/PA4 polymer blend, aiming to address the limitations of PA4, such as its narrow thermal processing window (△T = Td5−Tm) and high brittleness. The results demonstrated that the addition of LHBPx enhanced the △T of PA4 from 1.6 °C to 14.5 °C (LHBP3/PA4), increasing the rheological properties of LHBPx/PA4 polymer blends, thereby improving its thermal processability. Compared with PA4, the elongation at the break of the LHBP3/PA4 polymer blend was increased by 20.4%, and the brittle fracture was changed into a ductile fracture. The crystallinity of PA4 was greatly decreased, from 54.41% to 37.42%, owing to the incorporation of LHBPx, whereas Tm of PA4 had almost no change. It was explained that LHBPx hindered the crystal growth stage, whereas it promoted the nucleation stage of PA4, resulting in no significant change in crystal type. Moreover, the longer the branched chain of LHBPx was, the more pronounced the improvement in the thermal processability and toughness of PA4 became. Above all, this work was meaningful for the potential application of PA4 in industrial plastics.
1. Introduction
PA4 (Polyamide 4) can be completely degraded naturally within a few months, making it a renewable raw material with environmentally friendly and other characteristics, but it also has heat resistance, impact resistance, and gas barrier characteristics [1] as good as the market PA6 and PA66 [2], which makes it useful for biological tissue engineering [3], packaging engineering [4], and as an engineering plastic [1] in the construction, automotive, and other fields, with very good advantages in those applications. However, the dense distribution of amide groups along the PA4 molecular chain results in strong intermolecular hydrogen bonding, leading to challenges such as a narrow processing temperature range and brittleness [5,6]. Therefore, enhancing thermal processability and toughness represents a crucial focus in PA4 research.
Some researchers have used various techniques, such as copolymerization [7,8,9,10,11] and splicing or end sealing [12], to modify the molecular chain structure of PA4. However, these intrinsic modifications may compromise PA4′s biodegradability and have some disadvantages such as a complex synthesis process and wide molecular weight distribution of products. Additionally, simpler blending methods like solution-blending film layering [13,14], solution electrostatic spinning [15,16], or melt extrusion [17] have also been employed by researchers for non-intrinsic modification of PA4. Nevertheless, doping modifications frequently encounter compatibility issues, which further result in phase separation within the polymer blend, adversely affecting their mechanical properties [18].
Long-chain hyperbranched polymer (LCHBP) combines the advantageous features of hyperbranched polymers (HBPs) [19,20] and linear polymers, having benefits such as reduced hydrodynamic volume, lower viscosity, and enhanced inter-molecular entanglement [21,22]. Incorporating LCHBP into linear polymer matrices can effectively enhance both toughness and processability [23,24]. Yang et al. [22] found that linear triblock copolymer macromolecules have a distinct long-range ordered structure, whereas the macromolecular monomers generate LHBP without a distinct long-range ordered structure. Li et al. [25] studied the rheological properties and aggregation morphology of LHBP. They found that an increase in the degree of aggregation of LHBP is not conducive to intermolecular chain entanglement and that LHBP in different solvents may collapse or aggregate. Konkel et al. [24] obtained LCHBP by linking a large number of fatty acid long chains to the ends of hyperbranched polyesters and extruded LCHBP with PLA/PPC melt blending, and it was found that the addition of LCHBP improved the compatibility between PLA and PPC, and the addition of 2% of LCHBP increased the impact strength by 61% and the elongation at the break by 367%. LHBP combines the advantages of both hyperbranched polymer and linear polymers, retaining the benefits of small hydrodynamic volume and low viscosity, but also increasing intermolecular chain entanglement [21]. When modified to linear polymers, it effectively inhibits the long-range ordering of polymer microphase separation structure, reduces the crystallinity of polymers, and improves the toughness of polymers. Moreover, LHBPx exhibits remarkable designability, enabling the synthesis of three-dimensional typologies with diverse structures through different synthetic approaches [20,26]. Furthermore, the thiol-ene click [27,28] reaction is widely employed to synthesize HBP due to its high selectivity, rapid kinetics, and mild reaction conditions [29].
In this study, the LHBPx with varying branched chain lengths was synthesized by dienyl monomer (A2-X), tetrasulfhydryl monomer (B4), and trans-methyl crotonate (TMC). The structures of LHBPx were characterized using 1hydrogen-nuclear magnetic resonance (1H-NMR). Subsequently, LHBPx was incorporated into PA4 via a solution method to obtain the LHBPx/PA4 polymer blend. The inter-molecular hydrogen bonds of LHBPx/PA4 polymer blend were analyzed by a Fourier transform infrared spectrometer (FTIR). The thermal, rheological, and mechanical properties of the LHBPx/PA4 polymer blend were evaluated. The tensile fracture type was analyzed by scanning electron microscope (SEM). Furthermore, an X-ray diffractometer (XRD) was employed to characterize the crystal structure of the LHBPx/PA4 polymer blend, and non-isothermal kinetic calculations were performed to study its crystallization process.
2. Experimental
2.1. Materials
Gamma-aminobutyric acid (GABA) (Wanxiang Hongrun Biotechnology Co., Ltd., Chengdu, China); benzoyl chloride (Aladdin Biochemical Technology Co., Ltd., Shanghai, China); formic acid (Titan Scientific Co., Ltd., Shanghai, China); pentaerythritol tetra(3-mercapto propionate) (B4), polyethylene glycol 200 diacrylate (A2-1), polyethylene glycol 400 diacrylate (A2-2), polyethylene glycol 1000 diacrylate (A2-3), trans-methyl crotonate (TMC), 2,2-azobis(2-methylpropionitrile) (AIBN), tetrahydrofuran, hexafluoroisopropanol, and potassium tert-butoxide were supplied by Adamas Reagent Co., Ltd. (Shanghai, China); acetone, ethanol, methylbenzene, diethyl ether, and trifluoroethanol were supplied by China National Medicines Co., Ltd. (Beijing, China).
2.2. Preparation of LHBPx/PA4 Polymer Blend
2.2.1. Synthesis of PA4
GABA was slowly heated from 190 °C to 220 °C under nitrogen protection, dehydrated at 220 °C for 2 h, and then purified at 130 °C under reduced pressure to obtain transparent biobutylactam (BBY) (Scheme 1) [30].
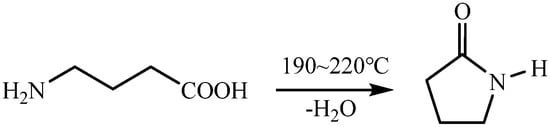
Scheme 1.
