Tailored SAPO-34/Graphite Adsorbent Composite Coatings on Aluminum Substrate for Energy Sustainable Sorption Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sulfonated PEEK Preparation
2.3. Composite Coating Preparation
2.4. Coating Characterization
3. Results and Discussion
4. Conclusions
- The SP-Z90-G5 batch demonstrated suitable adhesive capabilities (average pull-off strength equal to 1.00 MPa), which is consistent with the performances of the other batches. However, its adhesion strength was superior to that of similar composite adsorbent coatings used in AHPs.
- The coatings with exfoliated graphite exhibited lower scratch resistance than those without due to graphite’s lower density and weaker interfacial adhesion with S-PEEK. This effect was more pronounced at higher filler contents (90–95 wt.%). The matrix, which acts as a binding agent, has a key role in maintaining the coating’s structural integrity. At high filler contents, the reduced matrix volume compromises the coating’s cohesive strength, leading to increased scratch sensitivity.
- The SP-Z90-G5 batch, comprising composite coatings with embedded EG, exhibited the highest water absorption capacity, reaching a maximum of 26.34% during the adsorption/desorption experiments. This makes this coating a promising candidate for use in sustainable sorption technologies.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, B. Grand Challenges in Sorption Technologies. Front. Environ. Chem. 2020, 1, 6. [Google Scholar] [CrossRef]
- Xu, J.; Wang, P.; Bai, Z.; Cheng, H.; Wang, R.; Qu, L.; Li, T. Sustainable Moisture Energy. Nat. Rev. Mater. 2024, 9, 722–737. [Google Scholar] [CrossRef]
- Singh, S.K.; Rakshit, D.; Kumar, K.R.; Agarwal, A. Recent Advancements and Sustainable Solutions in Adsorption-Based Cooling Systems Integrated with Renewable Energy Sources and Industrial Waste Heat: A Review. Clean. Eng. Technol. 2024, 23, 100827. [Google Scholar] [CrossRef]
- Muttakin, M.; Pal, A.; Rupa, M.J.; Ito, K.; Saha, B.B. A Critical Overview of Adsorption Kinetics for Cooling and Refrigeration Systems. Adv. Colloid. Interface Sci. 2021, 294, 102468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R. Sorption Thermal Energy Storage: Concept, Process, Applications and Perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption Heat Storage: State-of-the-Art and Future Perspectives. Nanomaterials 2018, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ma, Z. Thermochemical Energy Storage. In Storing Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 651–683. [Google Scholar]
- Tatlier, M. Theoretical Investigation of Performances of Zeolite Y and SAPO-34 Coatings for Adsorption Heat Pump Applications. Heat Mass Transf. 2021, 57, 975–984. [Google Scholar] [CrossRef]
- Strelova, S.V.; Solovyeva, M.V.; Gordeeva, L.G.; Aristov, Y.I. Dynamics of Water Adsorption on SAPO-34: Comparison of Three Adsorbent Bed Configurations of Adsorption Chillers. Appl. Therm. Eng. 2024, 256, 124058. [Google Scholar] [CrossRef]
- Khatibi, M.; Mohammadzadeh Kowsari, M.; Golparvar, B.; Niazmand, H. Optimum Loading of Aluminum Additive Particles in Unconsolidated Beds of Finned Flat-Tube Heat Exchangers in an Adsorption Cooling System. Appl. Therm. Eng. 2021, 196, 117267. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging Offers of Material Science for Adsorption Heat Transformation: A Review. In Proceedings of the Applied Thermal Engineering, Pergamon, Turkey, 1 February 2013; Volume 50, pp. 1610–1618. [Google Scholar]
- Bauer, J.; Herrmann, R.; Mittelbach, W.; Schwieger, W. Zeolite/Aluminum Composite Adsorbents for Application in Adsorption Refrigeration. Int. J. Energy Res. 2009, 33, 1233–1249. [Google Scholar] [CrossRef]
