Impact of Hydrogen Bonding in Natural Cellulose Fibers on Plasmonic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soda AQ Pulping Process Description
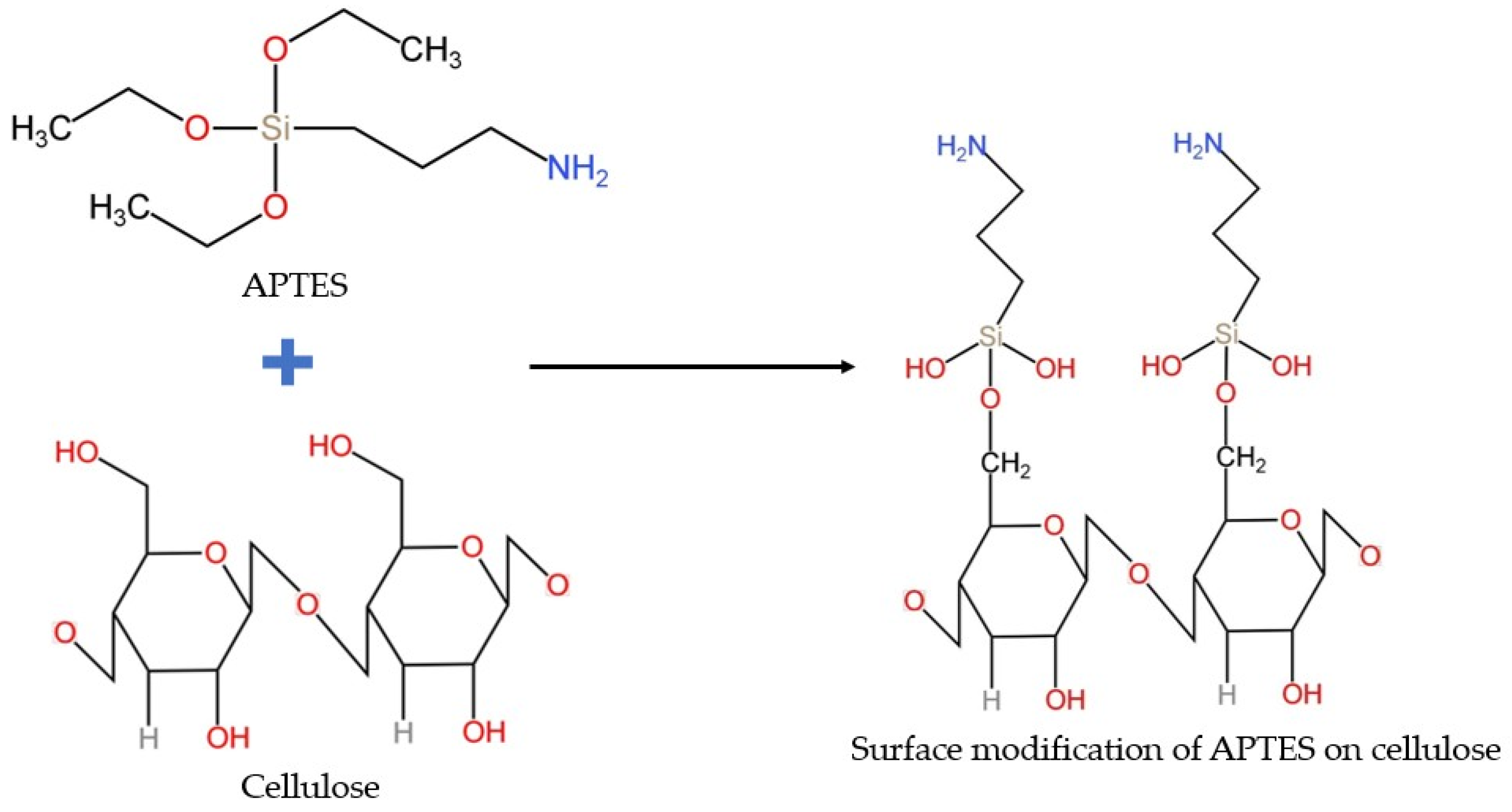
2.3. Silanization Process
2.4. Photophysical Properties
2.5. Atomic Force Microscopy (AFM)
2.6. FT-IR
2.7. Particle Charge Detector (PCD)
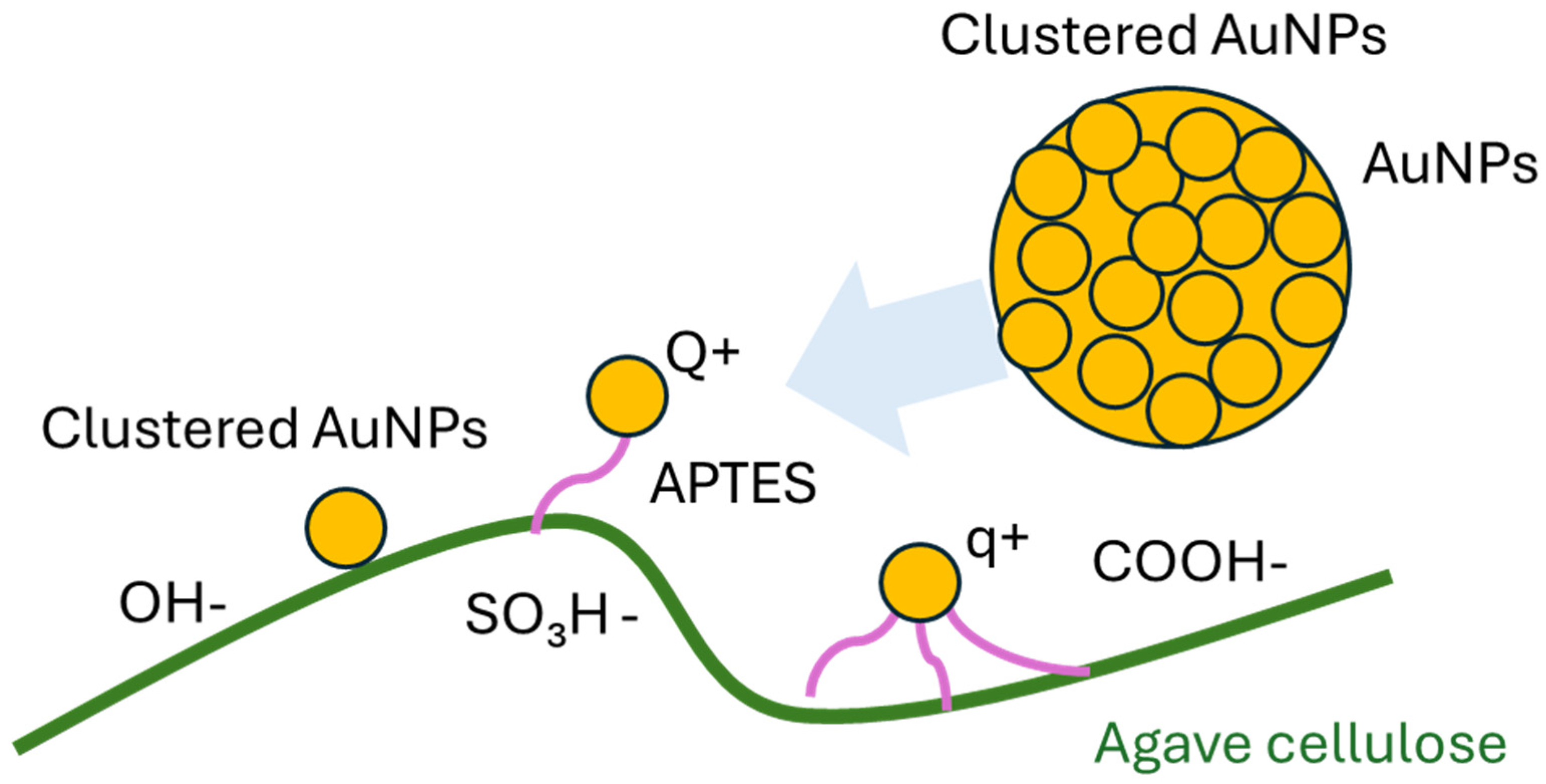
3. Results and Discussion
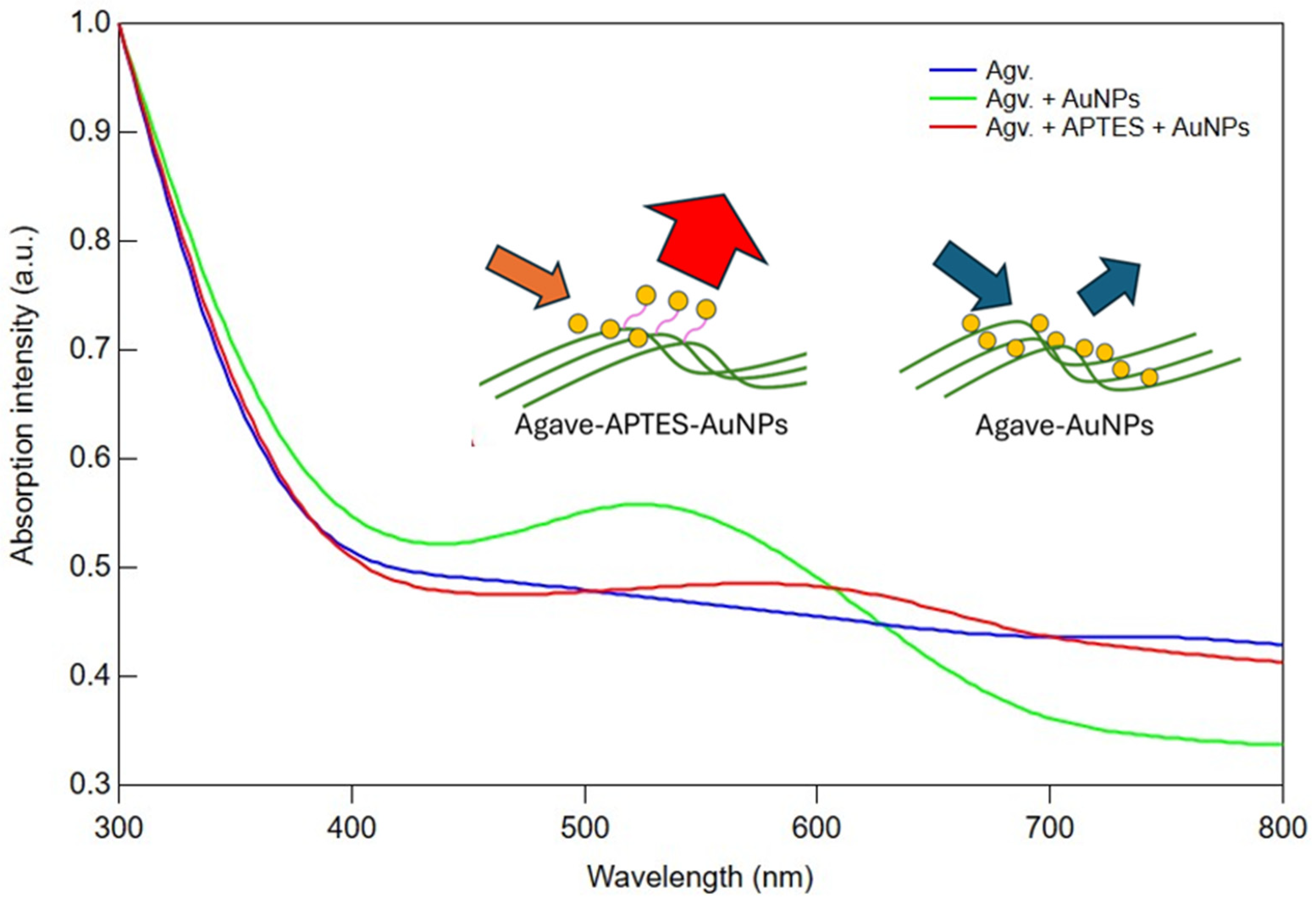
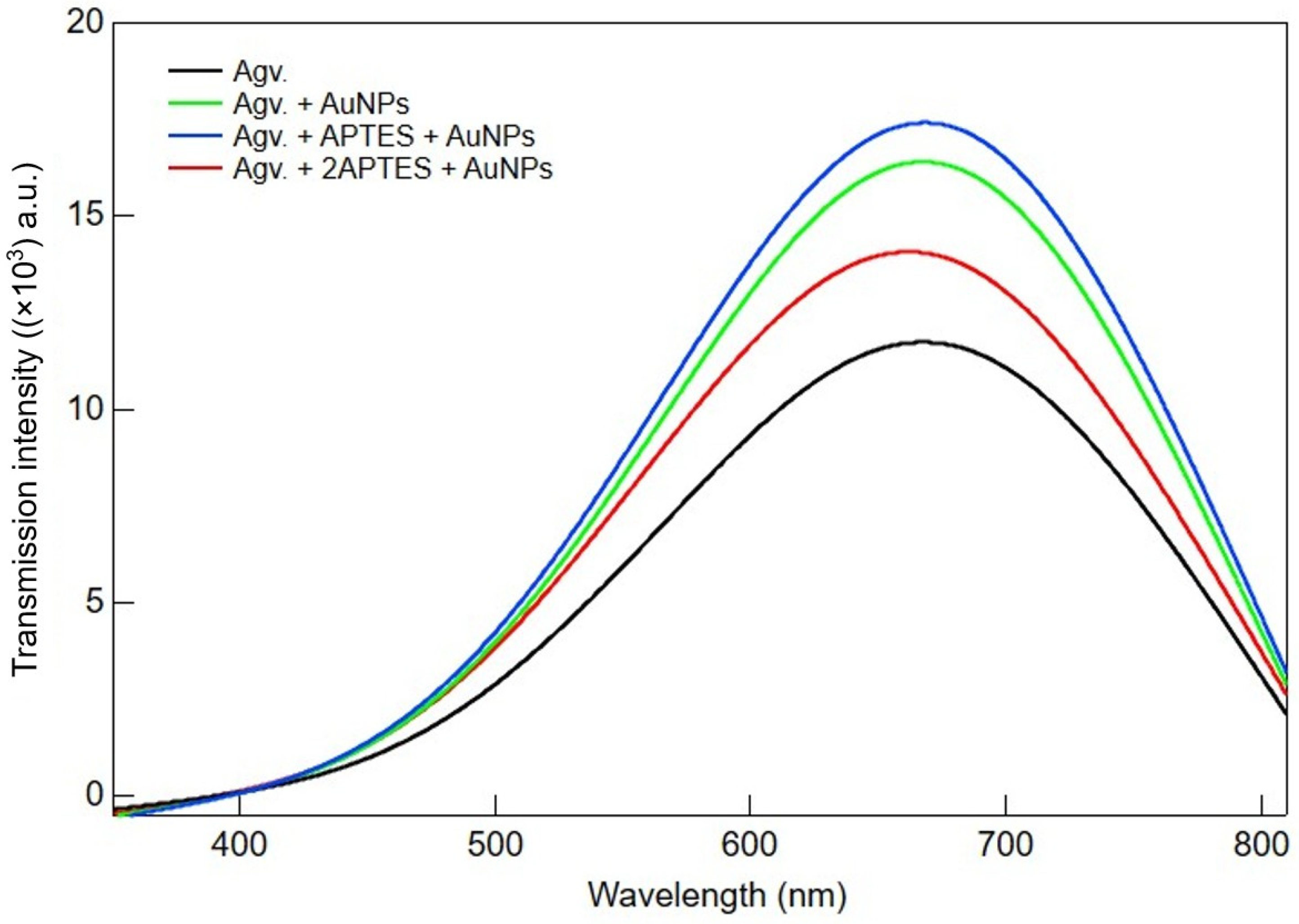
3.1. Photophysical Properties from Absorption and Transmission Spectroscopy
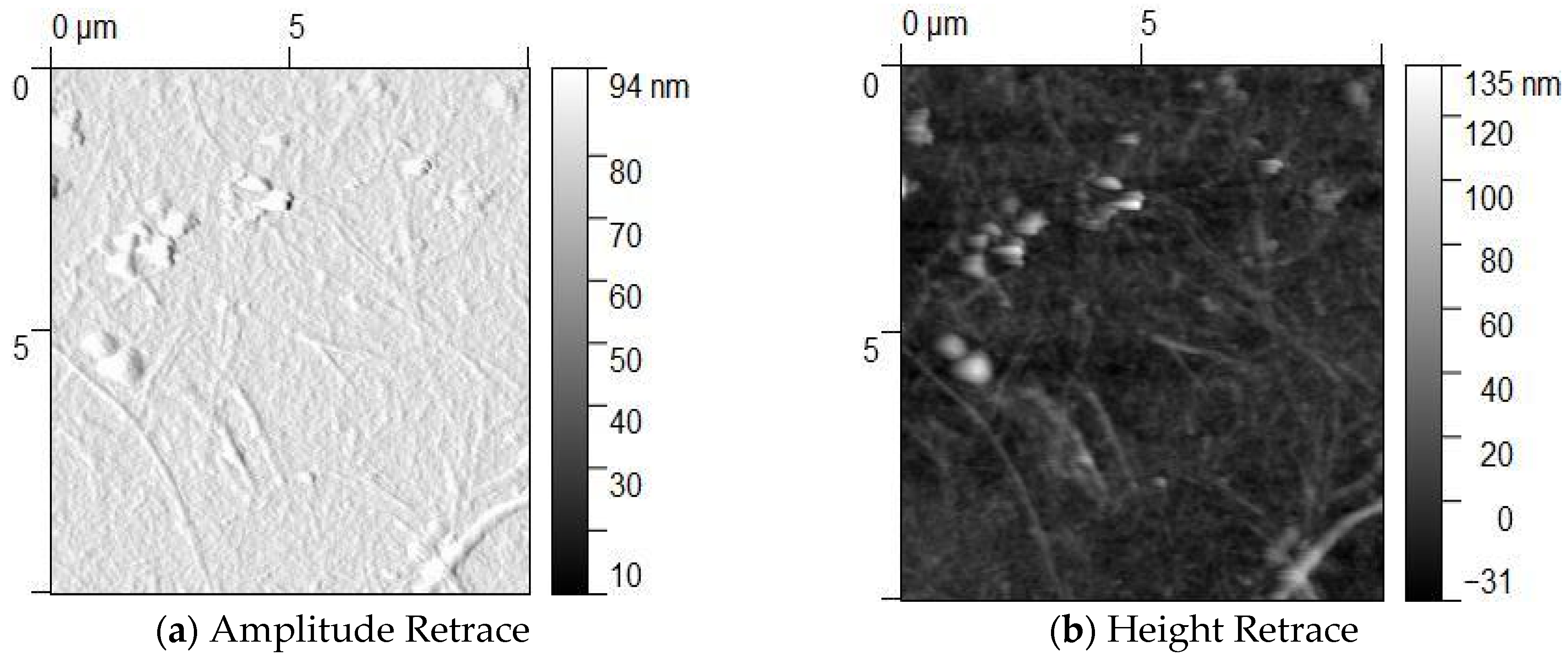
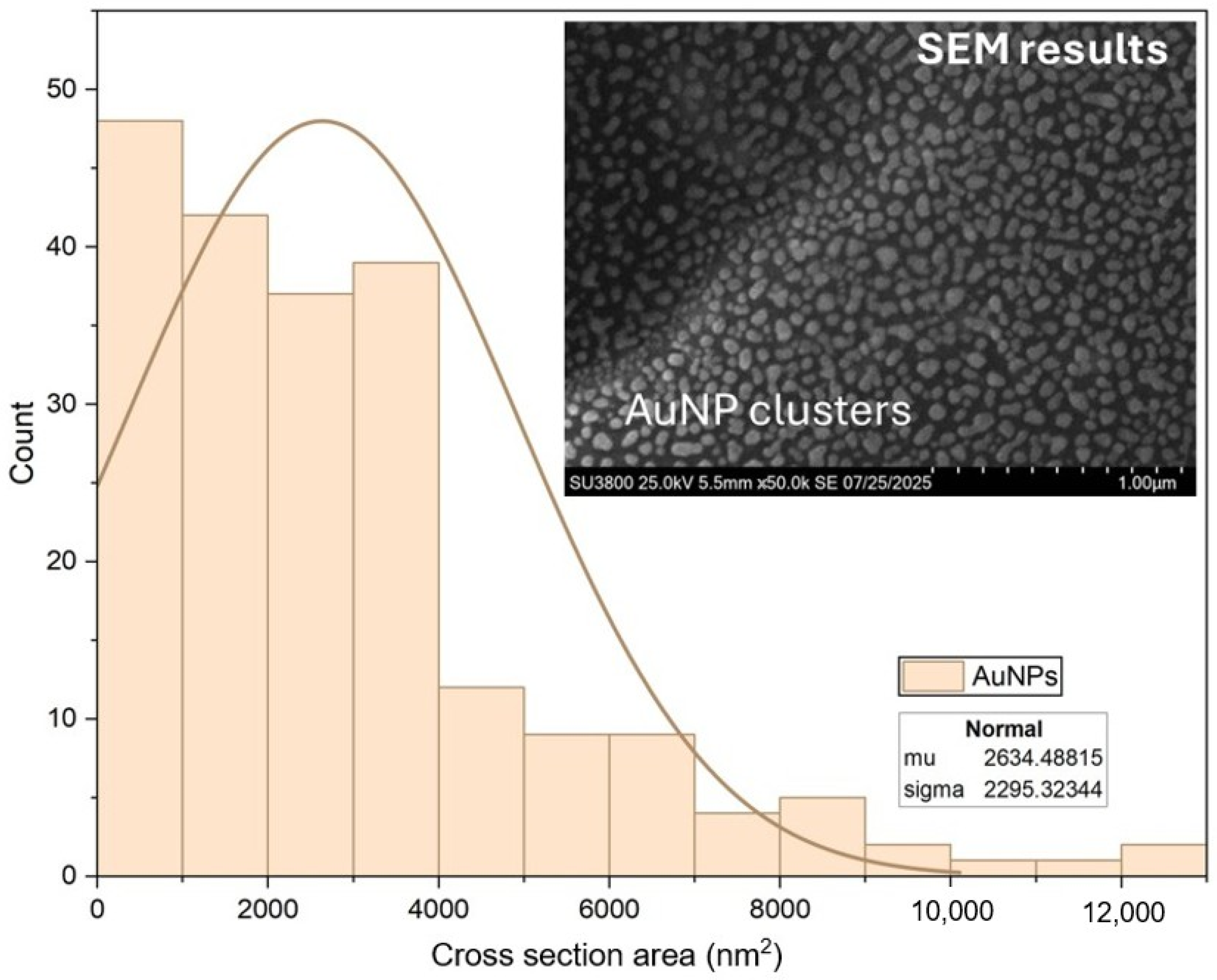
3.2. Morphological Studies Using Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM) Analysis
3.3. Functional Analysis and Net Charge Analysis Using FTIR and PCD
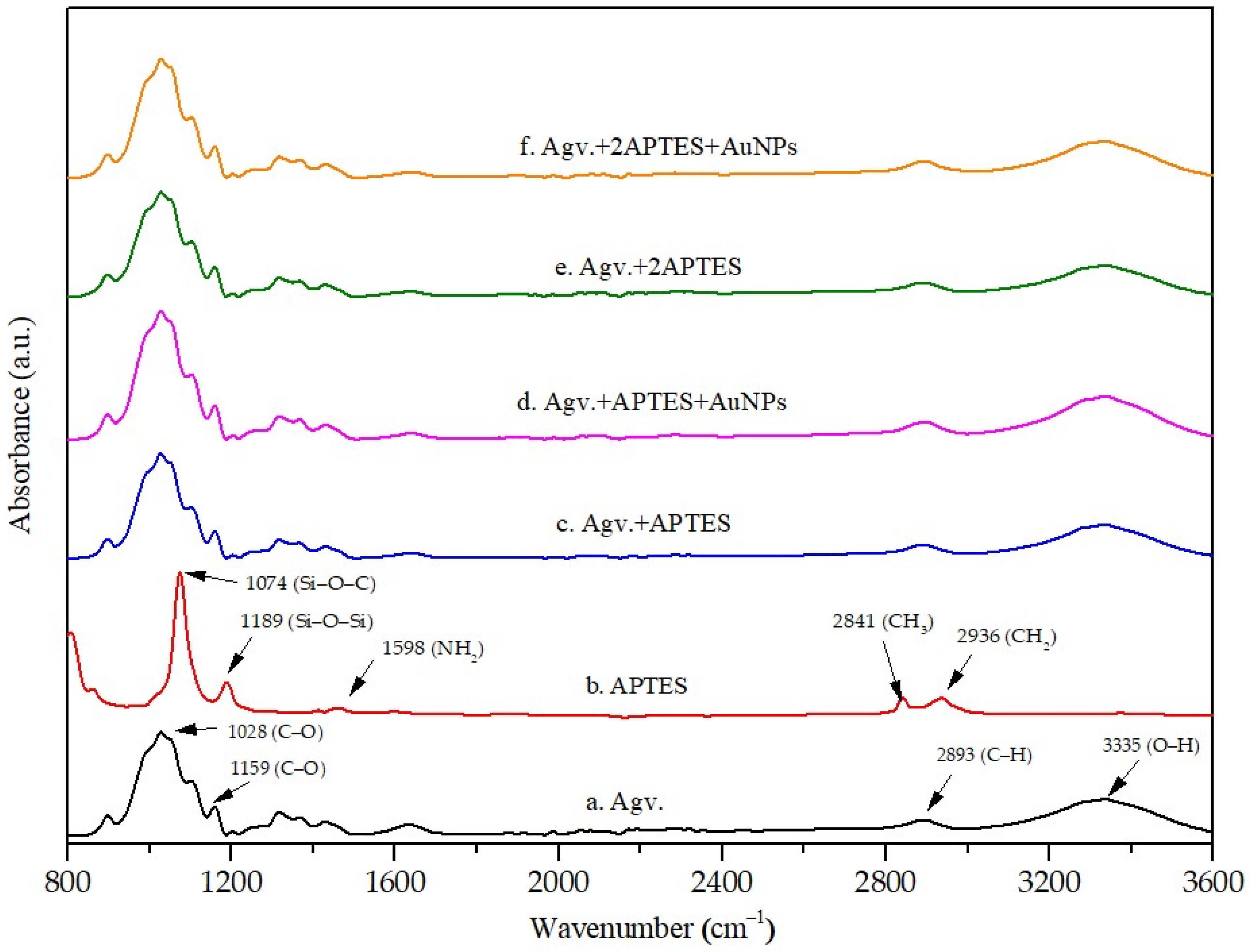
3.3.1. FTIR Analysis of Cellulose Modified with APTES and AuNPs
3.3.2. Particle Charge Detection (PCD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Zhu, E.; Li, T.; Zhang, L.; Wang, Z. High-Value Utilization of Cellulose: Intriguing and Important Effects of Hydrogen Bonding Interactions—A Mini-Review. Biomacromolecules 2024, 25, 6296–6318. [Google Scholar] [CrossRef]