Synthesis equation of BBY.
The mixture of BBY (4 mL) and tert-butanol methyl (0.283 g) were reacted under vacuum for 2 h at 90 °C, and cooled to 40 °C. Then, a benzoyl chloride (160 μL) initiator was added to the nitrogen atmosphere for an anionic ring-opening reaction for 12 h, dissolved by formic acid (10 mL), and precipitated in acetone (50 mL) to obtain a flocculent precipitate. After washing with deionized water and ethanol, the PA4 product was obtained by vacuum drying at 80 °C for 12 h. The yield of PA4 was 83%, Mη ≈ 21,000.
2.2.2. Synthesis of LHBPx
The LHBPx was prepared via thiol-ene click reaction. A2-X, B4, AIBN (0.1 g), and toluene (100 mL) were added to a round-bottom flask and mixed evenly; then, the reaction was carried out at 70 °C under a nitrogen atmosphere for 2 h. The product was added to ether, stirred and mixed for 10 min, the supernatant was poured away, dichloromethane was added to dissolve the product, and repeated three times to remove excess reactant, and then the organic solvent was removed by rotary evaporation. Then, the TMC (20 mL) and AIBN (0.15 g) were added, and continued to react under nitrogen atmosphere at 70 °C for 2 h, adding ether and pouring off the supernatant, dissolving the precipitate with dichloride, and repeated three times to wash away the non-reactants, and then rotating evaporation was used to remove the organic solvent to obtain transparent and viscous LHBPx (Scheme 2). The types and amounts of A2-X and B4 required for LHBPx synthesis are shown in Table 1. The yield of LHBPx was higher than 94%.
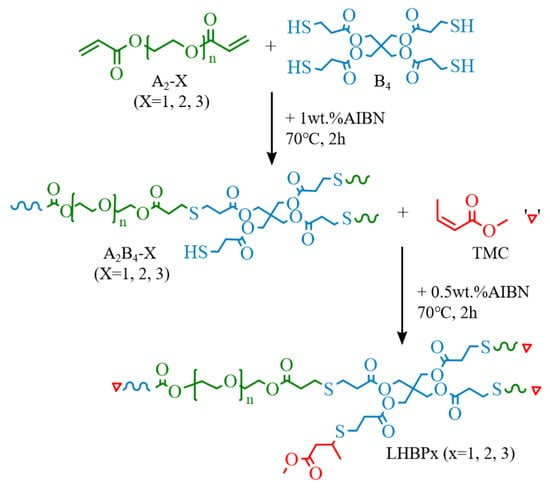
Scheme 2.
Synthesis route of LHBPx.

Table 1.
Types and amounts of A2-X and B4 required for LHBPx synthesis.
2.2.3. Solution Method to Prepare LHBPx/PA4 Polymer Blends
Each LHBPx/PA4 polymer blend was prepared via a solution-blended process. PA4 and LHBPx were added into the trifluoroethanol solution (solution concentration 10 wt.%) at a 9:1 ratio, stirred at room temperature for 24 h, poured into the silicone mold, and dried at room temperature for 4 h, and then dried overnight in a drying oven at 80 °C. A LHBPx/PA4 polymer blend with a mass fraction of 10 wt.% was obtained. The composite material was placed in a hot-pressing mold, preheated for 1 min at an atmospheric pressure at 230 °C, and then held under 6 MPa pressure for 1 min. After pressure release, the sample was taken out for rapid cooling, and a translucent LHBPx/PA4 polymer blend was obtained for subsequent testing. The compositions of the blends are summarized in Table 2.

Table 2.
Compositions of the LHBPx/PA4 polymer blends.
2.3. Characterization of LHBPx/PA4 Polymer Blends
2.3.1. Hydrogen Nuclear Magnetic Resonance (1H-NMR)
The structures of A2, B4, TMC, and LHBPx were determined by a 1H-NMR (AVANCE III, 500 MHz, Bruker Co., Ltd., Billerica, Germany) at concentrations of 25 mg·mL−1 in deuterated trifluoroacetic acid (TFA-d) for PA4 dissolution and deuterated chloroform (CDCl3) for LHBPx dissolution.
2.3.2. Fourier Transform Infrared Spectrometer (FTIR)
The functional groups of the LHBPx/PA4 polymer blends were characterized by a FTIR (Nicolet IS 50, Thermo Fischer Technology Co., Ltd., Waltham, MA, USA) in attenuated total reflection mode. The test range was 4000~400 cm−1 and the scanning times were 32.
2.3.3. Thermogravimetric Analyzer (TGA)
The thermal stability of the LHBPx/PA4 polymer blends was assessed by a TGA (TGA-2, Mettler Toledo Co., Ltd., Zurich, Switzerland). The 6~8 mg samples were placed in aluminum crucibles under N2 atmosphere, and the system was heated from 30 °C to 600 °C at a heating rate of 10 °C·min−1, with a gas flow rate of 50 mL·min−1.
2.3.4. Differential Scanning Calorimeter (DSC)
The thermal properties of the LHBPx/PA4 polymer blends were evaluated by DSC (DSC-200, Netzsch Co., Ltd., Selb, Germany). The 6~8 mg samples were placed in aluminum crucibles under Ar atmosphere, and the system was heated from 30 °C to 270 °C at a heating rate of 10 °C·min−1. The non-isothermal crystallization kinetics of the polymer blends were examined via first being heated from 30 to 270 °C at a rate of 10 °C·min−1 and then cooled at five different cooling rates (2.5, 5, 10, 20, 40 °C·min−1).
2.3.5. Relative Viscosity
The relative viscosity of the LHBPx and LHBPx/PA4 polymer blends was measured by using a rotary viscometer (VISCO, Atago Co., Ltd., Tokyo, Japan) at a concentration of 1 g·dL−1 in m-cresol at 25 °C.
2.3.6. Electronic Universal Testing Machine
The mechanical properties of the LHBPx/PA4 polymer blends were evaluated by an electronic universal testing machine (CMT4204, Heng Yu Co., Ltd., Hong Kong, China) at a stretching rate of 50 μm·s−1 and a temperature of 25 °C. The samples were cut as follows: the total length was 27 mm and width was 10 mm; the parallel part was 10 mm long and 5 mm wide, and 3~5 groups of each sample were tested.
2.3.7. Scanning Electron Microscope (SEM)
The tensile section morphology of the LHBPx/PA4 polymer blends was characterized by a cold SEM (SU8010, Hitachi Co., Ltd., Tokyo, Japan). The sample was placed on an electron microscope flat plate with conductive tape, sprayed with gold by an ion-sputtering instrument (E-1010, Hitachi Co., Ltd., Japan), and observed with a SEM at an operating voltage of 5 kV.
