Enhanced Adsorption of Bisphenol a on Lignin-Derived Biochars: Role of Thermal and Phosphoric Acid Activation in Surface Functionalization and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Biochar Adsorbents
2.2.1. Thermal Activation
2.2.2. Chemical Activation
2.3. Characterization of the Biochars
2.3.1. Elemental Analysis
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Scanning Electron Microscopy
2.3.4. Specific Surface Area
2.3.5. Point of Zero Charge (PZC)
2.4. Analytical Determination of the BPA
2.5. Adsorption Experiments
2.5.1. Kinetic Studies
2.5.2. Adsorption Isotherms
2.6. Effect of Operating Parameters on BPA Adsorption onto LB450-H3PO4
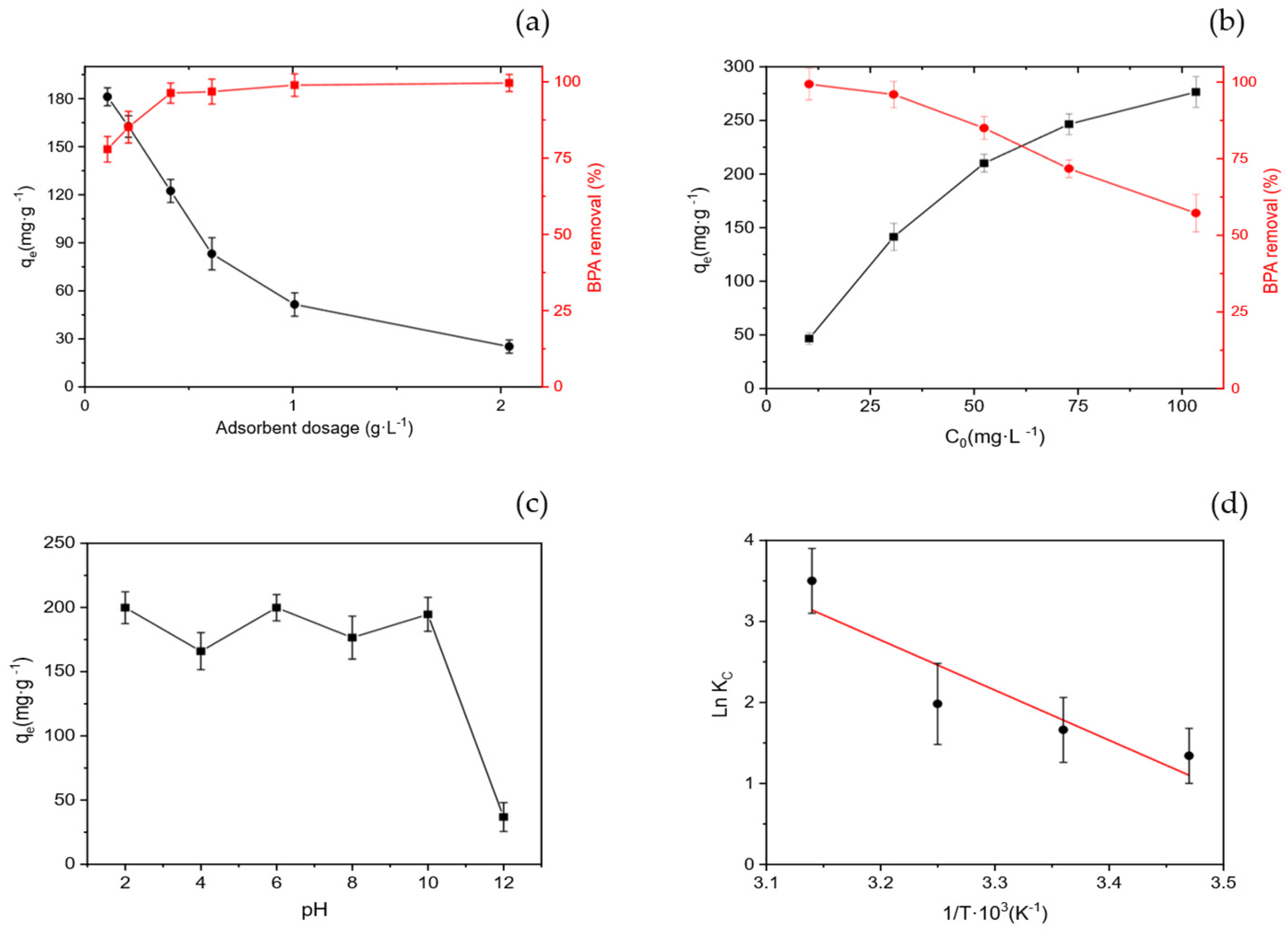
2.6.1. Effect of the Adsorbent Dosage
2.6.2. Effect of the Initial BPA Concentration
2.6.3. Effect of pH
2.6.4. Effect of the Temperature
3. Results and Discussion
3.1. Characterization of Lignin-Derived Biochars
3.1.1. Elemental Composition and Yield
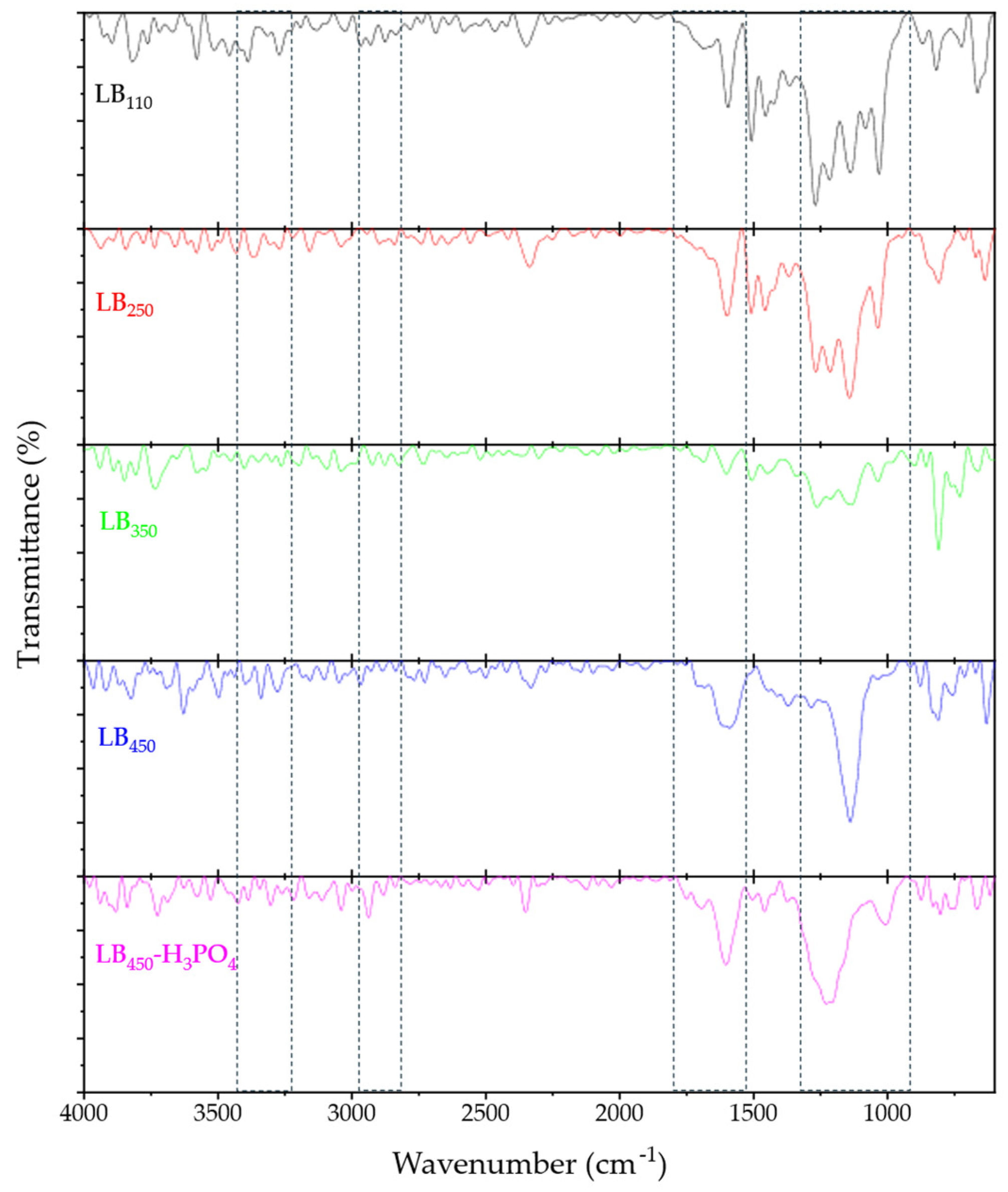
3.1.2. FTIR
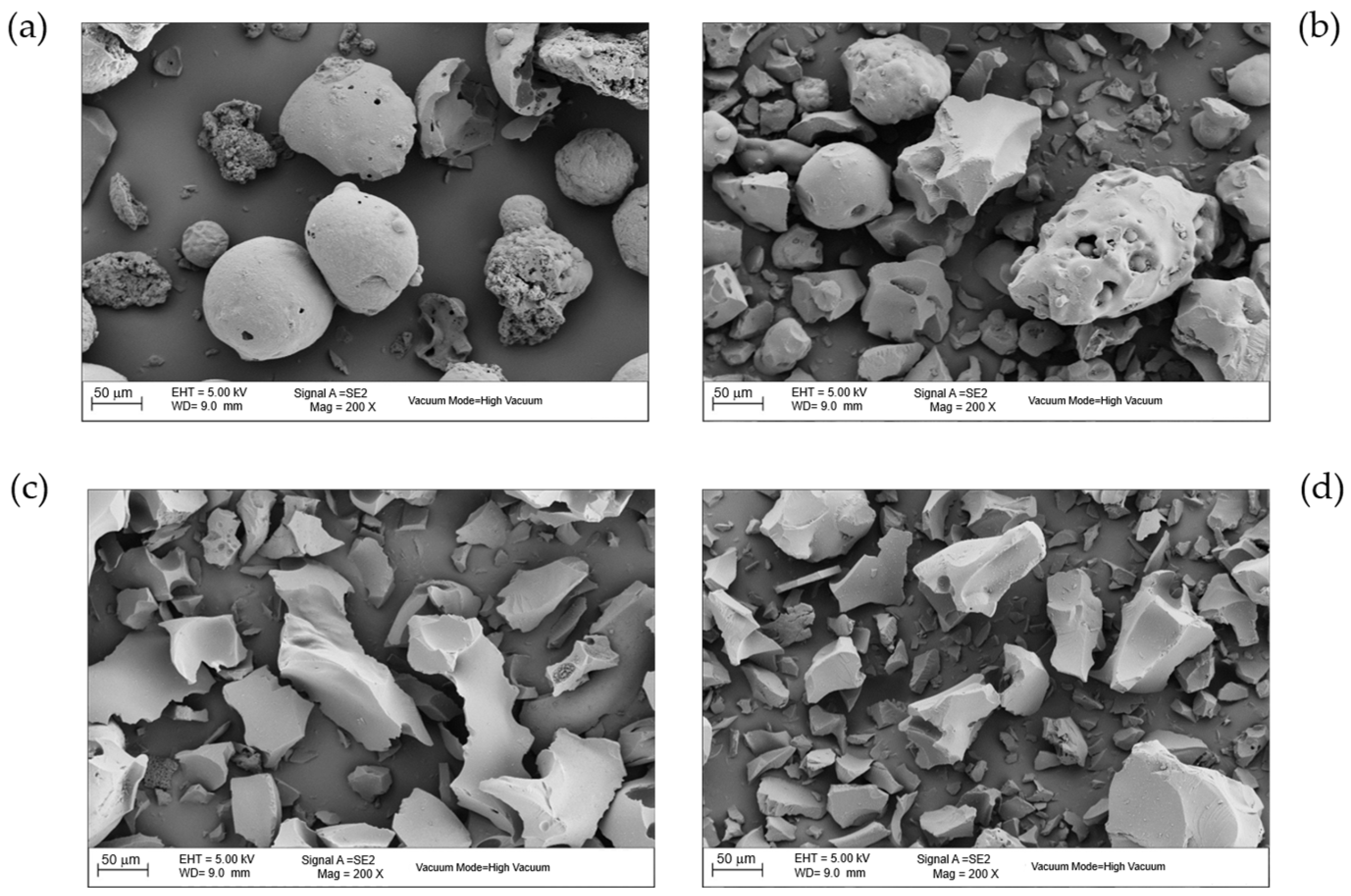
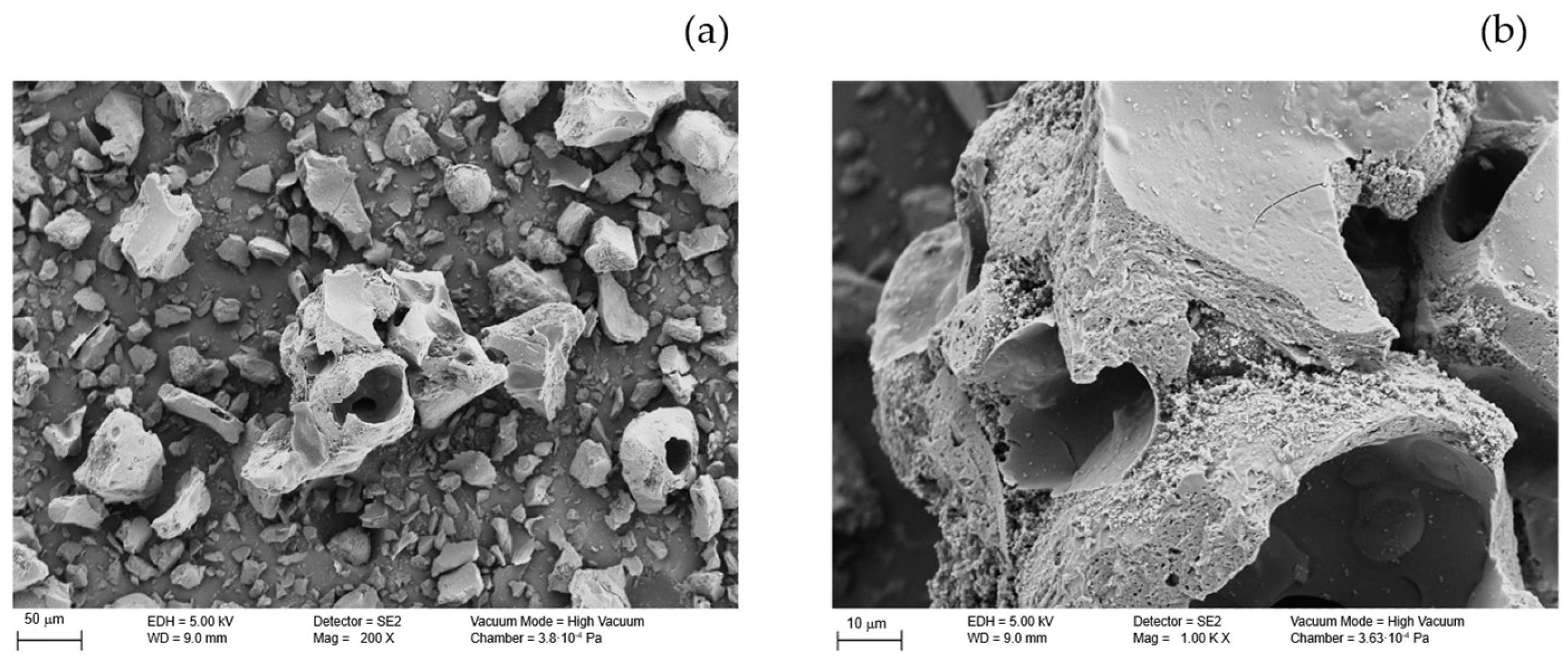
3.1.3. Morphological Analysis
3.1.4. BET Surface Area and Pore Structure
3.1.5. Point of Zero Charge (PZC)
3.2. Adsorption Behavior of BPA
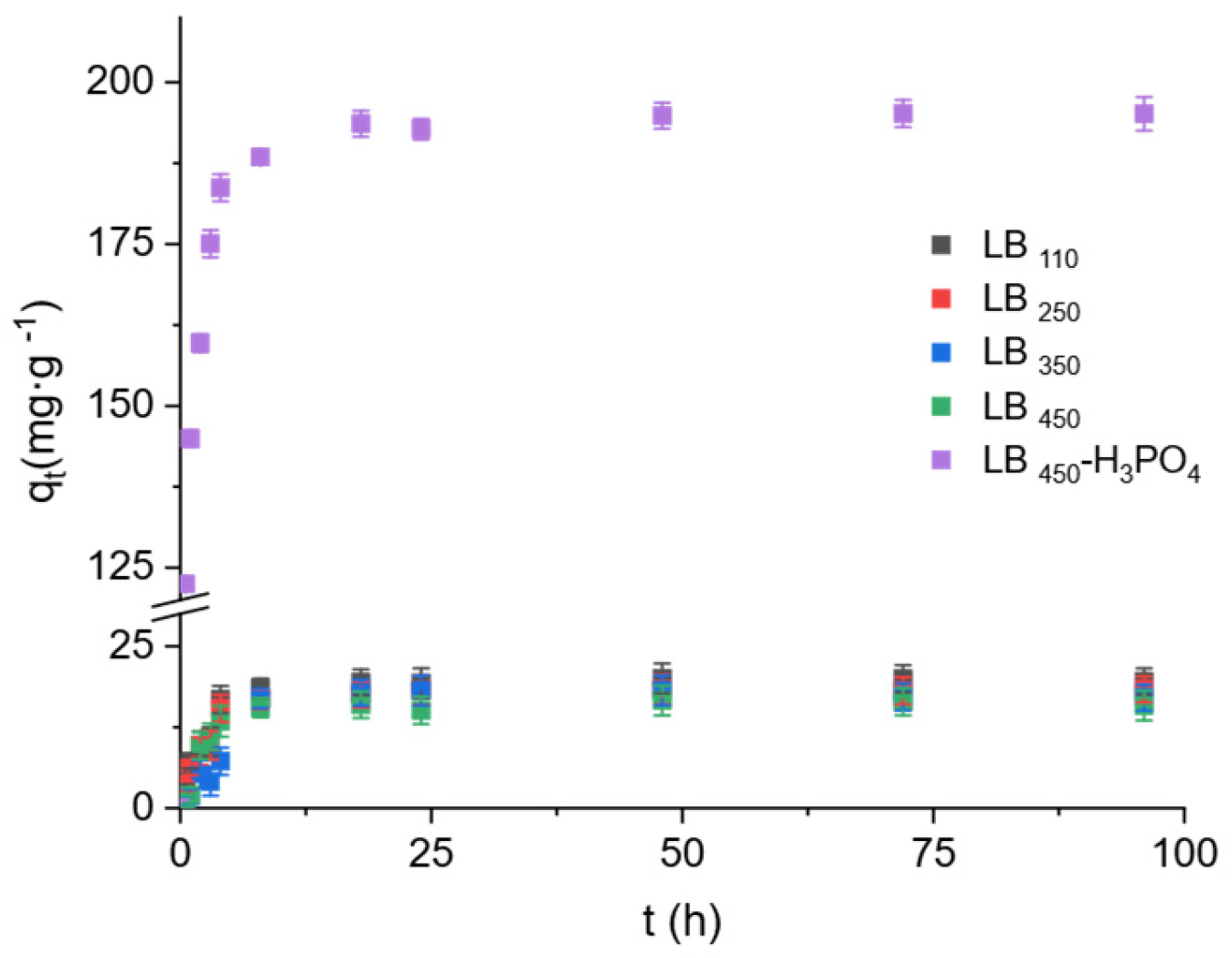
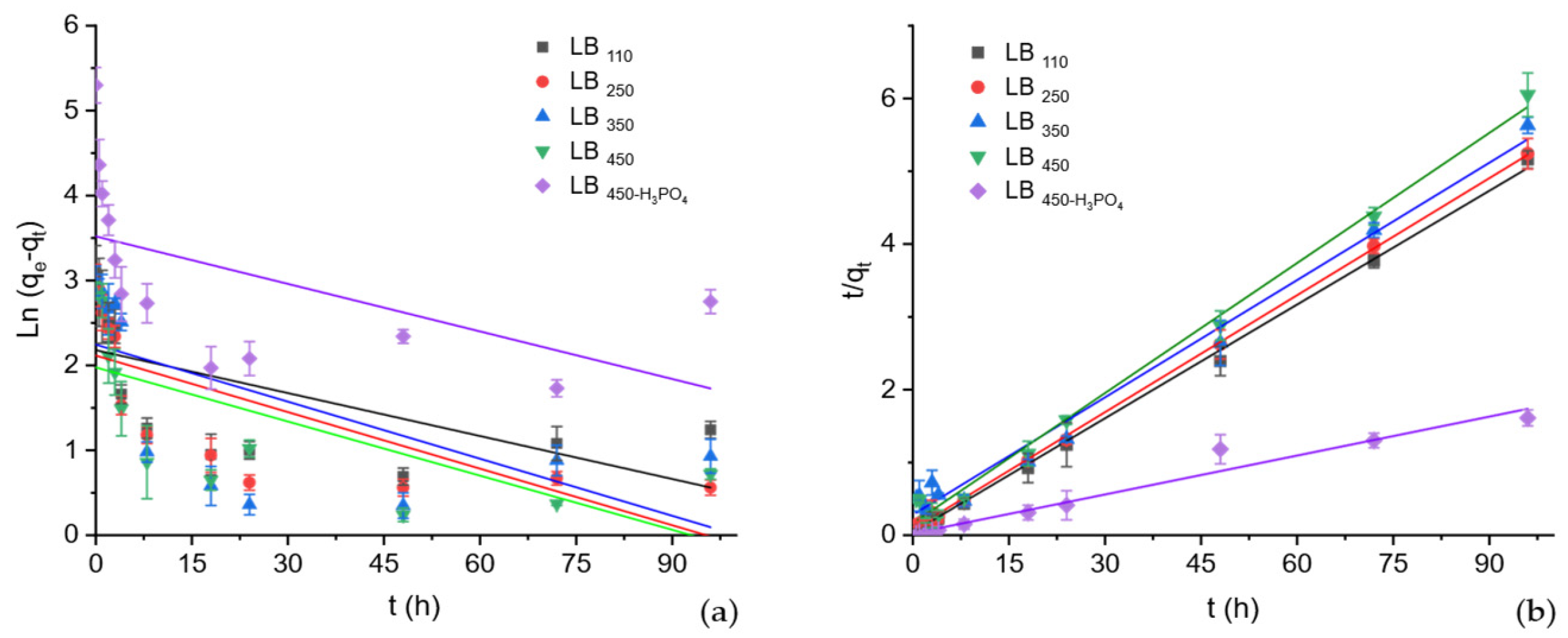
3.2.1. Adsorption Kinetics
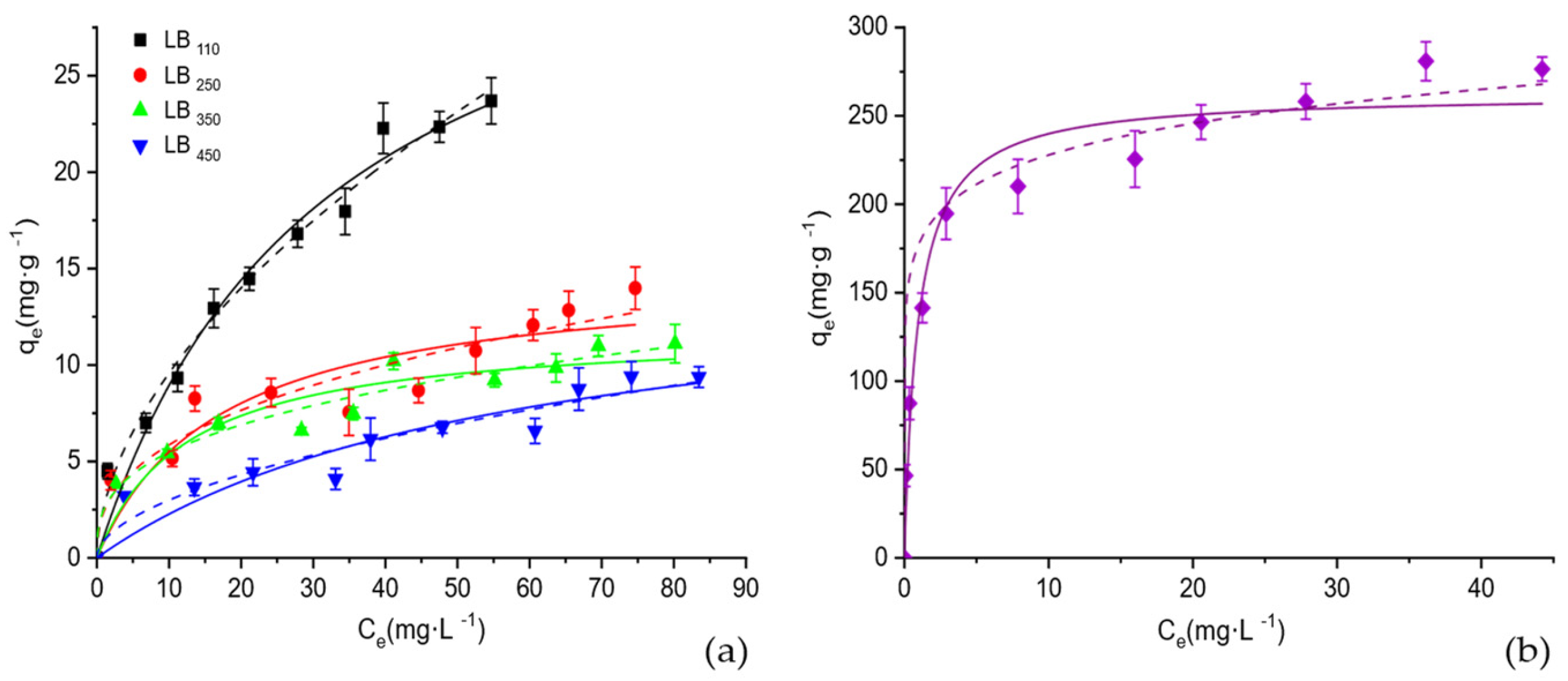
3.2.2. Adsorption Isotherms
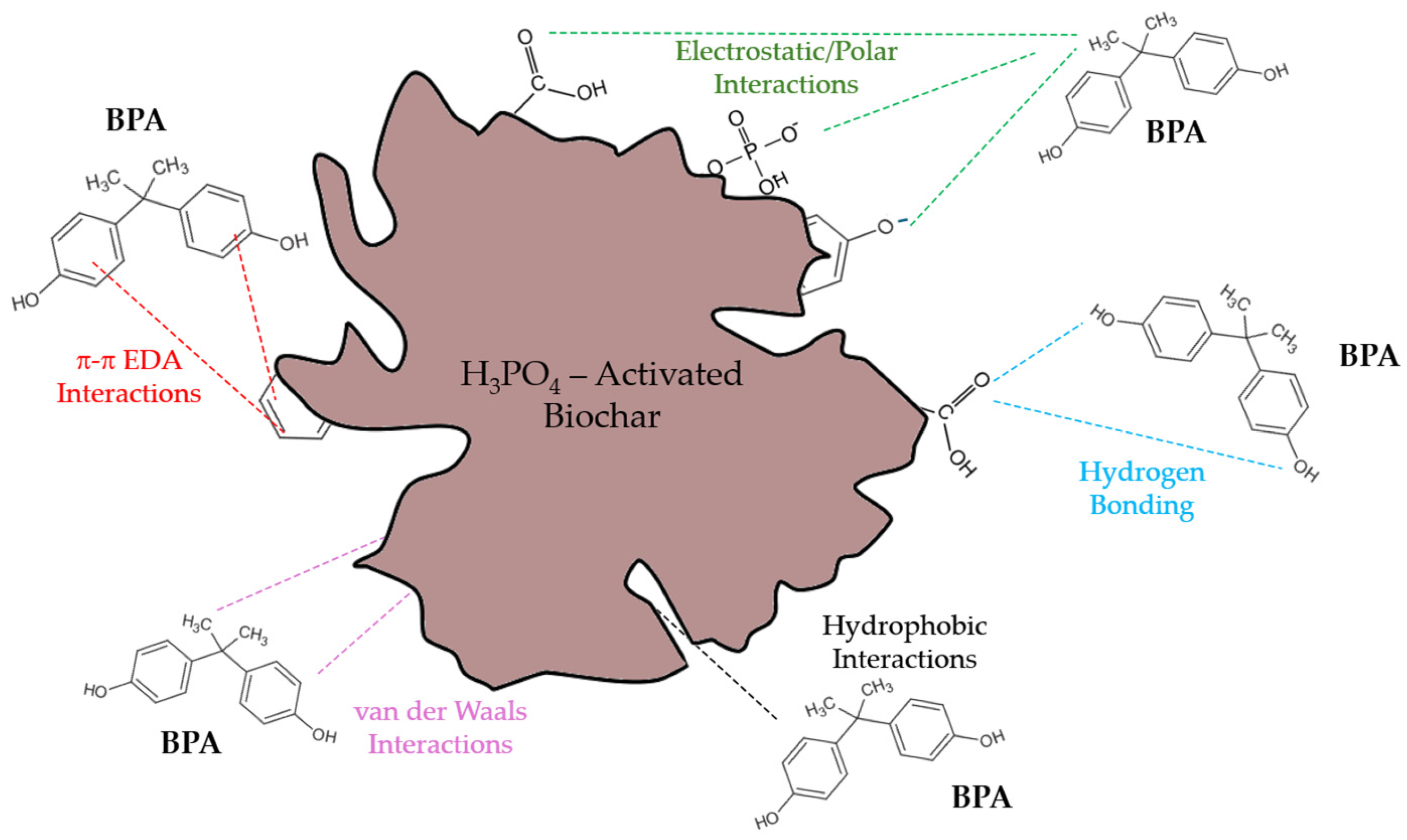