- Pinto, R.J.; Carlos, L.D.; Marques, P.A.; Silvestre, A.J.; Freire, C.S. An overview of luminescent bio-based composites. J. Appl. Polym. Sci. 2014, 131, 41169. [Google Scholar] [CrossRef]
- Kulpinski, P.; Namyslak, M.; Grzyb, T.; Lis, S. Luminescent cellulose fibers activated by Eu3+-doped nanoparticles. Cellulose 2012, 19, 1271–1278. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef]
- Musino, D.; Rivard, C.; Landrot, G.; Novales, B.; Rabilloud, T.; Capron, I. Hydroxyl groups on cellulose nanocrystal surfaces form nucleation points for silver nanoparticles of varying shapes and sizes. J. Colloid Interface Sci. 2021, 584, 360–371. [Google Scholar] [CrossRef]
- Geethalakshmi, K.; Ng, T.Y.; Crespo-Otero, R. Tunable optical properties of OH-functionalised graphene quantum dots. J. Mater. Chem. C 2016, 4, 8429–8438. [Google Scholar] [CrossRef]
- Miao, C.; Mauran, D.; Hamad, W.Y. How hydrogen-bonding interactions and nanocrystal aspect ratios influence the morphology and mechanical performance of polymer nanocomposites reinforced with cellulose nanocrystals. Soft Matter 2022, 18, 4572–4581. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Rauwel, E.; Estephan, E.; Soares, M.R.; Rauwel, P. Significance of hydroxyl groups on the optical properties of ZnO nanoparticles combined with CNT and PEDOT: PSS. Nanomaterials 2022, 12, 3546. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Aminopropyltriethoxysilane as a linker for cellulose-based functional materials: New horizons and future challenges. Curr. Opin. Green Sustain. Chem. 2021, 30, 100480. [Google Scholar] [CrossRef]
- Amit, S.K.; Gomez-Maldonado, D.; Bish, T.; Peresin, M.S.; Davis, V.A. Properties of APTES-Modified CNC Films. ACS Omega 2024, 9, 16572–16580. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Kim, D.H. Surface plasmon resonance mediated photoluminescence properties of nanostructured multicomponent fluorophore systems. Nanoscale 2014, 6, 4966–4984. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Nair, B.U.; Sreeram, K.J. Effect of functionalized gold nanoparticle on collagen stabilization for tissue engineering application. Int. J. Biol. Macromol. 2019, 123, 1211–1220. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shamsuzzaman, M.; Rahman, M.M.; Moeiz, S.I.; Ni, Y. Effect of pre-extraction on soda-anthraquinone (AQ) pulping of rice straw. Ind. Crops Prod. 2012, 37, 164–169. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, H.-J. Effect of H2O2 bleaching with ultrasonication on the properties of thermomechanical pulp and unbleached kraft pulp. Ultrason. Sonochem. 2015, 23, 347–353. [Google Scholar] [CrossRef]