2.3.8. X-Ray Diffractometer (XRD)
The crystal structure of a LHBPx/PA4 polymer blend was analyzed by an XRD (Smar/SmartLa, Rigaku Co., Ltd., Tokyo, Japan) operating at CuK (λ = 1.542 A). The scanning range was set from 5 to 60 ° at a scanning speed of 10 °·min−1.
2.3.9. Gel Permeation Chromatography (GPC) Analysis
The apparent number average (Mn) and weight average molecular weight (Mw) of the samples were characterized by a gel permeation chromatography (GPC) analyzer, model PL-GPC50, from Agilent, Santa Clara, CA, USA. The testing temperature was 25 °C, THF was used as the eluent, the flow rate was 1 mL/min, and linear polystyrene was used as the standard sample.
3. Results and Discussion
3.1. Chemical Structures and Thermal Properties of LHBPx
3.1.1. Chemical Structures of LHBPx
1H-NMR spectroscopy was used to characterize the structure of LHBPx (Figure 1) [31]. The ‘a’ and ‘b’ were the CH2 peak of the LHBPx linear unit, ‘c’ was the CH2 peak of the ether repeat unit of the LHBPx linear unit, ‘d’ and ‘e’ were the CH2 peak of the LHBPx dendritic unit, and ‘f’ was the CH3 peak in the LHBPx terminal unit.
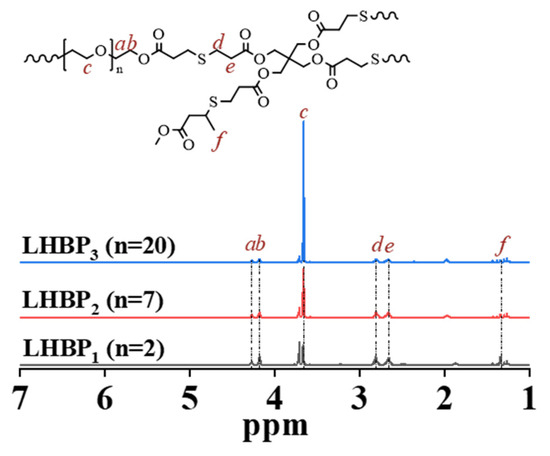
Figure 1.
1H-NMR spectra of LHBPx.
The CH2=CH2 peak (5.2~6.2 ppm) of A2-X disappeared (Figure S1), the ‘d’ and ‘e’ peaks of LHBPx appeared, indicating a thiol-ene reaction between A2-X and B4 had occurred. At 1.65 ppm, the S-H peak (1.5~1.6 ppm) disappeared (Figure S1), and the ‘f’ peak belonging to the terminal reagent TMC appeared, confirming the successful synthesis of LHBPx terminated with TMC. As the branched chain length of A2-X increased, Ad/Ac gradually decreased in LHBPx, suggesting an increase in branched chain length and successful synthesis of LHBPx (LHBP1, LHBP2, and LHBP3) with different branched chain lengths.
3.1.2. Gel Permeation Chromatography (GPC) of LHBPx
Gel permeation chromatography was used to characterize the molecular weight and molecular weight distribution of LHBPx. Figure 2 shows the GPC efflux time curve of LHBPx. The figure shows that the GPC curves of all hyperbranched polysulfides were single peaks, indicating that the relative molecular weight distribution of the synthesized LHBPx was relatively concentrated, and there was no reactant residue in the reaction system.
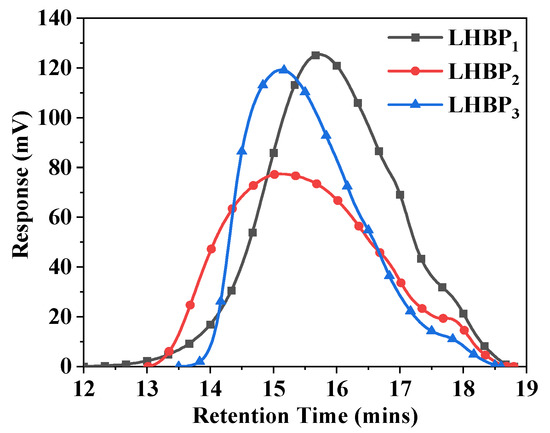
Figure 2.
GPC flow curves of LHBPx.
The relative molecular weights and molecular weight distributions of the prepared LHBPx were fitted from the GPC outflow curves (the results are shown in Table 3), from which it can be seen that the number average molecular weights of LHBPx increased gradually with the increase in the branched chain lengths. The weight-average molecular weights increased first and then decreased. The polydispersity coefficients of PDI were all greater than 3 and showed a trend of increasing first and then decreasing, which indicated that the molecular weight distributions of LHBPx were wider. The three kinds of weight-average molecular weights of LHBPx with different chain lengths were greater than 15,000.

Table 3.
Mw, Mn, and PDI of LHBPx.
3.1.3. Thermal Property of LHBPx
LHBPx had excellent thermal stability, and the Td5 of LHBPx with three different branched-chain lengths was greater than 345 °C. With the increase in the branched-chain length, the Td5 of LHBPx gradually increased, and the temperature Tmax corresponding to the maximum thermal decomposition rate also gradually increased. The peak area in the 360 to 435 °C interval in the DTG profile of Figure 3b increased with the increase in the length of the branched chain, while the peak area in the 435 to 500 °C interval decreased with the increase in the length of the branched chain.
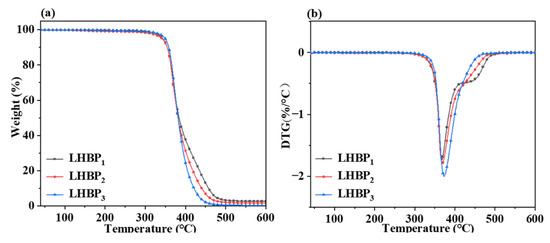
Figure 3.
(a) TG curve of LHBPx, (b) DTG curve of LHBPx.