3.2.3. Proposed Adsorption Mechanism of BPA on the Biochars
3.3. Effect of Operational Parameters on BPA Adsorption Using Acid-Activated Biochar
3.3.1. Effect of Adsorbent Dose
3.3.2. Effect of Initial BPA Concentration
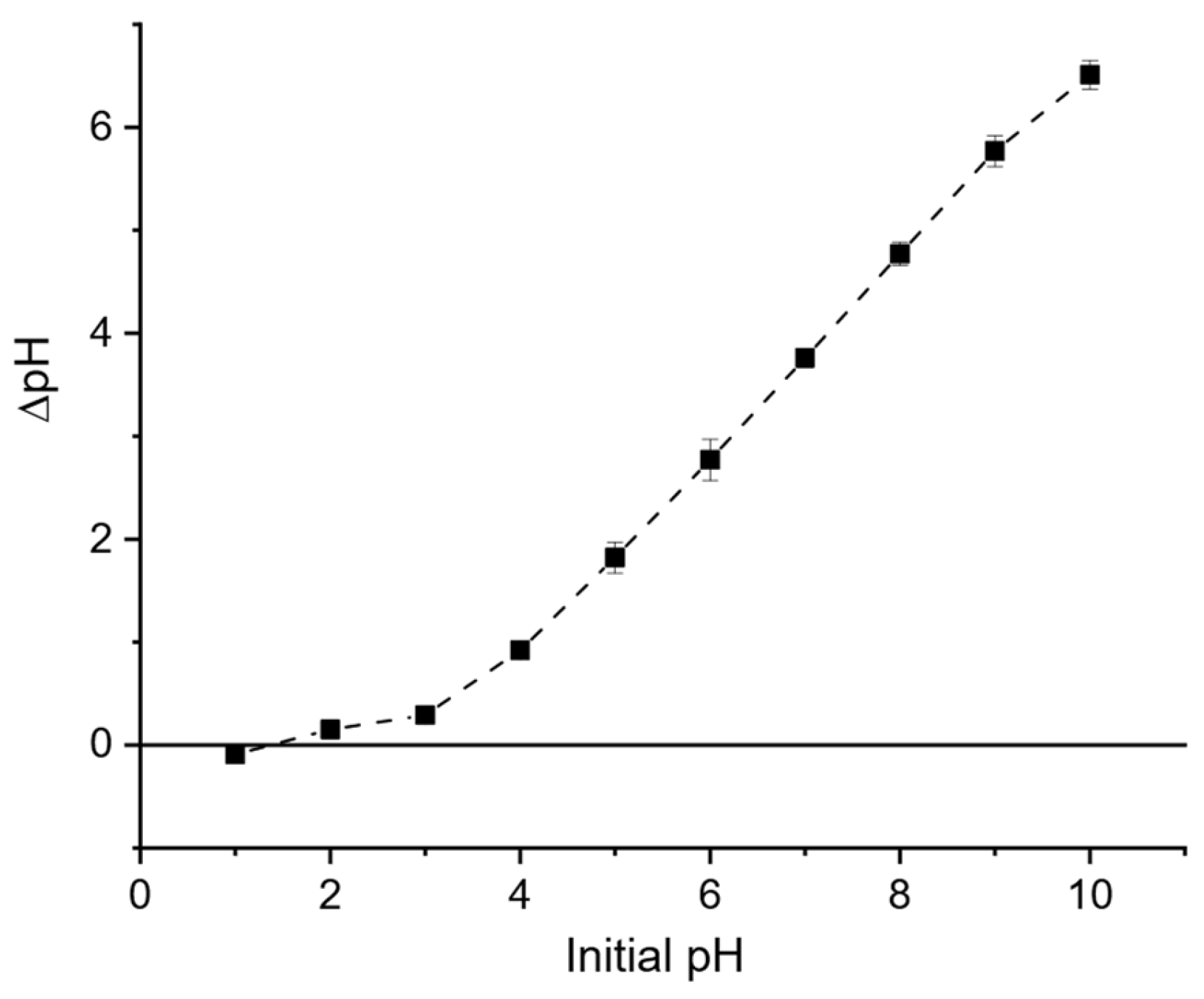
3.3.3. Effect of Solution pH
3.3.4. Effect of Temperature and Thermodynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, M.; He, L.; Jiang, M.; Zhu, Y.; Wang, J.; Gustave, W.; Wang, S.; Deng, Y.; Zhang, X.; Wang, Z. Biochar for the Removal of Emerging Pollutants from Aquatic Systems: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1679. [Google Scholar] [CrossRef] [PubMed]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; Nor, M.Z.M.; Azis, R.S. An Insight into a Sustainable Removal of Bisphenol A from Aqueous Solution by Novel Palm Kernel Shell Magnetically Induced Biochar: Synthesis, Characterization, Kinetic, and Thermodynamic Studies. Polymers 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Angaru, G.K.R.; Pal, C.A.; Koduru, J.R.; Karri, R.R.; Mubarak, N.M.; Chang, Y.-Y. Insights into Kinetics, Thermodynamics, and Mechanisms of Chemically Activated Sunflower Stem Biochar for Removal of Phenol and Bisphenol-A from Wastewater. Sci. Rep. 2024, 14, 4267. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Zhang, Y.; Sun, K.; Liu, X.; Si, Y.; Pan, B.; Steinberg, C.E.W. Inherent Minerals Facilitated Bisphenol A Sorption by Biochar: A Key Force by Complexation. ACS EST Water 2021, 2, 184–194. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, M.; Qi, K.; Pan, J. The Adsorption of Bisphenol A by Biochars Modified with Potassium Phosphate. Desalin. Water Treat. 2024, 319, 100444. [Google Scholar] [CrossRef]