- Riaz, N.; Sultan, M.; Noor, S.; Sajjad, U.; Farooq, M.; Khan, M.U.; Hanif, S.; Riaz, F. Recent Developments in Adsorption Heat Pumps for Heating Applications. Adv. Mech. Eng. 2022, 14, 168781322210894. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 Coated Adsorbent Heat Exchanger for Adsorption Chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Calabrese, L.; Mittelbach, W.; Bonaccorsi, L.; Freni, A. An Industrial Approach for the Optimization of a New Performing Coated Adsorber for Adsorption Heat Pumps. Energies 2022, 15, 5118. [Google Scholar] [CrossRef]
- Gordeeva, L.; Aristov, Y. Adsorbent Coatings for Adsorption Heat Transformation: From Synthesis to Application. Energies 2022, 15, 7551. [Google Scholar] [CrossRef]
- Frazzica, A.; Füldner, G.; Sapienza, A.; Freni, A.; Schnabel, L. Experimental and Theoretical Analysis of the Kinetic Performance of an Adsorbent Coating Composition for Use in Adsorption Chillers and Heat Pumps. Appl. Therm. Eng. 2014, 73, 1022–1031. [Google Scholar] [CrossRef]
- Caprì, A.; Frazzica, A.; Calabrese, L. Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings 2020, 10, 855. [Google Scholar] [CrossRef]
- Malara, A.; Bonaccorsi, L.; Frontera, P.; Freni, A.; Calabrese, L. Microfiber Textiles of Adsorbing Materials for Heat Transformations. Heat. Transf. Eng. 2022, 43, 1652–1663. [Google Scholar] [CrossRef]
- van Heyden, H.; Munz, G.; Schnabel, L.; Schmidt, F.; Mintova, S.; Bein, T. Kinetics of Water Adsorption in Microporous Aluminophosphate Layers for Regenerative Heat Exchangers. Appl. Therm. Eng. 2009, 29, 1514–1522. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Proverbio, E.; Freni, A. SAPO-34 Based Zeolite Coatings for Adsorption Heat Pumps. Energy 2019, 187, 115981. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and Characterization of Silane-Zeolite Adsorbent Coatings for Adsorption Heat Pump Applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Ammann, J.; Michel, B.; Ruch, P.W. Characterization of Transport Limitations in SAPO-34 Adsorbent Coatings for Adsorption Heat Pumps. Int. J. Heat Mass Transf. 2019, 129, 18–27. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruzzaniti, P.; Palamara, D.; Freni, A.; Proverbio, E. New SAPO-34-SPEEK Composite Coatings for Adsorption Heat Pumps: Adsorption Performance and Thermodynamic Analysis. Energy 2020, 203, 117814. [Google Scholar] [CrossRef]
- Brancato, V.; Calabrese, L.; Palomba, V.; Frazzica, A.; Fullana-Puig, M.; Solé, A.; Cabeza, L.F. MgSO4·7H2O Filled Macro Cellular Foams: An Innovative Composite Sorbent for Thermo-Chemical Energy Storage Applications for Solar Buildings. Sol. Energy 2018, 173, 1278–1286. [Google Scholar] [CrossRef]
- Palamara, D.; Palomba, V.; Calabrese, L.; Frazzica, A. Evaluation of Ad/Desorption Dynamics of S-PEEK/Zeolite Composite Coatings by T-LTJ Method. Appl. Therm. Eng. 2022, 208, 118262. [Google Scholar] [CrossRef]
- Bendix, P.; Füldner, G.; Möllers, M.; Kummer, H.; Schnabel, L.; Henninger, S.; Henning, H.-M. Optimization of Power Density and Metal-to-Adsorbent Weight Ratio in Coated Adsorbers for Adsorptive Heat Transformation Applications. Appl. Therm. Eng. 2017, 124, 83–90. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Wang, R.Z. A Consolidated Calcium Chloride-Expanded Graphite Compound for Use in Sorption Refrigeration Systems. Carbon 2007, 45, 390–396. [Google Scholar] [CrossRef]
- Bahrehmand, H.; Khajehpour, M.; Bahrami, M. Finding Optimal Conductive Additive Content to Enhance the Performance of Coated Sorption Beds: An Experimental Study. Appl. Therm. Eng. 2018, 143, 308–315. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and Characterization of Sulfonated Poly(Ether Ether Ketone) for Proton Exchange Membranes. J. Memb. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef]
- Palamara, D.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Effect of Degree of Sulfonation on the Performance of Adsorbent SAPO-34/S-PEEK Composite Coatings for Adsorption Heat Pumps. Prog. Org. Coat. 2021, 154, 106193. [Google Scholar] [CrossRef]
- EN ISO 1518-1; Paints and Varnishes—Determination of Scratch Resistance Part 1: Constant-Loading Method. ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/83330.html (accessed on 18 January 2025).