3.2. Intermolecular Hydrogen Bonds of LHBPx/PA4 Polymer Blends
The FTIR spectra of pure-PA4 and LHBPx/PA4 polymer blends is shown in Figure 4 [32]. The peaks observed at 1631 and 1530 cm−1 correspond to the amide I band (C=O stretching) and the amide II band (N-H bending and C-N stretching) of PA4, respectively. The peak at 1738 cm−1 correspond to the carbonyl (C=O stretching) of LHBPx. The main chain of the PA4 molecule contained a significant number of amide groups, and it exhibited intermolecular hydrogen bonds between N-H and C=O. Upon incorporation of LHBPx, a red shift was observed in the C=O stretching vibration peak of PA4 from 1631 cm−1 to 1633 cm−1 (LHBP3/PA4); a similar red shift was also noted in the N-H stretching vibration peak from 1530 cm−1 to 1536 cm−1 (LHBP3/PA4). These observations suggest that hydrogen bond interaction between PA4 molecular chains were weakened by the addition of LHBPx. The carbonyl (C=O) peak on LHBP3 shifted from 1740 to 1738 cm−1 (LHBP3/PA4), indicating an increased polarity of the carbonyl (C=O) group on LHBPx due to the formation of “N-H···O” hydrogen bonds with PA4. The establishment of a hydrogen bond network between LHBPx and PA4 at the molecular level enhanced their compatibility.
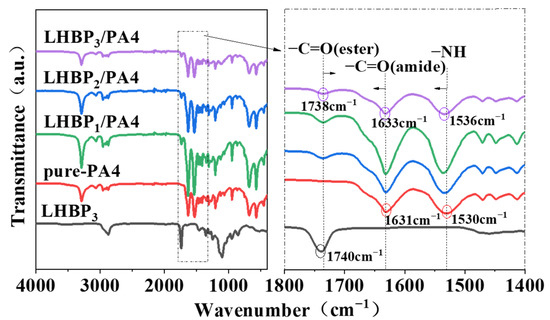
Figure 4.
FTIR spectra of LHBP3, pure-PA4, and LHBPx/PA4 polymer blends.
3.3. Thermal Properties of LHBPx/PA4 Polymer Blend
Figure 5a–c shows the DSC, TGA, and DTG curves of pure-PA4 and LHBPx/PA4 polymer blends. Figure 5d shows the changes in Tm and Td5 of LHBPx/PA4 polymer blends with the different types of LHBPx. With the increase in the branched-chain length of LHBPx, Tm gradually decreased from 260.9 °C (pure-PA4) to 260.5 °C (LHBP1/PA4), 260.4 °C (LHBP2/PA4), and 260.0 °C (LHBP3/PA4), and the melting point decreased slightly; Td5 gradually increased from 262.5 °C (pure-PA4) to 269.5 °C (LHBP1/PA4), 271 °C (LHBP2/PA4), and 274.5 °C (LHBP3/PA4), and the thermal stability was improved; △T (Td5−Tm) gradually widened from 1.6 °C (pure-PA4) to 9.0 °C (LHBP1/PA4), 10.6 °C (LHBP2/PA4), and 14.5 °C (LHBP3/PA4), and △T widened significantly. With the increase in branch chain length, the △T value increased due to the enhanced thermal stability of LHBPx with longer branches (Figure S2).
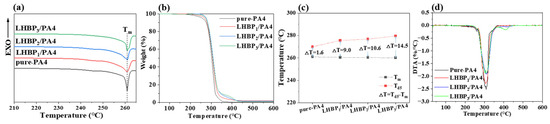
Figure 5.
(a) DSC curves of pure-PA4 and LHBPx/PA4 polymer blends, (b) TGA curves of pure-PA4 and LHBPx/PA4 polymer blends, (c) DTG curves for pure-PA4 and LHBPx/PA4 polymer blends, (d) Tm and Td5 changes of pure-PA4 and LHBPx/PA4 polymer blends.
As can be seen from Table 4, the addition of LHBP1 to the polymerization blends increased the Td5 and Td50 of LHBP1/PA4 by 7 °C and 4 °C, respectively, and that of LHBP3/PA4 by 12 °C and 6 °C, respectively, compared to pure-PA4. In contrast, adding LHBPx increased the degradation temperatures of the materials. The degradation temperature of the material increased after mixing with LHBPx. This indicates that the branch length of LHBPx affects the thermal properties of the PA4 matrix, and the longer branch length of LHBPx is more effective in improving the thermal stability of PA4.

Table 4.
Td5, Td50, and DTG peaks of pure-PA4 and LHBPx/PA4 polymer blends.
Therefore, the highly thermally stable LHBPx formed a physical barrier in PA4, hindering heat conduction, and thereby improving the thermal stability of the LHBPx/PA4 polymer blends.
3.4. Rheological Properties of LHBPx/PA4 Polymer Blends
The thermal processability of the polymer is not only related to its thermal properties but is also concerned with its rheological properties. The relative viscosity of the solutions of pure-PA4 and LHBPx/PA4 polymer blends is shown in Figure 6.
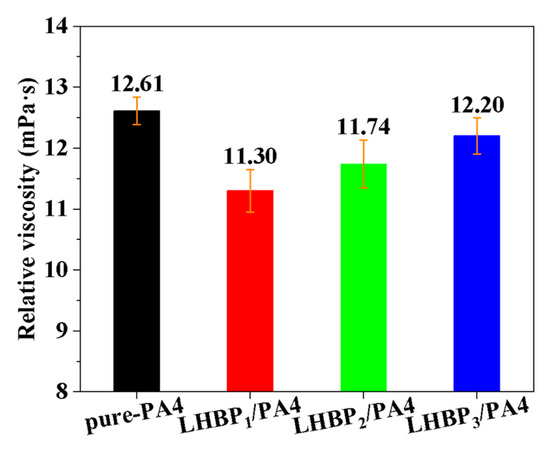
Figure 6.
Relative viscosity of pure-PA4 and LHBPx/PA4 polymer blends.
The addition of LHBPx reduced the ηr of PA4 from 12.61 mPa·s (pure-PA4) to 11.30 mPa·s (LHBP1/PA4) at first, and with the increase in LHBPx branched-chain length, ηr gradually rose to 11.74 mPa·s (LHBP2/PA4) and 12.20 mPa·s (LHBP3/PA4). This indicates that a LHBPx with low viscosity could improve the rheology of PA4. The increase in the length of the LHBPx branched chains led to a higher formation of physical entanglements with PA4, thereby enhancing the viscosity of PA4 when a LHBPx with a longer branched chain was added.