- Zhao, H.J.; Xu, J.K.; Yan, Z.H.; Ren, H.Q.; Zhang, Y. Microplastics Enhance the Developmental Toxicity of Synthetic Phenolic Antioxidants by Disturbing the Thyroid Function and Metabolism in Developing Zebrafish. Environ. Int. 2020, 140, 105750. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Fattahi, N.; Fattahi, T.; Kashif, M.; Ramazani, A.; Jung, W.K. Lignin: A Valuable and Promising Bio-Based Absorbent for Dye Removal Applications. Int. J. Biol. Macromol. 2024, 276, 133763. [Google Scholar] [CrossRef]
- Yang, Z.; Wan, X.; Chen, Y.; Chen, X.; Wang, Z.; Zhang, C.; Kumar, V.; Varrone, C.; Xiao, X.; Yu, P.; et al. A Review of Lignin-Based Adsorbent Materials: Synthesis and Applications in the Remediation of Heavy Metal/Antibiotic-Containing Wastewater. J. Environ. Chem. Eng. 2025, 13, 119080. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, K.; Wang, B.; Ren, W.; Sun, D.; Shao, C.; Sun, R. Advances in Lignin-Based Biosorbents for Sustainable Wastewater Treatment. Bioresour. Technol. 2024, 395, 130347. [Google Scholar] [CrossRef]
- Hernández-Abreu, A.B.; Álvarez-Torrellas, S.; Águeda, V.I.; Larriba, M.; Delgado, J.A.; Calvo, P.A.; García, J. Enhanced Removal of the Endocrine Disruptor Compound Bisphenol A by Adsorption onto Green-Carbon Materials. Effect of Real Effluents on the Adsorption Process. J. Environ. Manag. 2020, 266, 110604. [Google Scholar] [CrossRef]