- Fergusson, J.; Wallace, G.Q.; Sloan-Dennison, S.; Carland, R.; Shand, N.C.; Graham, D.; Faulds, K. Plasmonic and photothermal properties of silica-capped gold nanoparticle aggregates. J. Phys. Chem. C 2023, 127, 24475–24486. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Al-Harthi, S.H.; Sellai, A.; Dutta, J. Self-organization of gold nanoparticles on silanated surfaces. Beilstein J. Nanotechnol. 2015, 6, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: London, UK, 2006. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Dulkeith, E.; Morteani, A.; Niedereichholz, T.; Klar, T.; Feldmann, J.; Levi, S.; Van Veggel, F.; Reinhoudt, D.; Möller, M.; Gittins, D. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef]
- Persson, B.N.J.; Lang, N.D. Electron-hole-pair quenching of excited states near a metal. Phys. Rev. B 1982, 26, 5409–5415. [Google Scholar] [CrossRef]
- Kim, K.-S.; Zakia, M.; Yoon, J.; Yoo, S.I. Metal-enhanced fluorescence in polymer composite films with Au@Ag@SiO2 nanoparticles and InP@ZnS quantum dots. RSC Adv. 2019, 9, 224–233. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, P.; Li, S.; Garai, M.; Hong, M.; Xu, Q.-H. Plasmon-enhanced fluorescence in coupled nanostructures and applications in DNA detection. ACS Appl. Bio Mater. 2018, 1, 118–124. [Google Scholar] [CrossRef]