3.5. Mechanical Properties of LHBPx/PA4 Polymer Blends
The effect of the type of LHBPx of the LHBPx/PA4 polymer blend on mechanical properties is shown in Figure 7a,b and Table 5. The addition of LHBPx reduced the tensile strength of PA4 from 43.7 MPa (pure-PA4) to 42.3 MPa (LHBP1/PA4) at first, and with the increase in LHBPx branched-chain length, tensile strength gradually rose to 42.6 MPa (LHBP2/PA4) and 43.1 MPa (LHBP3/PA4); elongation at break gradually increased from 10.8% (pure-PA4) to 10.9 (LHBP1/PA4), 11.1 (LHBP2/PA4), and 13.0 (LHBP3/PA4); the elongation at break was improved. Therefore, when an appropriate amount of LHBPx was added, the toughness of PA4 could be improved to a certain extent, and the mechanical strength did not decrease significantly.
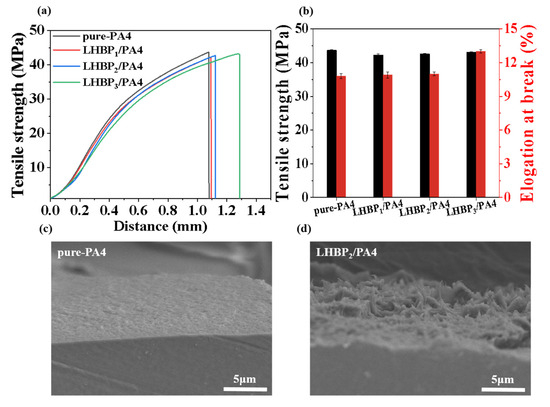
Figure 7.
(a,b) Tensile strength and elongation at break of pure-PA4 and LHBPx/PA4 polymer blend, (c) SEM pattern of pure-PA4, (d) SEM pattern of LHBP2/PA4.

Table 5.
Tensile properties data sheet for pure-PA4 and LHBPx/PA4 samples.
The microstructure of the tensile section of pure-PA4 and the LHBPx/PA4 polymer blends was observed by SEM micrographs (Figure 7c,d). The tensile section of pure-PA4 had no obvious deformation, and the fracture was flat and smooth, which was an obvious brittle fracture. With the addition of 10 wt.% LHBPx, the LHBPx/PA4 composite tensile section had obvious plastic deformation, the fracture was rough, and the fiber area was visible, which was due to the occurrence of holes in the section, and the hole-induced ductile fracture made the section rough. The SEM test further proved that with the addition of LHBPx, the mode of tensile fracture transitions changed from brittle to ductile. These results indicate that LHBPx could obviously improve the toughness of PA4.
3.6. Crystallization Behavior of LHBPx/PA4 Polymer Blend
The properties of PA4 are mainly determined by the crystallization behavior, which can control the formation of the microstructure. The crystallization parameters of pure PA4 and LHBPx/PA4 polymer blends were analyzed by XRD, as shown in Figure 8, and the fitting results are shown in Table 6. The crystallinity data of LHBPx/PA4 samples were calculated by substituting the fitted data into Equation (1) [33,34].
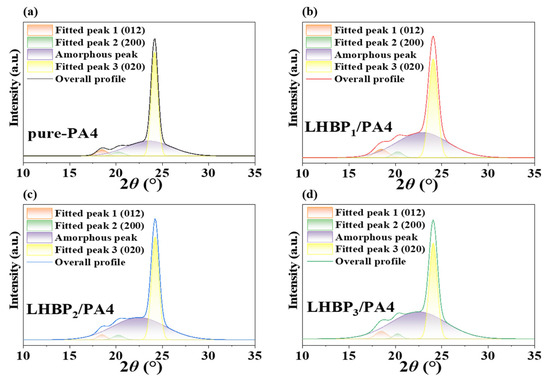
Figure 8.
XRD spectra of pure-PA4 and LHBPx/PA4 polymer blends, (a) pure-PA4, (b) LHBP1/PA4, (c) LHBP2/PA4, (d) LHBP3/PA4.

Table 6.
Peak-fitting area proportion and crystallinity of pure-PA4 and LHBPx/PA4 polymer blends.
LHBPx/PA4 polymer blends were fitted with three crystal-plane diffraction peaks and one amorphous peak. The diffraction peaks of the three crystal planes were located at 2θ ≈ 18.5°, 2θ ≈ 20.3°, and 2θ ≈ 24.1°, respectively, corresponding to the (012) crystal plane, (200) crystal plane, and (020) crystal plane of the PA4. The (200) crystal plane and (020) crystal plane belonged to the α crystal form of PA4. The α crystal form was a relatively stable structure formed by hydrogen bonds between antiparallel molecular chains [35,36,37]. The (200) crystal faces were connected by hydrogen bonds, and the spacing of the crystal faces was related to the strength of the PA4 hydrogen bond [33]. With the increase in the branched-chain length of LHBPx, the (012) and (200) crystal plane diffraction peak area ratio A1/A2 gradually decreased, indicating that increasing the branched-chain length of LHBPx promoted the growth of the (200) crystal planes and inhibited the growth of the (012) crystal planes. The crystallinity of LHBPx/PA4 also decreased as the branched-chain length of LHBPx increased. The addition of LHBPx reduced the crystallinity, but promoted the growth of a stable α crystal form of PA4 [38,39], so the melting point of the composite did not drop significantly.
3.7. Non-Isothermal Crystallization Kinetic Analysis of LHBPx/PA4 Polymer Blends
To understand the crystallization process of the LHBPx/PA4 polymer blends, they were crystallized at five different cooling rates (2.5, 5, 10, 20, 40 °C·min−1, respectively). The DSC curves of pure-PA4 and LHBPx/PA4 polymer blends under various cooling rates are shown in Figure 9a–d.
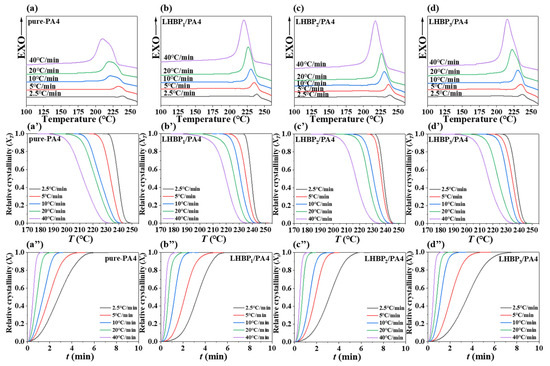
Figure 9.
(a–d) DSC curves of pure-PA4 and LHBPx/PA4 polymer blends at different cooling rates, (a’–d’) relationship between relative crystallinity XT and crystallization temperature T of pure-PA4 and LHBPx/PA4 polymer blends, (a”–d”) relationship between relative crystallinity Xt and crystallization temperature t of pure-PA4 and LHBPx/PA4 polymer blends.