- de Lima, H.H.C.; Llop, M.E.G.; dos Santos Maniezzo, R.; Moisés, M.P.; Janeiro, V.; Arroyo, P.A.; Guilherme, M.R.; Rinaldi, A.W. Enhanced Removal of Bisphenol A Using Pine-Fruit Shell-Derived Hydrochars: Adsorption Mechanisms and Reusability. J. Hazard. Mater. 2021, 416, 126167. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric Acid Pretreatment Enhances the Specific Surface Areas of Biochars by Generation of Micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.T.; Nguyen, T.B.; Chen, W.H.; Chen, C.W.; Kumar Patel, A.; Bui, X.T.; Chen, L.; Singhania, R.R.; Dong, C.D. Phosphoric Acid-Activated Biochar Derived from Sunflower Seed Husk: Selective Antibiotic Adsorption Behavior and Mechanism. Bioresour. Technol. 2023, 371, 128593. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Sun, C.; Stevens, L.; Liu, H. Synthesis and Characterization of Advanced Bio-Carbon Materials from Kraft Lignin with Enhanced CO2 Capture Properties. J. Environ. Chem. Eng. 2022, 10, 107471. [Google Scholar] [CrossRef]
- Adamovic, T.; Zhu, X.; Perez, E.; Balakshin, M.; Cocero, M.J. Understanding Sulfonated Kraft Lignin Re-Polymerization by Ultrafast Reactions in Supercritical Water. J. Supercrit. Fluids 2022, 191, 105768. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural Characterization of Lignin from D. Sinicus by FTIR and NMR Techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Naydenova, I.; Radoykova, T.; Petrova, T.; Sandov, O.; Valchev, I. Utilization Perspectives of Lignin Biochar from Industrial Biomass Residue. Molecules 2023, 28, 4842. [Google Scholar] [CrossRef]
- Chen, W.; Shi, S.; Nguyen, T.; Chen, M.; Zhou, X. Effect of Temperature on the Evolution of Physical Structure and Chemical Properties of Bio-Char Derived from Co-Pyrolysis of Lignin with High-Density Polyethylene. Bioresources 2016, 11, 3923–3936. [Google Scholar] [CrossRef]
- Pereira, L.H.; Boas, E.C.V.; Ferreira, O.P.; Avelino, F. Development of Kraft Lignin-Based Biochars for Adsorption of Ciprofloxacin: Insights about the Effects of Activation on the Adsorption Mechanism. Int. J. Biol. Macromol. 2025, 329, 147845. [Google Scholar] [CrossRef]
- Xiang, W.; Gan, L.; Wang, Y.; Yang, N.; Wang, W.; Li, L.; Liu, Z.; Feng, Y.; Chen, D.; Wang, R. Enhanced Adsorption Performance of Phosphoric Acid Activated Biochar from In-Situ Pre-Carbonized Bamboo Shoot Shells. Ind. Crops Prod. 2025, 226, 120719. [Google Scholar] [CrossRef]
- Chatir, E.M.; El Hadrami, A.; Ojala, S.; Brahmi, R. Production of Activated Carbon with Tunable Porosity and Surface Chemistry via Chemical Activation of Hydrochar with Phosphoric Acid under Oxidizing Atmosphere. Surf. Interfaces 2022, 30, 101849. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Din, S.U.; Ngueagn, P.T.; Al-Ahmary, K.M.; AlMohamadi, H.; Al-Mhyawi, S.R.; Elamin, N.Y.; Alshdoukhi, I.F.; Alrashood, J.S.; Ofudje, E.A. Adsorption of Bisphenol-A by Banana Biochar: Kinetic, Isotherms and Thermodynamics. Sci. Rep. 2025, 15, 31659. [Google Scholar] [CrossRef]