- Auimviriyavat, J.; Changkhamchom, S.; Sirivat, A. Development of Poly(Ether Ether Ketone) (Peek) with Inorganic Filler for Direct Methanol Fuel Cells (DMFCS). Ind. Eng. Chem. Res. 2011, 50, 12527–12533. [Google Scholar] [CrossRef]
- Lin, L.; Ecke, N.; Huang, M.; Pei, X.Q.; Schlarb, A.K. Impact of Nanosilica on the Friction and Wear of a PEEK/CF Composite Coating Manufactured by Fused Deposition Modeling (FDM). Compos. Part B Eng. 2019, 177, 107428. [Google Scholar] [CrossRef]
- Chuesutham, T.; Sirivat, A.; Paradee, N.; Changkhamchom, S.; Wattanakul, K.; Anumart, S.; Krathumkhet, N.; Khampim, J. Improvement of Sulfonated Poly(Ether Ether Ketone)/Y Zeolite -SO3H via Organo-Functionalization Method for Direct Methanol Fuel Cell. Renew Energy 2019, 138, 243–249. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Wang, Q.; Wang, T.; Liang, Y. Effects of Individual and Sequential Irradiation with Atomic Oxygen and Protons on the Surface Structure and Tribological Performance of Polyetheretherketone in a Simulated Space Environment. RSC Adv. 2015, 5, 83065–83073. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Amine Functionalized Carbon Nanotube (ACNT) Filled in Sulfonated Poly(Ether Ether Ketone) Membrane: Effects of ACNT in Improving Polymer Electrolyte Fuel Cell Performance under Reduced Relative Humidity. Compos. Part