At different crystallization rates, the crystallization peaks of the polymer blends exhibit unimodality, which is the crystallization temperature attributed to the α crystal type of PA4 [11]. With an increased cooling rate, the exothermic peak of the crystal broadened and shifted toward lower temperatures. Similarly, the initial, peak and final temperatures at the crystallization of all polymer blends also shifted toward lower temperatures. The observed phenomenon could be attributed to a delay in the movement of PA4 molecular chains related to the cooling rate. Consequently, these molecular chains were unable to sufficiently integrate into the lattice structure and become immobilized, resulting in imperfect crystallization. Conversely, when the cooling rate slowed, there was ample time for molecular chain adjustment within the lattice structure, leading to relatively flawless crystal formation. As a result, narrower crystallization peaks were observed with higher initiation and peak temperatures. For the LHBPx/PA4 blend system, as the length of the LHBPx branched chain decreased, the crystallization peak of the PA4 phases became sharper and moved toward the high-temperature direction, indicating that the LHBPx/PA4 polymer blends with a shorter branched chain of LHBP were more easily crystallized.
The relative crystallinity of the blends at a specified temperature can be calculated using Equation (2) [40]:
where is the temperature at the beginning of crystallization, is the temperature when the crystallization is complete, and are the heat released when the crystallinity is and the crystallinity is , and is the heat flow at temperature .
The relationship between crystallization time () and crystallization temperature () is shown in Equation (3) [40], as follows:
where is the cooling rate. By bringing Equation (3) into Equation (2), the relative crystallinity with respect to crystallization time () is obtained.
The and diagrams of LHBP/PA4 polymer blends obtained using Equations (2) and (3) are shown in Figure 9a’–d’ and Figure 9a”–d”, respectively.
With the increase in cooling rate, the curves of the four samples all moved towards lower temperatures. At 234 °C, the of pure-PA4 was 100% when cooled at a rate of 2.5 °C·min−1, whereas it dropped to 0% when cooled at a rate of 20 °C·min−1. This clearly demonstrated that the cooling rate significantly influenced the crystallization process of PA4.
The curves of the four samples were all ‘S’ curves, which were because the crystal growth mainly went through three stages: crystal nucleus growth, crystal growth, and crystal stability. With the increase in cooling rate, the slope of the crystal growth stage gradually increased, indicating that the crystallization rate of the polymer increased with the increase in cooling rate. The slope of PA4 added with LHBPx became larger in the crystal growth stage, that is, at the same cooling rate, the time required for = 50% was shorter. The addition of LHBPx led to an increased number of heterophase points and promoted the formation of crystal nuclei in PA4, thereby enhancing the crystallization rate during the crystal nucleus growth stage. However, the entanglement of long branched-chain LHBP hindered the further arrangement of PA4 molecular chains and, consequently, reduced the crystallinity of PA4.
Mo’s (Equation (6)) [41,42] method was combined with the Avrami (Equation (4)) [41] and Ozawa (Equation (5)) [43] methods to obtain a wider range of non-isothermal crystallization kinetics methods. So, the crystallization kinetic parameters of the polymer blend were fitted and calculated using Mo’s method, and the results are shown in Figure 10 and Table 7, as follows:
where is the Avrami index, which is related to the nucleation mechanism and crystal growth dimension, is the crystallization rate constant, is the relative crystallinity of different times, and is the time, and the following:
where is the function of temperature, is the cooling rate, is the Ozawa index, and is the relative crystallinity of different temperatures, and the following:
where is the cooling rate required to reach a certain relative crystallinity per unit of time; , , and are the Avrami and Ozawa indexes for the non-isothermal crystallization process, respectively.
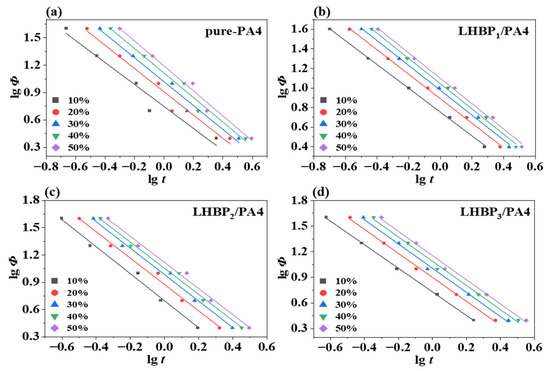
Figure 10.
curves of Mo’s method, (a) pure-PA4, (b) LHBP1/PA4, (c) LHBP2/PA4, (d) LHBP3/PA4.

Table 7.
Mo’s method data for pure-PA4 and LHBPx/PA4 polymer blends.
The value can represent the crystallization rate. Under the same relative crystallinity, the higher the value, the slower the crystallization rate. When the LHBP of the shorter-chain branch was added, became smaller. With the gradual increase in the length of the branched chain, became bigger. The results indicated that the crystallization rate of PA4 was first accelerated and then slowed down with the increase in LHBP branched chain length; , from which the Ozawa index, could be calculated (Table 7). This result proved that pure-PA4 grew in two dimensions, while PA4 with LHBPx added grew in three dimensions, and both homogeneous nucleation and heterophase nucleation occurred in the crystallization process of the four materials.
The Kissinger (Equation (7)) [43] method was used to calculate the non-isothermal crystallization activation energy of the polymer blends, as follows:
where is the cooling rate, is the temperature corresponding to the cooling crystallization peak, is the frequency factor independent of the cooling rate, is the ideal gas constant ( = 8.314 J·(K·mol)−1), and is the crystallization activation energy.
Using ln() to plot 1/, a linear correlation line could be obtained (Figure 11), which could be used to calculate from the slope of the line. Therefore, 0 (pure-PA4) = −42.82 KJ·mol−1, 1 (LHBP1/PA4) = −68.14 KJ·mol−1, 2 (LHBP2/PA4) = −59.07 KJ·mol−1, and 3 (LHBP3/PA4) = −53.65 KJ·mol−1. The value of crystallization activation energy was negative, indicating that the process could be a spontaneous exothermic process. The crystallization activation energy of PA4 with LHBPx added was lower, which further indicates that PA4 with LHBP added was more prone to crystallization. However, since crystal growth included the nucleation and growth processes, LHBPx promoted the nucleation of PA4, but hindered the growth process of PA4, resulting in an increase in crystallization rate but a decrease in crystallinity. With the increase in LHBPx branched-chain length, the crystallization activation energy of the LHBPx/PA4 polymer blends gradually increased and became more difficult to crystallize. The reason for this was that when the additional amount was the same, hyperbranched polymers with longer branched chains had a higher relative molecular weight.