- Lekene, R.B.N.; Ntep, T.M.M.; Fetzer, M.N.A.; Strothmann, T.; Nsami, J.N.; Janiak, C. The Efficient Removal of Ibuprofen, Caffeine, and Bisphenol A Using Engineered Egusi Seed Shells Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics, and Mechanism. Environ. Sci. Pollut. Res. 2023, 30, 100095–100113. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, Q.; Jia, Z.; Hou, L.; Wang, Z.L. Adsorption of Typical Endocrine Disrupting Chemicals by Wheat Straw Biochars and the Effects of the Steric Structure. Desalin. Water Treat. 2020, 182, 395–404. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption Characteristics and Mechanism of Bisphenol A by Magnetic Biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef]
| Biochar | N [%] | C [%] | H [%] | S [%] | O [%] * | H/C | O/C | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| LB110 | 0.46 | 61.18 | 5.33 | 1.70 | 31.33 | 0.08 | 0.51 | 96.18 |
| LB250 | 0.37 | 66.84 | 4.12 | 1.43 | 27.24 | 0.06 | 0.40 | 66.26 |
| LB350 | 0.49 | 67.87 | 4.75 | 1.47 | 25.42 | 0.06 | 0.37 | 48.33 |
| LB450 | 0.65 | 76.66 | 3.18 | 1.45 | 18.05 | 0.04 | 0.23 | 45.87 |
| LB450-H3PO4 | 0.37 | 65.31 | 3.61 | 0.38 | 30.33 | 0.05 | 0.46 | 77.23 |
| Biochar | Surface Area BET (m2·g−1) |
|---|---|
| LB110 | 1.46 ± 0.11 |