B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Rahnavard, A.; Rowshanzamir, S.; Parnian, M.J.; Amirkhanlou, G.R. The Effect of Sulfonated Poly (Ether Ether Ketone) as the Electrode Ionomer for Self-Humidifying Nanocomposite Proton Exchange Membrane Fuel Cells. Energy 2015, 82, 746–757. [Google Scholar] [CrossRef]
- Swier, S.; Chun, Y.S.; Gasa, J.; Shaw, M.T.; Weiss, R.A. Sulfonated Poly(Ether Ketone Ketone) Ionomers as Proton Exchange Membranes. Polym. Eng. Sci. 2005, 45, 1081–1091. [Google Scholar] [CrossRef]
- Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Proton Exchange Membranes from Sulfonated Poly(Ether Ether Ketone) Reinforced with Silica Nanoparticles. High. Perform. Polym. 2013, 25, 854–867. [Google Scholar] [CrossRef]
- Sonpingkam, S.; Pattavarakorn, D. Mechanical Properties of Sulfonated Poly (Ether Ether Ketone) Nanocomposite Membranes. Int. J. Chem. Eng. Appl. 2014, 5, 181–185. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone Composite Foams for Adsorption Heat Pump Applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Vadivel, S.; Tejangkura, W.; Sawangphruk, M. Graphite/Graphene Composites from the Recovered Spent Zn/Carbon Primary Cell for the High-Performance Anode of Lithium-Ion Batteries. ACS Omega 2020, 5, 15240–15246. [Google Scholar] [CrossRef]
- Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Structurally Modified Aromatic Sulfone Polymer Nanocomposites as Polyelectrolyte Membranes. High Perform. Polym. 2014, 26, 550–560. [Google Scholar] [CrossRef]