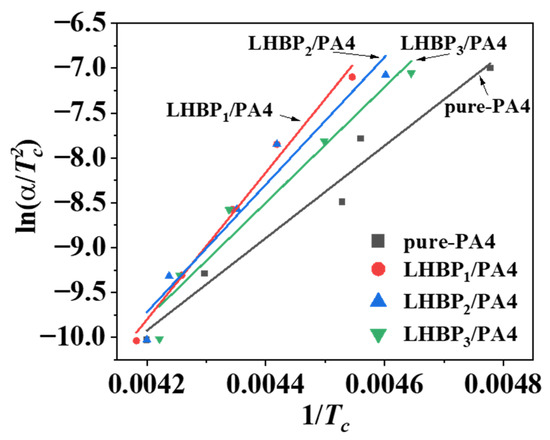
Figure 11.
ln() − 1/ curves of Kissinger equation.
4. Conclusions
In this study, the incorporation of LHBPx enhanced the thermal processability of PA4, improving its toughness while maintaining mechanical strength. Moreover, the longer the branched chain of LHBPx was, the more pronounced the improvement in the thermal processability and toughness of PA4 became. Compared with PA4, the thermal processability window was enhanced to 14.5 °C (LHBP3/PA4), and the rheological effect was increased; thereby, the thermal processability of LHBPx/PA4 was improved. The elongation at the break of the LHBP3/PA4 polymer blend was increased by 20.4% compared with PA4, and the brittle fracture was changed into a ductile fracture. The crystallinity of PA4, when blended with LHBPx, was reduced from 54.41% to 37.42%, but the crystal type did not change significantly, so the Tm of PA4 almost had no change. In summary, this study provided a research idea to modify the thermal processability and toughness of PA4, which was meaningful in promoting the further development of PA4 in industrial plastics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym17030318/s1, Figure S1: 1H-NMR spectra of A2-1, B4, TMC and LHBP1; Figure S2: TGA pattern of LHBPx.
Author Contributions
Conceptualization, L.S., J.L. and X.Z.; Methodology, X.Z.; Software, X.G. and Z.L.; Validation, X.Z.; Formal analysis, X.G. and J.L.; Investigation, L.S.; Resources, X.Z.; Data curation, L.S. and X.G.; Writing—original draft, L.S.; Writing—review & editing, X.G. and X.Z.; Visualization, X.G.; Supervision, Q.Z., G.C. and X.Z.; Project administration, X.Z.; Funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Xiamen, China (3502Z20227040, 3502Z20234025), the National Natural Science Foundation of China (52303124), the Natural Science Foundation of Fujian Province (2023I0016, 2021H6027), the Fundamental Research Funds for the Central Universities (ZQN-1109). The author also thanks the Xiamen Key Laboratory of Polymer and Electronic Functional Materials.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Zhang, D.; Zhang, Z.; Li, C.; Liu, J.; Chen, T.; Zhao, L. Research Progress of Biodegradability of Polybutyrolactam. Packag. Eng. 2021, 42, 136–142. [Google Scholar] [CrossRef]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Significance of landfill microbial communities in biodegradation of polyethylene and nylon 6,6 microplastics. J. Hazard. Mater. 2024, 462, 132786. [Google Scholar] [CrossRef]
- Blazkova, L.; Malinova, L.; Benesova, V.; Roda, J.; Brozek, J. Nanofibers prepared by electrospinning from solutions of biobased polyamide 4. J. Polym. Sci. Part A-Polym. Chem. 2017, 55, 2203–2210. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Q.; Zhao, L.; Jiang, S.; Chen, T. Analyzing the Properties of Bio-based Polybutyrolactam Sausage Casing. Food Sci. 2021, 42, 236–242. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X. Research progress of biodegradable polybutyrolactam. Synth. Fiber China 2023, 52, 7–11. [Google Scholar] [CrossRef]
- Lee, J.A.; Ahn, J.H.; Kim, I.; Li, S.; Lee, S.Y. Synthesis, characterization, and application of fully biobased and biodegradable nylon-4, 4 and-5, 4. ACS Sustain. Chem. Eng. 2020, 8, 5604–5614. [Google Scholar] [CrossRef]
- Nakayama, A.; Yamano, N.; Kawasaki, N.; Nakayama, Y. Synthesis and biodegradation of poly (2-pyrrolidone-co-ε-caprolactone) s. Polym. Degrad. Stab. 2013, 98, 1882–1888. [Google Scholar] [CrossRef]
- Kim, N.C.; Kamal, T.; Park, S.-Y.; Cho, C.-G.; Kim, J.-H.; Yoo, Y.-T.; Nam, S.W.; Jeon, B.S.; Kim, Y.J. Preparation, chemical, and thermal characterization of nylon 4/6 copolymers by anionic ring opening polymerization of 2-Pyrrolidone and ε-Caprolactam. Fibers Polym. 2014, 15, 899–907. [Google Scholar] [CrossRef]
- Kang, K.-S.; Hong, Y.-K.; Kim, Y.J.; Kim, J.H. Synthesis and properties of nylon 4/5 copolymers for hydrophilic fibers. Fibers Polym. 2014, 15, 1343–1348. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Mayes, H.B.; Maurya, A.K.; Tassone, C.J.; Beckham, G.T.; Chen, E.Y.-X. Redesigned hybrid nylons with optical clarity and chemical recyclability. J. Am. Chem. Soc. 2022, 144, 5366–5376. [Google Scholar] [CrossRef]
- Galimberti, D.; Quarti, C.; Milani, A. Polymorphism of even nylons revisited through periodic quantum chemical calculations. Polymer 2015, 67, 167–173. [Google Scholar] [CrossRef]
- Tachibana, K.; Hashimoto, K.; Tansho, N.; Okawa, H. Chemical modification of chain end in nylon 4 and improvement of its thermal stability. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2495–2503. [Google Scholar] [CrossRef]
- Nie, R. Study on Blending Modification Technology of Polybutyrolactam and Polylactic Acid. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2020. [Google Scholar]
- Chen, J.; Liu, J.; Wang, L.; Chen, T.; Chen, Q.; Zhao, L. Thermal properties modification of polybutyrolactam. Polym. Mater. Sci. Eng. 2021, 37, 1–6. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, T.; Zhao, L. Preparation and properties of polybutyrolactam/chitosan electrospun composite fiber membranes with nano-cobweb structure. J. Funct. Polym. 2022, 35, 244–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, Y.; Chen, T.; Zhao, L.; Qiu, Y. Preparation and properties of PLLA/PBL electrospun fibers with core-sheath structure. J. Funct. Polym. 2022, 35, 137–145. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Xin, R.; Qiu, Y.; Chen, T.; Jiang, L.; Zhao, L. Study on the thermoplastic modification of polybutyrolactam by LiCl. J. Funct. Polym. 2022, 35, 548–553. [Google Scholar] [CrossRef]