- Minopoli, A.; Della Ventura, B.; Campanile, R.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Randomly positioned gold nanoparticles as fluorescence enhancers in apta-immunosensor for malaria test. Microchim. Acta 2021, 188, 88. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Ornaghi, H.; Zattera, A. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Kawalerczyk, J.; Walkiewicz, J.; Dziurka, D.; Mirski, R.; Brózdowski, J. APTES-modified nanocellulose as the formaldehyde scavenger for UF adhesive-bonded particleboard and strawboard. Polymers 2022, 14, 5037. [Google Scholar] [CrossRef] [PubMed]
- Prodan, D.; Moldovan, M.; Furtos, G.; Saroși, C.; Filip, M.; Perhaița, I.; Carpa, R.; Popa, M.; Cuc, S.; Varvara, S. Synthesis and characterization of some graphene oxide powders used as additives in hydraulic mortars. Appl. Sci. 2021, 11, 11330. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Lu, Q.; Moore, J.M.; Huang, G.; Mount, A.S.; Rao, A.M.; Larcom, L.L.; Ke, P.C. RNA polymer translocation with single-walled carbon nanotubes. Nano Lett. 2004, 4, 2473–2477. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Xie, J.; Wang, Y.; Wang, T.-J. Surface amination of silica nanoparticles using tris (hydroxymethyl) aminomethane. Ind. Eng. Chem. Res. 2020, 59, 21383–21392. [Google Scholar] [CrossRef]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size correlation of optical and spectroscopic properties for gold nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Colloidal silver nanoplates. State of the art and future challenges. J. Mater. Chem. 2008, 18, 1724–1737. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Fan, C.; Wang, S.; Hong, J.W.; Bazan, G.C.; Plaxco, K.W.; Heeger, A.J. Beyond superquenching: Hyper-efficient energy transfer from conjugated polymers to gold nanoparticles. Proc. Natl. Acad. Sci. USA 2003, 100, 6297–6301. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, F.; Zuo, P.; Tian, H. Principles and applications of resonance energy transfer involving noble metallic nanoparticles. Materials 2023, 16, 3083. [Google Scholar] [CrossRef] [PubMed]
- Campora, L.; Metzger, C.; Dähnhardt-Pfeiffer, S.; Drexel, R.; Meier, F.; Fürtauer, S. Fluorescence Labeling of Cellulose Nanocrystals A Facile and Green Synthesis Route. Polymers 2022, 14, 1820. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Hyun, J.H.; Kim, J.; Jung, K.O.; Kim, D. Recent progress in the development of cellulose-derived organic-nanopolymer and coordination network platforms for application as optical chemosensors. Adv. Compos. Hybrid Mater. 2025, 8, 30. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]


| Sample | Equivalent (µeq/L) | Start Potential (mV) | Surface Interpretation |
|---|---|---|---|
| Agv. | −16 | −245 | Slightly negative surface charge due to deprotonated hydroxyl groups [39]. |
| Agv. + APTES | −148 | −85 | Amino groups from APTES can be protonated (–NH3+), compensating for the negative charges; however, residual –OH and Si–O− groups maintain the overall negative charge of the surface [40,41]. |
| Agv. + AuNPs | −362 | −227 | Strongly negative surface charge resulting from citrate-stabilized AuNPs [4,42]. |
| Agv. + APTES + AuNPs | −141 | −35 | APTES partially neutralizes the negative charges on citrate-coated AuNPs and facilitates their immobilization on cellulose, resulting in an almost neutral surface [43]. |
| Agv. + 2APTES + AuNPs | −43 | −31 | A double-layer APTES coating increases the density of amino-silane groups, further compensating negative charges and approaching electrical neutrality [44]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Techasuksakul, K.; Khamphakun, P.; Srichola, P.; Lobyaem, K.; Sampoompuang, C.; Wangkham, T.; Kataphiniharn, C.; Phanphak, S. Impact of Hydrogen Bonding in Natural Cellulose Fibers on Plasmonic Nanoparticles. Polymers 2025, 17, 3152. https://doi.org/10.3390/polym17233152
Techasuksakul K, Khamphakun P, Srichola P, Lobyaem K, Sampoompuang C, Wangkham T, Kataphiniharn C, Phanphak S. Impact of Hydrogen Bonding in Natural Cellulose Fibers on Plasmonic Nanoparticles. Polymers. 2025; 17(23):3152. https://doi.org/10.3390/polym17233152
Chicago/Turabian StyleTechasuksakul, Kunwara, Prapakorn Khamphakun, Preeyanuch Srichola, Keowpetch Lobyaem, Chaiyaporn Sampoompuang, Thidarat Wangkham, Chanwit Kataphiniharn, and Sorasak Phanphak. 2025. "Impact of Hydrogen Bonding in Natural Cellulose Fibers on Plasmonic Nanoparticles" Polymers 17, no. 23: 3152. https://doi.org/10.3390/polym17233152
APA StyleTechasuksakul, K., Khamphakun, P., Srichola, P., Lobyaem, K., Sampoompuang, C., Wangkham, T., Kataphiniharn, C., & Phanphak, S. (2025). Impact of Hydrogen Bonding in Natural Cellulose Fibers on Plasmonic Nanoparticles. Polymers, 17(23), 3152. https://doi.org/10.3390/polym17233152