- Knauth, P.; Hou, H.; Bloch, E.; Sgreccia, E.; Di Vona, M.L. Thermogravimetric Analysis of SPEEK Membranes: Thermal Stability, Degree of Sulfonation and Cross-Linking Reaction. J. Anal. Appl. Pyrolysis 2011, 92, 361–365. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion Aspects of Hydrophobic Silane Zeolite Coatings for Corrosion Protection of Aluminium Substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Effect of Silane Matrix Composition on Performances of Zeolite Composite Coatings. Prog. Org. Coat. 2016, 101, 100–110. [Google Scholar] [CrossRef]
- Jones, R.; Pollock, H.M.; Geldart, D.; Verlinden, A. Inter-Particle Forces in Cohesive Powders Studied by AFM: Effects of Relative Humidity, Particle Size and Wall Adhesion. Powder Technol. 2003, 132, 196–210. [Google Scholar] [CrossRef]
- Freni, A.; Dawoud, B.; Bonaccorsi, L.; Chmielewski, S.; Frazzica, A.; Calabrese, L.; Restuccia, G. Characterization of Zeolite-Based Coatings for Adsorption Heat Pumps; Springer International Publishing: Cham, Germany, 2015; ISBN 978-3-319-09326-0. [Google Scholar]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 Zeolite Filled Macrocellular Foams for Adsorption Heat Pump Applications: A Preliminary Study. Appl. Therm. Eng. 2017, 124, 1312–1318. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Frazzica, A. Experimental Evaluation of the Hydrothermal Stability of a Silicone/Zeolite Composite Foam for Solar Adsorption Heating and Cooling Application. J. Appl. Polym. Sci. 2020, 137, 48311. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Sapienza, A.; Frazzica, A.; Restuccia, G.; Vasta, S. Comparative Analysis of Promising Adsorbent/Adsorbate Pairs for Adsorptive Heat Pumping, Air Conditioning and Refrigeration. Appl. Therm. Eng. 2016, 104, 85–95. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.Z.; Ge, T.S.; Hu, L.M. Performance Study of SAPO-34 and FAPO-34 Desiccants for Desiccant Coated Heat Exchanger Systems. Energy 2015, 93, 88–94. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Synthesis of SAPO-34 on Graphite Foams for Adsorber Heat Exchangers. Appl. Therm. Eng. 2013, 61, 848–852. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Recent Advances in Metal-Organic Frameworks for Adsorption Heat Transformations. Renew. Sustain. Energy Rev. 2024, 198, 114411. [Google Scholar] [CrossRef]
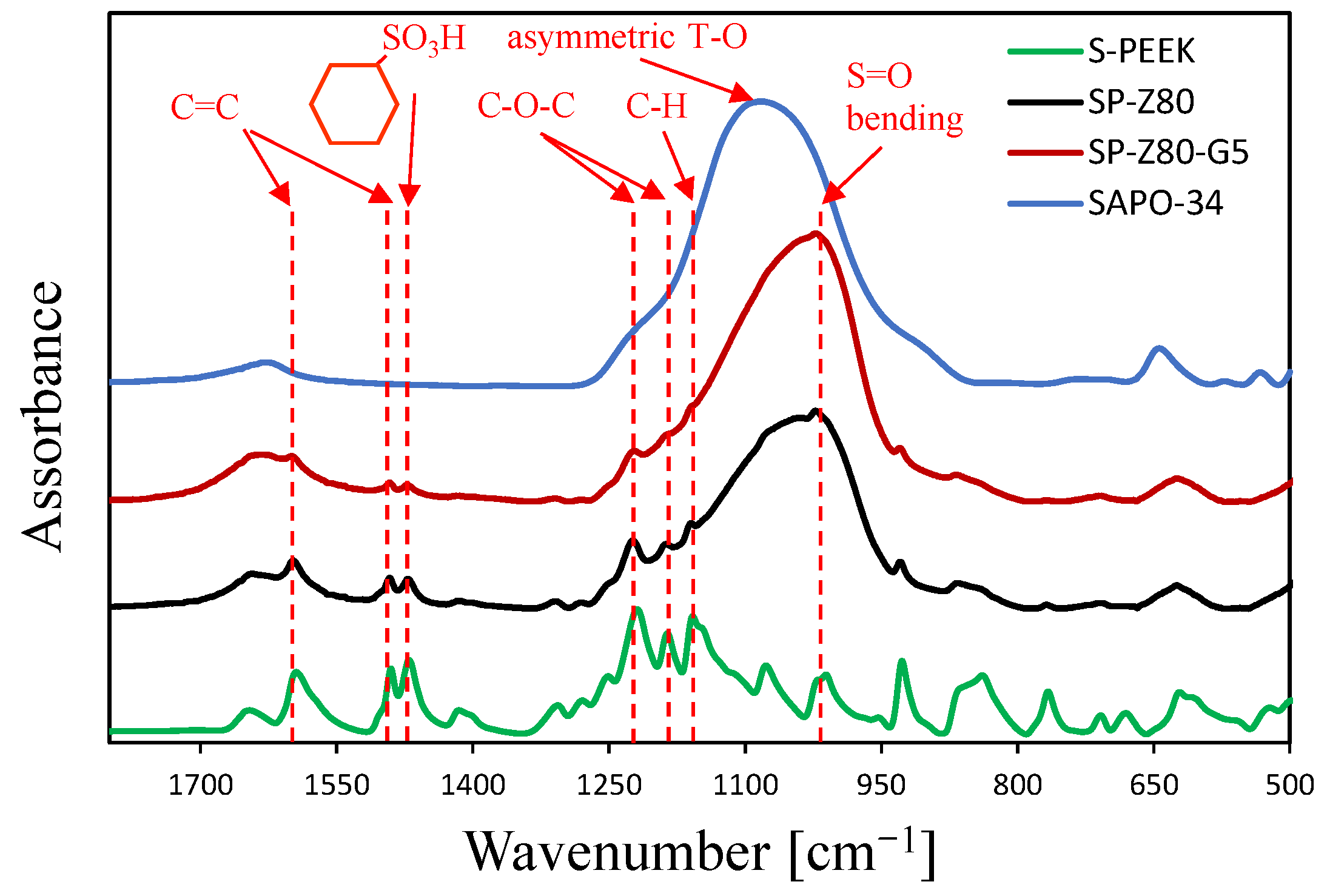
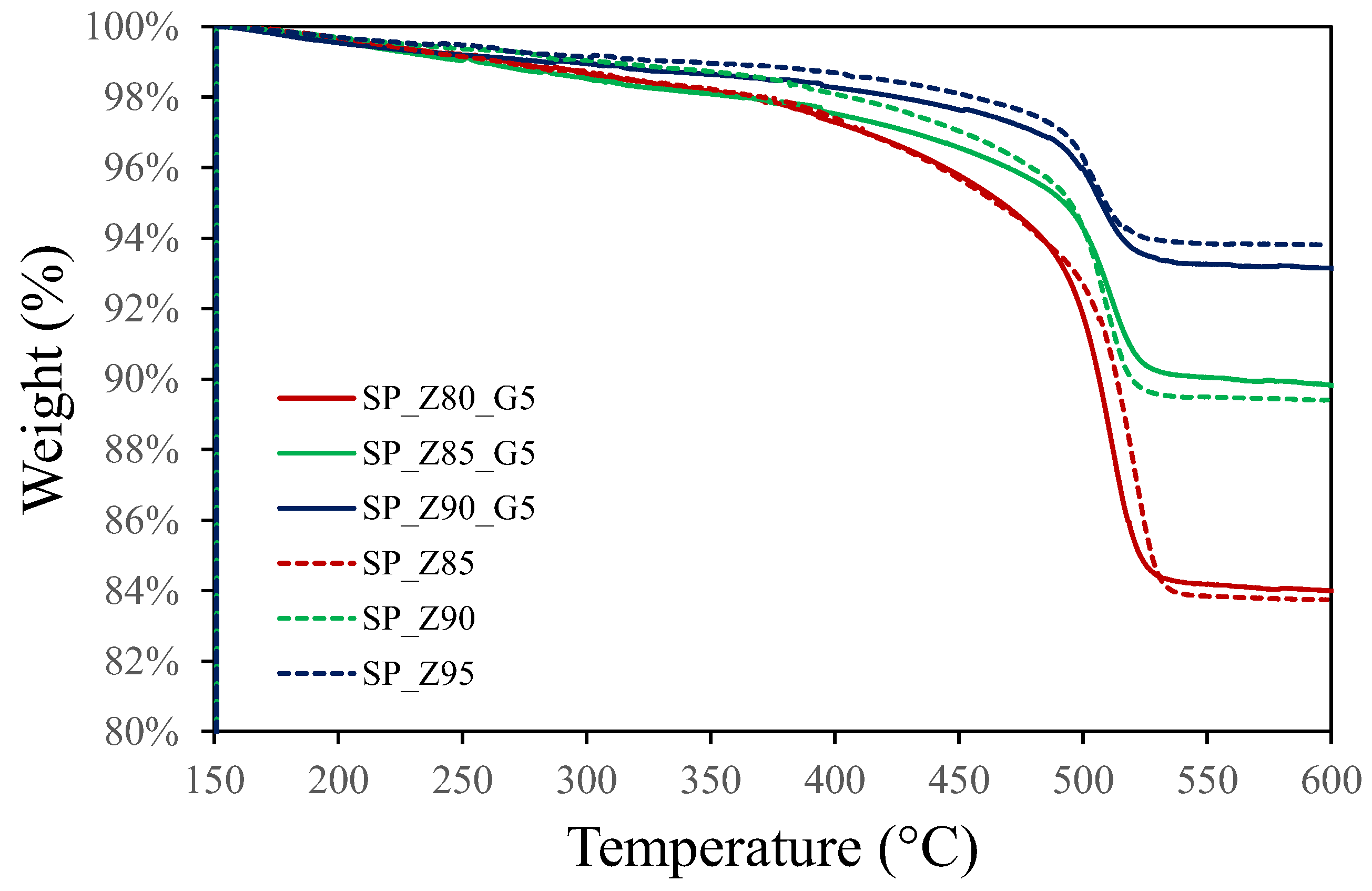


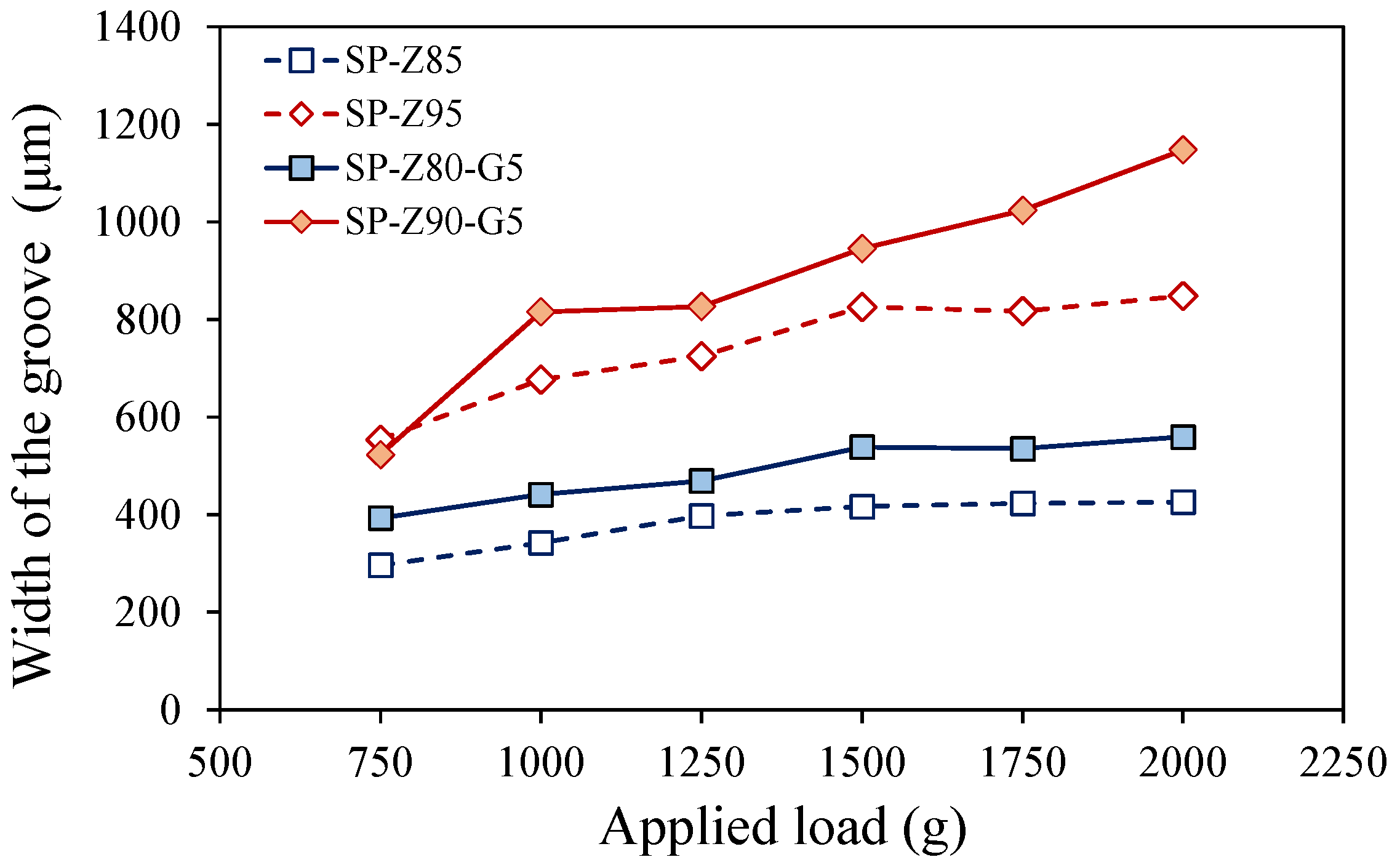


| Code | S-PEEK Content (wt. %) | Zeolite Content (wt. %) | Graphite Content (wt. %) |
|---|---|---|---|
| SP | 100 | 0 | 0 |
| SP-Z80 | 20 | 80 | 0 |
| SP-Z85 | 15 | 85 | 0 |
| SP-Z90 | 10 | 90 | 0 |
| SP-Z95 | 5 | 95 | 0 |