| LB250 | 0.31 ± 0.06 |
| LB350 | 0.44 ± 0.07 |
| LB450 | 13.03 ± 1.35 |
| LB450-H3PO4 | 522.17 ± 2.27 |
| Biochar | qe,exp (mg∙g−1) | Pseudo-First Order (PFO) * | Pseudo-Second Order (PSO) * | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg∙g−1) | k1·102 (h−1) | R2 | qe,cal (mg∙g−1) | k2·102 (g∙mg−1∙h−1) | R2 | ||
| LB110 | 19.74 ± 0.35 | 8.87 ± 2.27 | 1.6 ± 0.67 | 0.389 | 19.15 ± 0.29 | 6.6 ± 1.8 | 0.999 |
| LB250 | 18.24 ± 0.09 | 8.33 ± 2.05 | 2.2 ± 0.64 | 0.542 | 18.60 ± 0.14 | 3.7 ± 0.7 | 0.999 |
| LB350 | 17.63 ± 0.61 | 9.47 ± 3.03 | 2.2 ± 0.84 | 0.411 | 18.41 ± 0.71 | 1.1 ± 0.3 | 0.985 |
| LB450 | 16.01 ± 0.68 | 7.24 ± 1.93 | 2.1 ± 0.67 | 0.482 | 16.68 ± 0.47 | 2.4 ± 0.9 | 0.992 |
| LB450-H3PO4 | 193.48 ± 6.50 | 34.26 ± 11.60 | 1.7 ± 0.88 | 0.282 | 189.02 ± 38.22 | 47.8 ± 8.8 | 0.999 |
| Biochar | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| KL·102 (L∙mg−1) | qm (mg∙g−1) | RL | R2 | n | KF (L∙mg−1) | R2 | |
| LB110 | 3.2 ± 0.2 | 37.14 ± 0.7 | 0.24 ± 0.06 | 0.977 | 0.54 ± 0.20 | 2.70 ± 0.30 | 0.981 |
| LB250 | 5.8 ± 0.6 | 14.84 ± 1.3 | 0.14 ± 0.07 | 0.855 | 0.38 ± 0.11 | 2.40 ± 0.21 | 0.855 |
| LB350 | 8.2 ± 0.7 | 11.87 ± 0.8 | 0.11 ± 0.05 | 0.909 | 0.33 ± 0.07 | 2.53 ± 0.21 | 0.908 |
| LB450 | 1.7 ± 0.2 | 15.29 ± 1.2 | 0.37 ± 0.10 | 0.876 | 0.52 ± 0.13 | 0.89 ± 0.10 | 0.865 |
| LB450-H3PO4 | 107 ± 12 | 262.28 ± 14.3 | 0.01 ± 0.01 | 0.964 | 0.21 ± 0.05 | 128.43 ± 2.61 | 0.974 |
| Temperature (K) | ΔGº (kJ·mol−1) | ΔHº (kJ·mol−1) | ΔSº (J·mol−1·K−1) |