- Fattahi, A.M.; Najipour, A. Experimental study on mechanical properties of PE / CNT composites. J. Theor. Appl. Mech. 2017, 55, 719–726. [Google Scholar] [CrossRef][Green Version]
- Lee, E.; Kim, D.; Kim, H.; Yoon, J. Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci. Rep. 2015, 5, 15124. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, Z.; Shi, X. Latest research and developing tendency of hyperbranched polymers fabrication. J. Polym. Res. 2021, 28, 355. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Dodds, J.M.; Rees, D.; Kimani, S.M.; Wu, J.J.; Smith, E. HyperMacs to hyperblocks: A novel class of branched thermoplastic elastomer. Macromolecules 2009, 42, 8675–8687. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Li, L.; Jing, Z.; Ye, X.; Wu, C. Construction and properties of hyperbranched block copolymer with independently adjustable heterosubchains. Macromolecules 2014, 47, 8437–8445. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, N.; Shao, X.; Zhang, H.-L.; Liu, Z. Hyperbranched polymers for organic semiconductors. ChemPlusChem 2023, 88, e202300261. [Google Scholar] [CrossRef]
- Kang, K.; Jin, Y.; Wang, B.; Weng, Y. Study on compatibilizing modification of PLA/PPC blends with long-chain hyperbranched polymer. China Plast. 2019, 33, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Wu, C. Intrachain folding and interchain association of hyperbranched chains with long uniform subchains made of amphiphilic diblock copolymers. Macromolecules 2012, 45, 9391–9399. [Google Scholar] [CrossRef]
- Gunasekhar, R.; Indumathy, B.; Ponnan, S.; Arun, A.P. A mini-review on the synthesis, characterization and applications of end-capped hyperbranched polymers. ECS Trans. 2022, 107, 11351. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Zhang, J.; Miao, M.; Zhang, D. Influence of vinyl-terminated hyperbranched polyester on performance of films obtained by UV-initiated thiol-ene click reaction of A2 + B3 system. J. Coat. Technol. Res. 2018, 15, 1049–1057. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Zang, X.; Xu, X.; Cai, W.; Lin, Z.; Lai, Y. Judicious design and rapid manufacturing of a flexible, mechanically resistant liquid-like coating with strong bonding and antifouling abilities. Adv. Mater. 2022, 34, 2204581. [Google Scholar] [CrossRef]
- Varley, R.J.; Tian, W. Toughening of an epoxy anhydride resin system using an epoxidized hyperbranched polymer. Polym. Int. 2004, 53, 69–77. [Google Scholar] [CrossRef]
- Wu, D.; Tang, L.; Tang, S.; Qian, J.; Wei, J.; Zhao, L. Synthesis and properties of bio-based butyrolactam and polybutyrolactam. J. Funct. Polym. 2019, 32, 110–116. [Google Scholar] [CrossRef]
- Watanabe, N.; Imoto, H.; Matsukawa, K.; Naka, K. Thiol-ene polymerization of double-decker-shaped phenyl-substituted silsesquioxanes and thiol monomers. J. Polym. Sci. 2024, 62, 2921–2927. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Wang, C.; Durham, O.Z.; Norton, H.R.; Bowman, C.N.; Shipp, D.A. Radical mediated thiol-ene/yne dispersion polymerizations. Polymer 2016, 105, 180–186. [Google Scholar] [CrossRef]
- Hu, X.; Liu, D.; He, M.; Zheng, Q.; Yu, J.; Lu, S. Crystallization behaviors and confined mechanisms of LiCl modified polyamide 6. Acta Mater. Compos. Sin. 2016, 33, 495–502. [Google Scholar] [CrossRef]
- Ho, J.C.; Wei, K.H. Induced γ → α crystal transformation in blends of polyamide 6 and liquid crystalline copolyester. Macromolecules 2000, 33, 5181–5186. [Google Scholar] [CrossRef]
- Dencheva, N.; Braz, J.; Scheibel, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Polymer-Assisted Biocatalysis: Polyamide 4 Microparticles as Promising Carriers of Enzymatic Function. Catalysts 2020, 10, 767. [Google Scholar] [CrossRef]
- Hong, J.-H.; Mokudai, H.; Masaki, T.; Matsuno, H.; Tanaka, K. Water-Induced Crystal Transition and Accelerated Relaxation Process of Polyamide 4 Chains in Microfibers. Biomacromolecules 2022, 23, 3458–3468. [Google Scholar] [CrossRef]
- Wang, Z.; Zhan, F.; Zhang, D.; Wang, Y.; Qiu, Y.; Zhang, J.; Wang, L.; Zhao, L. Relative humidity-triggered polyamide 4/Cinnamaldehyde core-shell nanofibers for antibacterial packaging. J. Food Eng. 2023, 357, 111635. [Google Scholar] [CrossRef]
- Penel-Pierron, L.; Depecker, C.; Séguéla, R.; Lefebvre, J.-M. Structural and mechanical behavior of nylon 6 films part I. Identification and stability of the crystalline phases. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 484–495. [Google Scholar] [CrossRef]
- Penel-Pierron, L.; Séguéla, R.; Lefebvre, J.-M.; Miri, V.; Depecker, C.; Jutigny, M.; Pabiot, J. Structural and mechanical behavior of nylon-6 films. II. Uniaxial and biaxial drawing. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1224–1236. [Google Scholar] [CrossRef]
- Ge, M. A New Method for Study on Isothermal Crystallization Kinetics of Polymers. Plast. Sci. Technol. 2011, 39, 103–106. [Google Scholar] [CrossRef]
- Chen, G.; Tang, K.; Ma, S.; Zhang, L.; Feng, X. Nonisothermal crystallization kinetics of novel long-carbon chain polyamide 1211. J. Text. Res. 2018, 39, 1–6. [Google Scholar] [CrossRef]
- Jang, G.; Kim, J.; Kim, D.; Kim, Y.J.; Leet, T.S. Degradation behavior of nylon 4 in the presence of newly synthesized thermal stabilizers. Polymer 2014, 38, 314–319. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).