| SP-Z80-G5 | 15 | 80 | 5 |
| SP-Z85-G5 | 10 | 85 | 5 |
| SP-Z90-G5 | 5 | 90 | 5 |
| Code | ZMWU (wt.%) | Efficiency (%) |
|---|---|---|
| SP-Z80 | 29.72 | 93.76 |
| SP-Z85 | 29.95 | 94.48 |
| SP-Z90 | 30.55 | 96.37 |
| SP-Z80-G5 | 28.64 | 90.33 |
| SP-Z85-G5 | 29.34 | 92.54 |
| SP-Z90-G5 | 29.96 | 94.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palamara, D.; Proverbio, E.; Frazzica, A.; Calabrese, L. Tailored SAPO-34/Graphite Adsorbent Composite Coatings on Aluminum Substrate for Energy Sustainable Sorption Technologies. Polymers 2025, 17, 260. https://doi.org/10.3390/polym17030260
Palamara D, Proverbio E, Frazzica A, Calabrese L. Tailored SAPO-34/Graphite Adsorbent Composite Coatings on Aluminum Substrate for Energy Sustainable Sorption Technologies. Polymers. 2025; 17(3):260. https://doi.org/10.3390/polym17030260
Chicago/Turabian StylePalamara, Davide, Edoardo Proverbio, Andrea Frazzica, and Luigi Calabrese. 2025. "Tailored SAPO-34/Graphite Adsorbent Composite Coatings on Aluminum Substrate for Energy Sustainable Sorption Technologies" Polymers 17, no. 3: 260. https://doi.org/10.3390/polym17030260
APA StylePalamara, D., Proverbio, E., Frazzica, A., & Calabrese, L. (2025). Tailored SAPO-34/Graphite Adsorbent Composite Coatings on Aluminum Substrate for Energy Sustainable Sorption Technologies. Polymers, 17(3), 260. https://doi.org/10.3390/polym17030260