|---|---|---|---|
| 288 | −3.21 ± 0.81 | 51.14 ± 15.7 | 187.48 ± 51.57 |
| 298 | −4.11 ± 0.99 | ||
| 308 | −5.07 ± 1.12 | ||
| 318 | −9.25 ± 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Céspedes, F.; González-Fernández, I.; Fernández-Pérez, M.; García-Fuentes, L. Enhanced Adsorption of Bisphenol a on Lignin-Derived Biochars: Role of Thermal and Phosphoric Acid Activation in Surface Functionalization and Mechanism. Polymers 2025, 17, 3159. https://doi.org/10.3390/polym17233159
Flores-Céspedes F, González-Fernández I, Fernández-Pérez M, García-Fuentes L. Enhanced Adsorption of Bisphenol a on Lignin-Derived Biochars: Role of Thermal and Phosphoric Acid Activation in Surface Functionalization and Mechanism. Polymers. 2025; 17(23):3159. https://doi.org/10.3390/polym17233159
Chicago/Turabian StyleFlores-Céspedes, Francisco, Iván González-Fernández, Manuel Fernández-Pérez, and Luis García-Fuentes. 2025. "Enhanced Adsorption of Bisphenol a on Lignin-Derived Biochars: Role of Thermal and Phosphoric Acid Activation in Surface Functionalization and Mechanism" Polymers 17, no. 23: 3159. https://doi.org/10.3390/polym17233159
APA StyleFlores-Céspedes, F., González-Fernández, I., Fernández-Pérez, M., & García-Fuentes, L. (2025). Enhanced Adsorption of Bisphenol a on Lignin-Derived Biochars: Role of Thermal and Phosphoric Acid Activation in Surface Functionalization and Mechanism. Polymers, 17(23), 3159. https://doi.org/10.3390/polym17233159







